Translate this page into:
Ivermectin ameliorate the toxic effect of dimethylhydrazine in male Wistar rats
⁎Corresponding author. salkahtani@ksu.edu.sa (Saad Alkahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cancer is emerging as one of the most significant challenges to the human health. Ivermectin (IVM) is one of the most effective drugs in veterinary and human medicine. The purpose of this study was to evaluate the potential preventative effect of IVM against cytotoxicity induced by dimethylhydrazine (DMH) in Male Wistar rats. Three concentrations of IVM (0.25, 0.5 and 1 mg/kg) were utilized in this study. Liver function enzymes, inflammatory markers and histopathological alterations in liver and kidney tissues were investigated. Obtained data showed that the combination of DMH with each concentration of IVM increased the activity of liver enzymes (ALT, ALP, AST, and GGT) compared to control and DMH alone. RT-PCR findings showed upregulation in the level of INF-γ, IL-6, IL-1β, Cox1, and Cox2 genes compared to control. In contrast, IL-6 gene was down-regulated. The levels of genes expression were down-regulated with combined DMH with medium and high concentrations compared to DMH alone. Microscopic analysis demonstrated histological alterations in the liver and kidney of rats treated with DMH. Combinations of DMH either with medium or high concentration resulted in less pathological features in both liver and kidney tissues. In conclusion, this study showed that IVM mitigated the toxic effects of DMH on the liver and kidney of male Wistar rats.
Keywords
Ivermectin
Dimethylhydrazine
Liver
Kidney
Cytotoxicity
Rats
- IVM
-
Ivermectin
- DMH
-
Dimethylhydrazine
- CKD
-
Chronic kidney disease
- ROS
-
Reactive oxygen species
- ALT
-
Alanine transaminase
- AST
-
Aspartate transaminase
- ALP
-
Alkaline phosphatase
- GGT
-
Gamma-glutamyltransferase
- INF-α
-
Tumor necrosis factor-alpha
- IL-6
-
Interleukin-6
- IL-1β
-
Interleukin-1βeta
- Cox-1
-
Cyclooxygenase-1
- Cox-2
-
Cyclooxygenase-2
Abbreviations
1 Introduction
Cancer is one of the major causes of death globally and it develops due to uncontrolled proliferation of abnormal cells with altered genetic materials (Kooti et al., 2017). Cellular processes involving structure and function of cells including; cell growth and survival are regulated at the genetic level. In addition, there are environmental factors and others that cause cancer diseases (Tiffon, 2018). Liver is a largest organ in the human body that has a wide range of functions and is essential for survival. Hepatocellular carcinoma (HCC) is a type of cancer that starts in the liver and may develop and spread to other tissues. However, only cancers that start in the liver are described as liver cancer. Understanding the pathogenesis of hepatocellular carcinoma is essential for its prevention (Imawari, 2002). Kidney also, plays a vital role in human body. Chronic kidney disease (CKD) and cancer are connected either directly or indirectly through the adverse effects of therapies, thus, CKD may conversely be a risk factor for cancer. However, both CKD and cancer share common risk factors (Bénédicte, 2010). Ivermectin (IVM) is one of the most important drugs in veterinary and human medicine for the control of parasitic infection (Laing et al., 2017). Moreover, it is used in the treatment of human onchocerciasis and endoparasites (DelGiudice et al, 2003). The metabolism of IVM is primary via the oxidative pathways, and it has a high affinity to bind with proteins. The co-administration of IVM and Albendazole caused significant increase in serum urea and creatinine in rats. In contrast, the administration of vitamin E caused reduction in urea, creatinine level in rats (Arise and Malomo, 2009; Tavares de Almeida et al., 2012). Recently, it has been reported that two doses (300 μg/kg) of IVM and prophylaxis were associated with a reduction of SARS-CoV-2 infection among healthcare workers for weeks (Priyamadhaba et al., 2021). IVM has been shown to normalize blood sugar and cholesterol levels in diabetic mice (Jin et al., 2015), suppress proliferation in different types of cancer cell lines (Yin et al., 2015), reduce inflammatory and allergic (Schaller et al., 2017; Ventre et al., 2017). The antineoplastic activities of IVM against many types of cancer related to apoptosis and oxidative stress pathways. IVM induces generation of intracellular reactive oxygen species (ROS) in cancer cells leading to oxidative stress and DNA damage (Dominguez-Gomez et al., 2018; Juarez et al., 2018; Wang et al., 2018). A high prevalence of abnormal liver function parameters was observed for AST and ALT, respectively in population after treatment with IVM compared to healthy participants. The prevalence of abnormal kidney function profiles revealed (11.1 %) for creatinine, (16.2 %) for urea, and (9.1 %) for creatinine clearance these values indicated a significant association with IVM uptake (Shiynsa et al., 2021). However, despite extensive research, the mechanism of IVM remains unclear (Laing et al., 2017). As a model of cancer induction, dimethylhydrazine (DMH) has been used as a potent carcinogen in experimental animals (Perse and Cerar, 2011; Rubio, 2017). The objective of this study was to assess the effects of IVM against the cytotoxic effects of DMH in the liver and kidney of Male Wistar Rats.
2 Materials and methods
2.1 Animals and pharmacological treatments
Male rats weighing 120–150 g were provided from animal house and were housed under standard laboratory conditions. Ethical approval (KSU-SE-20–20) was obtained from King Saud University. DMH was purchased from Sigma-Aldrich (St. Louis, Missouri, USA) and dissolved in 0.001 M EDTA (pH 6.5) (Chari et al., 2018). Rats were injected subcutaneously with DMH (40 mg/kg) twice a week for 5 weeks. It was settled that the statistical accuracy of the fatal dosage of an ingredient recognized as eradicating half the animals in a trail which is the LD50 (Botham, 2002). Individual dosage was analyzed on 5 rats, and the following phase was chosen based on the fatality. LD50 of IVM dosage was observed to be 5–50 mg/Kg. experimented through initial 24 h. Based on LD50, three selected concentrations (0.25, 0.5, and 1 mg/kg) of IVM were used. Treated rats with IVM started before and during the course of DMH (Dadarkar et al., 2007).
3 Experimental design
A total of 40 male rats were divided into 8 groups (5 rats/group) as following: Group-1; negative control. Group-2, 3, and 4; treated with (0.25, 0.5 and 1 mg/kg) respectively of IVM for 10 weeks. Group-5; treated with 40 mg/kg of DMH (positive control) twice a week for five weeks. Group-6; treated daily with (0.25 mg/kg) of IVM for 10 weeks plus dose of DMH (40 mg/kg), twice a week for five weeks. Group-7: treated daily with (0.5 mg/kg) of IVM for 10 weeks plus dose of DMH (40 mg/kg), twice a week for five weeks. Group-8: treated daily with (1 mg/kg) of IVM for 10 weeks plus DMH (40 mg/kg) twice a week for five weeks. IVM was given for 1 week before the subcutaneous injection of DMH and continue for 4-weeks after the last DMH dose (Dadarkar et al., 2007).
3.1 Blood collection and tissue preparation
At the end of experiment, blood samples were collected via retro-orbital under ketamine/xylazine anesthesia (Paulose and Dakshinamurti, 1987). After clotting, samples were centrifuged at 3000 rpm for 15 min. Isolated serum was used for further biochemical analysis. For histopathological analysis, rats were sacrificed by cervical dislocation. The liver and kidney organs were immediately removed and fixed in 10 % buffered formalin till histological processing.
3.2 Biochemical analysis
Liver enzymes were assessed. Serum levels of alanine transaminase (ALT), aspartate transaminase (AST) (Reitman and Frankel, 1957), alkaline phosphatase (ALP) (Tietz et al., 1983) and gamma-glutamyltransferase (GGT) were measured (Abdelhalim, 2013).
3.3 Gene expression
For the purpose of this study, the activity of tumor necrosis factor-alpha (INF-α), interleukin-6 (IL-6), interleukin-1βeta (IL-1β), cyclooxygenase-1 (Cox-1), and cyclooxygenase-2 (Cox-2) genes were investigated. RNA was extracted using RNA extraction kit using previously published method (Khan et al., 2017). The cDNA was synthesized using superscript VILO cDNA Synthesis Kit. The qRT–PCR was performed using Syber green. The relative amount of mRNA was calculated with the cycle threshold (Ct) method. Fold changes in gene expression were analyzed by the 2−△△Ct method (Saquib et al., 2013).
3.4 Histopathological examination
Liver and kidney specimen were removed from the rats, fixed in 10 % buffered formalin, dehydrated in ethanol (50–100 %), cleared in xylene, and embedded in paraffin. Sections (4–5 μm thick) were prepared and then stained with hematoxylin and eosin (H&E). The sections were examined microscopically to detect any pathological alterations in the liver and kidney tissues.
3.5 Statistical analysis
The obtained results were analyzed using Statistical Package for Social Science (SPSS) version (SPSS Inc., Chicago) for windows. Result expressed as mean ± SE (n = 5). One-way ANOVA followed by Duncan test will use for analysis. P value<0.05 was considered significant.
4 Results
4.1 Liver functions
4.1.1 ALT activity
In order to investigate the effect of IVM on the liver function, the activity of ALT as an indicator for liver toxicity conditions was measured. As shown in (Fig. 1) the ALT activity was significantly decreased in rats treated with medium and high concentrations compared to control. Interestingly, each combination of DMH and the different doses of IVM decreased compared to DMH alone, which means all doses of IVM were reduced the negative effect of DMH on ALT activity.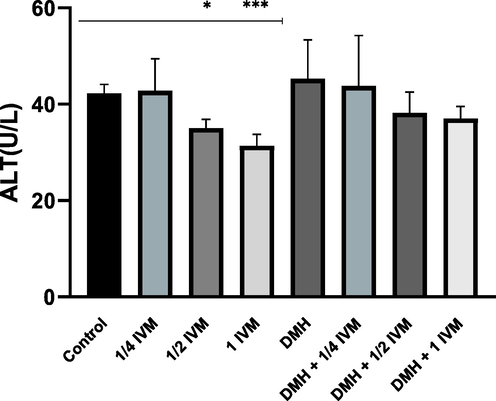
Shows the levels of ALT activity (u/l) in treated rats. Data represents the mean ± SE (n = 5).
4.1.2 ALP activity
The activity of ALP is associated with the toxicity and inflammatory conditions. The result showed significantly decreased ALP activities in rats treated with medium and high doses of IVM compared to control. Furthermore, ALP activity was significantly decreased in rats treated with combination of DMH and high dose of (IVM) compared to (DMH) alone (Fig. 2).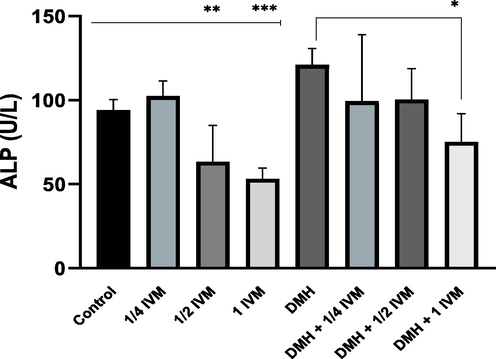
Shows the levels of ALP activity (u/l) in treated rats. Data represents the mean ± SE (n = 5).
4.1.3 AST activity
Cytoplasmic AST is used as a biomarker for liver damage. It is found in the cytoplasm of hepatocytes and other tissues. The present result demonstrated the level of AST significantly decreased in rats treated with high concentration of (IVM) compared to control and significantly decreased in rats treated with combination of DMH and medium concentration of (IVM) compared to DMH alone (Fig. 3). Like ALT, all doses of IVM reduced the side effect of DMH on the level of AST.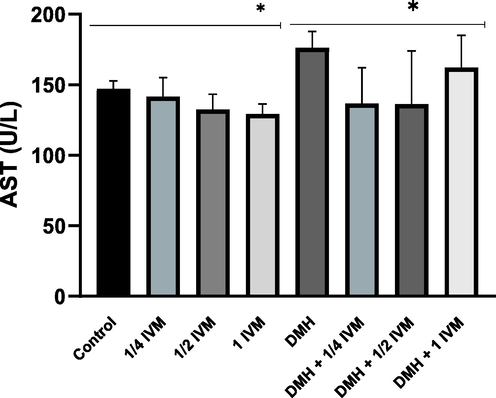
Shows the levels of AST activity (u/l) in treated rats. Data represents the mean ± SE (n = 5).
4.1.4 GGT activity
Serum value of GGT activity is elevated in all types of liver disease. Thus, the determination of GGT activity in serum is an essential indicator in the assessment of toxic effects. The obtained results indicated significant increase in rats treated with DMH alone compared to control. In contrast, the GGT level was significantly decreased in rats treated with combination of DMH and high dose of IVM compared to DMH alone (Fig. 4). Furthermore, our data demonstrated reduction in GGT level with each combined dose of IVM.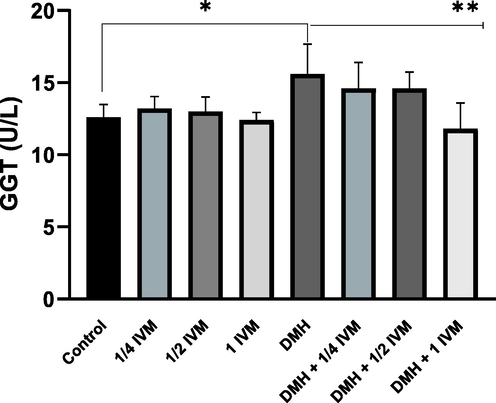
Shows the levels of GGT activity (u/l) in treated rats. Data represents the mean ± SE (n = 5).
4.1.5 Gene expression analysis
To investigate whether IVM, inhibit the effect of DMH in the treated rats, this study quantified the gene expression level of selected inflammatory markers genes including INF-α, IL-6, IL-1β, Cox-1, and Cox-2 using RT-PCR. The effects of DMH and IVM on the expression of inflammatory markers were shown in (Fig. 5 A-G). The levels of mRNA expression of INF-α and IL-1β, Cox-1, and Cox-2 genes were upregulated in rats treated with high and medium concentrations of IVM compared to control (Fig. 5 A and B). The expression levels of IL-6 and IL-1β were down-regulated at low concentration of IVM (Fig. 5 C). The levels of INF-α, IL-1β, Cox-1, and Cox-2 in rat treated with DMH alone and DMH combined with high and medium doses were upregulated. In contrast, the level of IL-6 was down-regulated in rats treated with DMH alone and DMH combined with high doses (Fig. 5 D, E, and F). Furthermore, the levels of all targeted genes were increased in rat treated with DMH and low dose of IVM (Fig. 5 G) while the level of IL-6 was down-regulated in rats treated with DMH combined with high and medium doses compared DMH alone. Based on genes expression, these findings showed clear effect of IVM on the targeted genes but the picture is not clear enough compared to DMH alone at tested doses.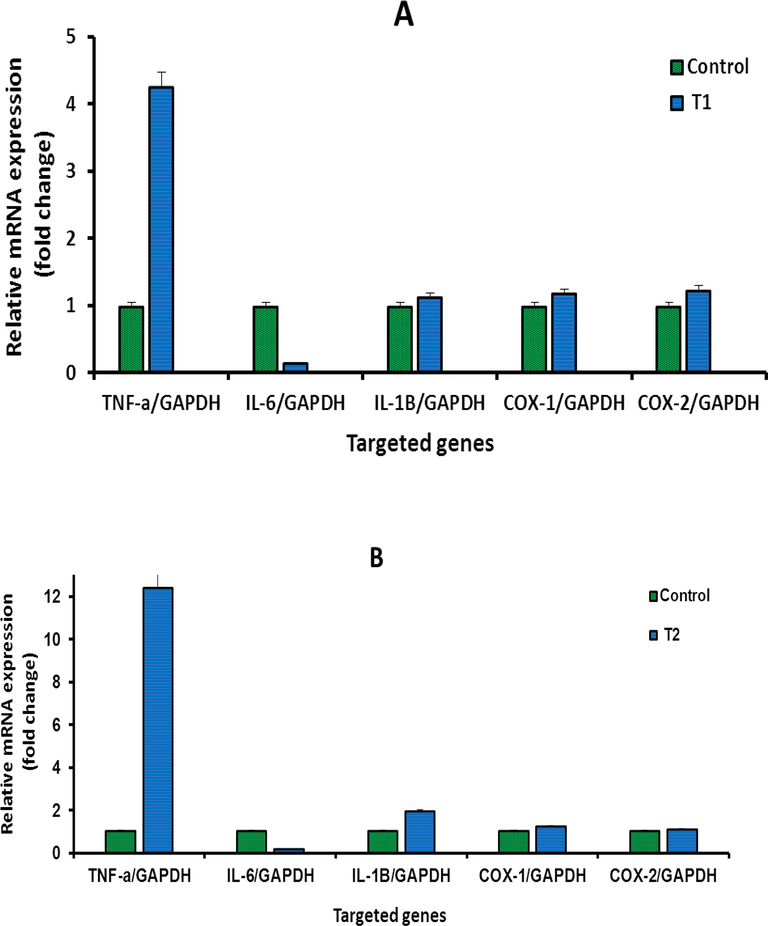
A-G: Shows the mRNA levels of inflammatory genes in the treated rats with high concentration (1.0 mg/kg) of IVM (A), with medium concentration (0.5 mg/kg) of IVM (B), with low concentration (0.25 mg/kg) of IVM (C), with 40 mg/kg of DMH (D), with combination of DMH and high dose of IVM (E), with combination of DMH and medium dose of IVM (F), with combination of DMH and low dose of IVM (G). Each value represents the mean ± SE. (n = 5), (*p < 0.05), (***p < 0.001) compared with untreated cells. T1: 1.0 mg/kg IVM. T2: 0.5 mg/kg IVM. T3: 0.25 mg/kg IVM. D: 40 mg/kg of DMH. TD1: 40 mg/kg of DMH + 1.0 mg/kg) of IVM. TD2: 40 mg/kg of DMH + 0.5 mg/kg) of IVM. TD3: 40 mg/kg of DMH + 0.25 mg/kg) of IVM.
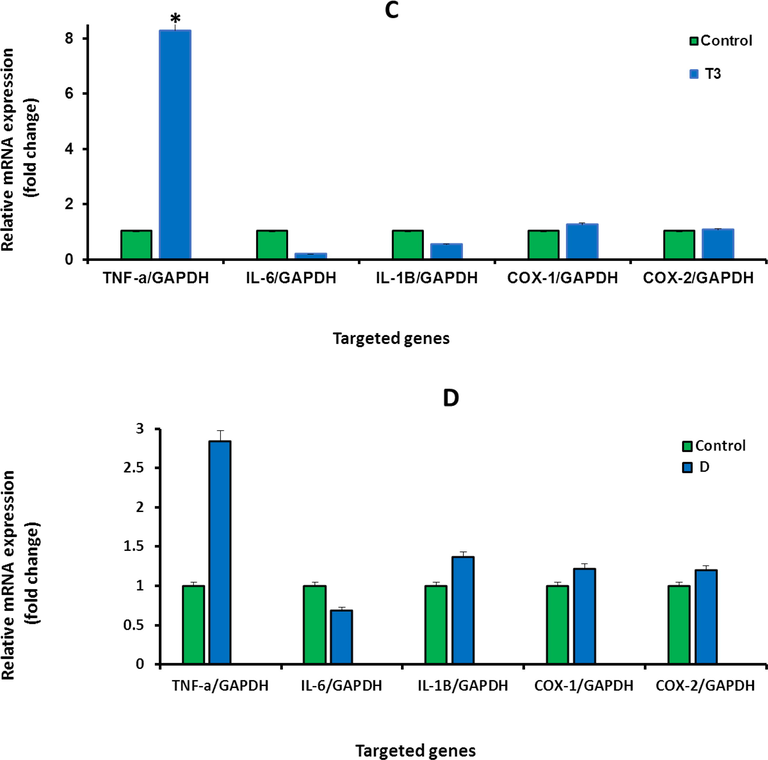
A-G: Shows the mRNA levels of inflammatory genes in the treated rats with high concentration (1.0 mg/kg) of IVM (A), with medium concentration (0.5 mg/kg) of IVM (B), with low concentration (0.25 mg/kg) of IVM (C), with 40 mg/kg of DMH (D), with combination of DMH and high dose of IVM (E), with combination of DMH and medium dose of IVM (F), with combination of DMH and low dose of IVM (G). Each value represents the mean ± SE. (n = 5), (*p < 0.05), (***p < 0.001) compared with untreated cells. T1: 1.0 mg/kg IVM. T2: 0.5 mg/kg IVM. T3: 0.25 mg/kg IVM. D: 40 mg/kg of DMH. TD1: 40 mg/kg of DMH + 1.0 mg/kg) of IVM. TD2: 40 mg/kg of DMH + 0.5 mg/kg) of IVM. TD3: 40 mg/kg of DMH + 0.25 mg/kg) of IVM.
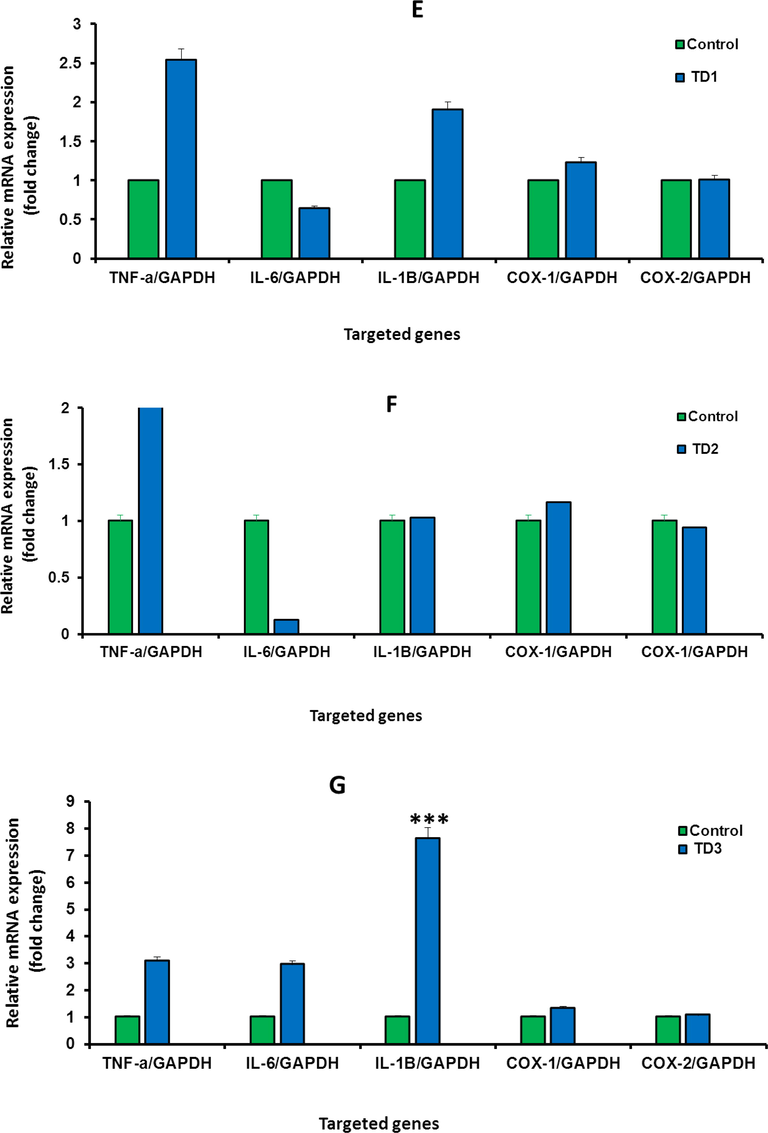
A-G: Shows the mRNA levels of inflammatory genes in the treated rats with high concentration (1.0 mg/kg) of IVM (A), with medium concentration (0.5 mg/kg) of IVM (B), with low concentration (0.25 mg/kg) of IVM (C), with 40 mg/kg of DMH (D), with combination of DMH and high dose of IVM (E), with combination of DMH and medium dose of IVM (F), with combination of DMH and low dose of IVM (G). Each value represents the mean ± SE. (n = 5), (*p < 0.05), (***p < 0.001) compared with untreated cells. T1: 1.0 mg/kg IVM. T2: 0.5 mg/kg IVM. T3: 0.25 mg/kg IVM. D: 40 mg/kg of DMH. TD1: 40 mg/kg of DMH + 1.0 mg/kg) of IVM. TD2: 40 mg/kg of DMH + 0.5 mg/kg) of IVM. TD3: 40 mg/kg of DMH + 0.25 mg/kg) of IVM.
5 Histopathological examination
5.1 Liver histopathology
Microscopic examination of liver sections of untreated control group showed normal hepatic architecture with central vein, portal triad, blood sinusoids between hepatic strands of hepatocytes. The hepatocytes are polygonal cells with large spherical pale nuclei (Fig. 6 A). Moreover, IVM- treated groups (0.25, 0.50 and 1 mg/kg) revealed normal liver tissue similar to untreated control group (Fig. 6 B, C and D) respectively. Liver of rats treated with DMH revealed significant pathological signs manifested by dilated vein congested with edema surrounded by infiltrative cells mixed with fibers, hepatocytes showed cytoplasmic degeneration (Fig. 7 A). However, rats -treated with 0.25 mg/kg of IVM to DMH displayed less pathological features as thickened wall surrounded with few numbers of infiltrative cells, while hepatocytes looked healthy (Fig. 7 B)., group -treated with 0.50 mg/kg of IVM to DMH showed healthy tissue except some foci of inflammation (Fig. 7 C). Furthermore, group -treated with 1 mg/kg of IVM to DHM exhibited healthy hepatic tissue (Fig. 7 D).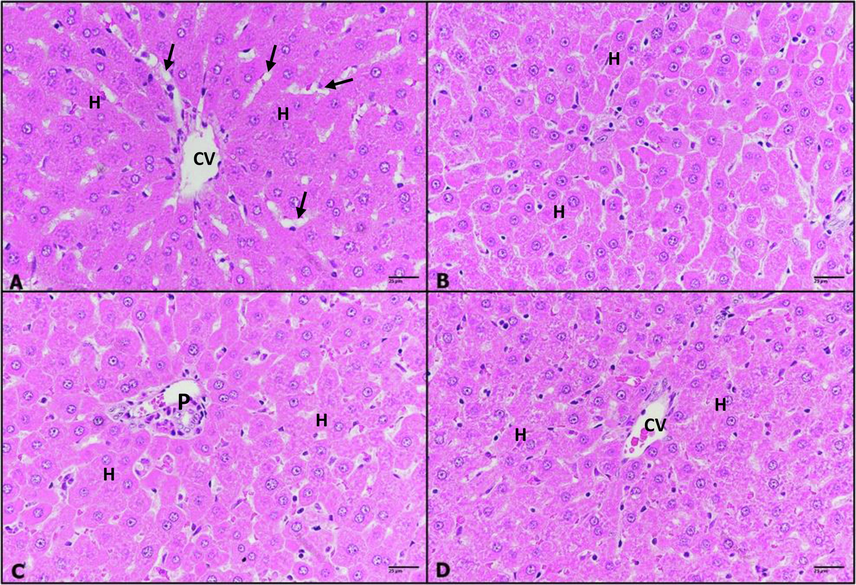
A–D: Photomicrographs of rat liver sections, untreated control (A), (0.25 mg/kg) IVM- treated group (B), (0.50 mg/kg) IVM- treated group (C), and (1 m g/kg) IVM- treated group (D) showing normal hepatic architecture with normal central vein (CV), hepatic strands of hepatocytes (H) with well-defined nuclei and cytoplasm, radiate from the central vein towards the periphery of the hepatic lobules and portal triads (P) and are separated by narrow blood sinusoids (arrows). H & E; 400 ×.
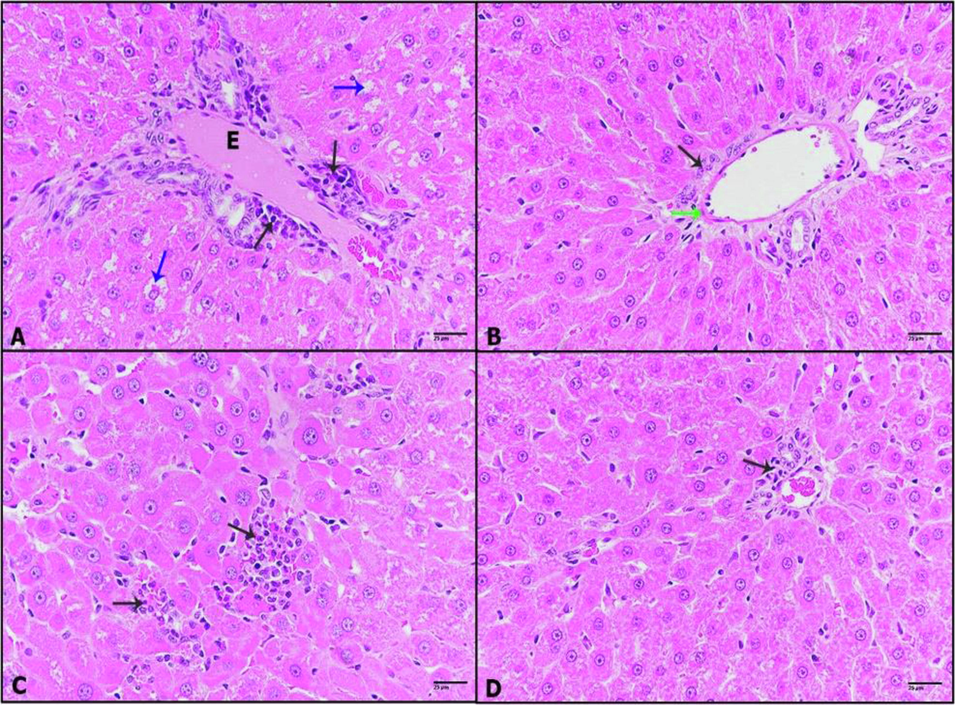
A-D: Photomicrographs of liver sections of (A): DHM-treated rats showing dilatation of congested blood vessel with inflammatory cells (black arrow), cytoplasmic degeneration of hepatocytes (blue arrows). (B): 0.25 mg/kg IVM plus DHM-treated rats displaying thickened vein wall (green arrow), a few inflammatory cells (black arrow). (C): 0.50 mg/kg IVM plus DHM-treated rats illustrating small foci of inflammation (black arrow). (D): 1 mg/kg IVM plus DHM-treated rats displaying healthy tissue with a very few inflammation foci (black arrow), (H&E-400×).
5.2 Kidney histopathology
Untreated control rats showed normal renal structure (Fig. 8 A). Similar to the liver, groups treated with 0.25, 0.50 and 1 mg/kg of IVM revealed healthy renal structure as untreated control kidney (Fig. 8 B, C, D). Histological examination of kidney sections of DHM-treated group exhausted minimized granuloma with low cellularity surrounded by dispersed carcinoma cells mixed with inflammatory cells (Fig. 9 A)., the kidney sections of 0.25 mg/kg IVM-treated group, displayed less incidence of carcinoma and inflammatory cells (Fig. 9 B). Additionally, 0.50 mg/kg IVM-treated rats showed healthy normal tissue (Fig. 9 C) while 1 mg/kg IVM-treated rats displayed healthy renal tissue with normal glomeruli (Fig. 9 D).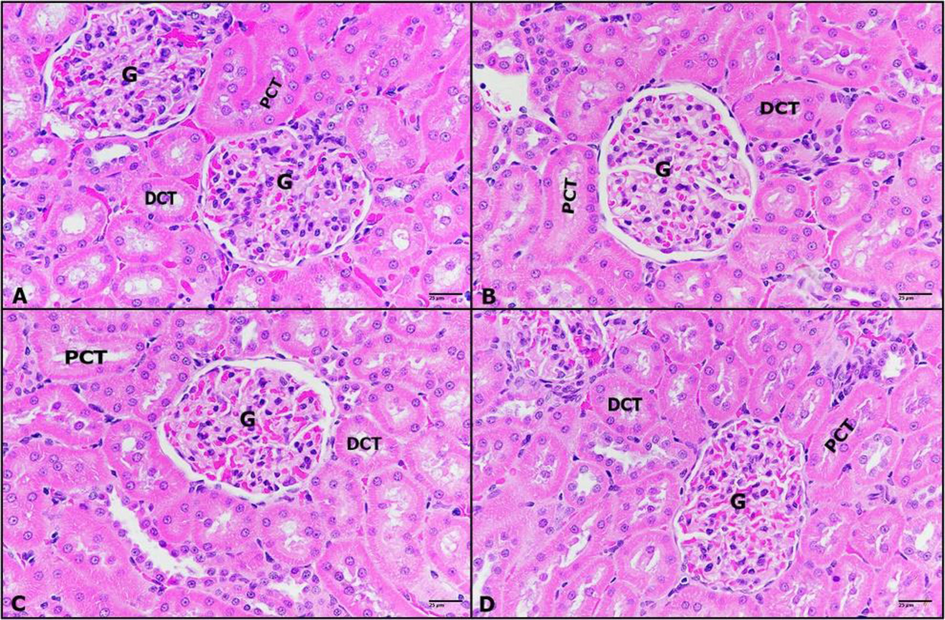
A-D: Photomicrographs of rat kidney sections untreated control (A), (0.25 mg/kg) IVM- treated group (B), (0.50 mg/kg) IVM- treated group (C), and (1 m g/kg) IVM- treated group (D) showing demonstrating normal histological architecture with normal glomeruli (G), normal tubular cells of proximal convoluted tubules (PCT) and distal convoluted tubules (DCT) associated with normal interstitial tissue structure. (H&E-400×).
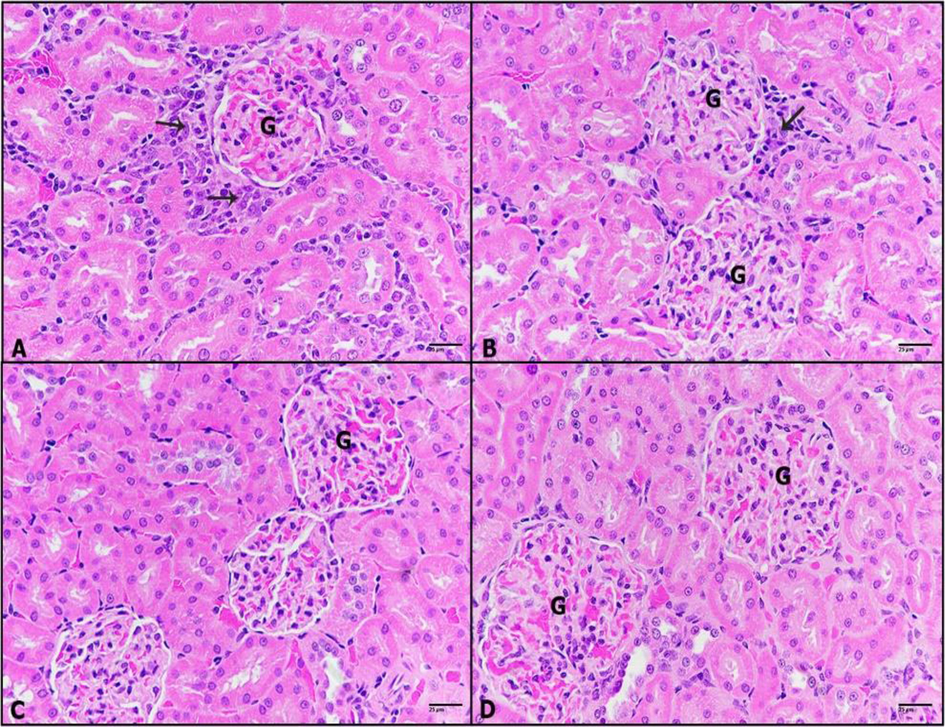
A-D: Photomicrographs of kidney sections of (A): DHM-treated rats showing carcinoma cells mixed with inflammatory cells (black arrow) surrounded glomeruli. (B): 0.25 mg/kg IVM plus DHM-treated rats displaying less incidence of carcinoma and inflammatory cells (black arrow). (C): 0.50 mg/kg IVM plus DHM-treated rats revealing healthy renal tissue. (D): 1 mg/kg IVM plus DHM-treated rats displaying healthy kidney tissue. Glomerulus (G), (H&E-400×).
6 Discussion
Malignant tumors are the main factors to cancer related mortality. Thus, chemotherapy is one of the most important strategies to treat tumors. There are several chemotherapeutic drugs in use, all of which are characterized with serious side effects. Therefore, the development of new drugs that have less side effects is highly required to improve patients’ quality of life and survival (Tang et al., 2021). Among different strategies of treatments, IVM, is a candidate drug that has been reconsidered as an anticancer drug (Zhou et al., 2021). Due to few available studies about the possible protective role of IVM on the cytotoxicity of liver and kidney, this study investigated the chemoprotective role of IVM against liver and kidney in rats, focusing on the liver enzymes, inflammatory markers, and histopathological alterations, Tawfeek et al. (2021) suggested that IVM induces oxidative stress and causes kidney damage. Similar study applied IVM with vitamin C on rats. This study showed that rat had more decline in oxidants activity in treated group (Atakisi et al., 2009). Wen-Jun and others reported that IVM drug has toxic effects on non-target organisms as shown by analyses of cyt P450 enzymes assay. Dose- and time-dependent decreases in the activities of cytr P450 were observed in the liver and kidney tissues of pigeons (Zhu et al., 2014). Another study conducted on baboons and monkeys infected with Trichinella zimbabwensis recorded few clinical signs such as increased levels of creatinine phosphokinase and lactate dehydrogenase which peaked on day 42 post-infection for both experimental animals. Also, lesions were recorded such as ascites, hydropericardium, congested liver and enlarged gall bladder. Furthermore, histopathological findings of various muscles reported a basophilic transformation of muscle cells, the disappearance of sarcomere myofibrils and basophilic sarcoplasm (Mukaratirwa et al., 2008). In addition, Sia et al. (2020) investigated effects of IVM on cytokines levels in cutaneous wounds. Results showed a significant increase in IL-1α and TNF- α level compared to control. Similar result was observed with the levels of IL-4 and IL-10. Same study illustrated those photomicrographs of picrosirius-stained sections taken on day 21 showed dose dependent decrease in collagen density with ivermectin treatment. Rats treated with IVM showed elevated kidney function markers and developed a significant increase in urea and creatinine. Furthermore, renal tissue analysis illustrated highly significant decrease in antioxidant markers such as CAT, SOD, and GPx with induction of ROS. These results suggest that IVM causes damage in the kidney tissue due to production of an oxidative stress (Tawfeek et al., 2021). Another published data reported that IVM treatment for 14 days recorded no significant pathological changes in the soft organs such as liver, spleen, kidney and brain of Wistar rats with low, moderate and high doses reaching 0.1 g/kg (Dong et al., 2020). The present work shows that rats treated with different low doses as 0.25, 0.50 and 1 mg/kg exhibited no pathological alterations in hepatic and renal tissues. On the contrary, DMH treatment significantly caused liver injury and apoptosis with increasing activities of the liver enzymes indicating hepatocytes damage (Punvittayagul et al., 2021). IVM is known for its wide safety margin at the recommended dose, but if taken frequently, it can induce cytotoxic effect, which will result in damage of the liver and other organs, compromising overall health (Ahmed et al., 2020). However, studies have shown that several toxic effects were induced in the liver and kidney with overdoses in experimental animals. A study by Chahrazed and others on rabbits showed that the repeated treatment with 2 mg/kg of IVM for three times/week for three weeks resulted in renal dysfunctions and histological changes in the structure of kidneys such as; cells infiltration, vascular congestion, expansion of the Bowmans’ spaces (Chahrazed et al., 2021). Also, further histological investigations were performed on the rat's kidney and showed recorded side effects after treatment with 0.4 mg/kg of IVM including; highly contractile glomeruli, irregular nuclei, swelled mitochondria, reduction in kidney function, elevation of antioxidant enzyme activity (Tawfeek et al., 2021). Microscopic analysis on rats treated with IVM (0.2 mg/kg) demonstrated focal degradation, accumulation of inflammatory cells, necrotic and apoptotic cells. Theses, findings were associated with increased levels of AST, ALT, and ALP (Hosseini Omshi et al., 2018; Ahmed et al., 2020). The current study revealed that DMH treatment induced hepatotoxic pathological features as cytoplasmic degeneration and inflammation in addition to appearance of carcinoma cells in the renal tissues. On the other hand, post-treatment with IVM to DMH resulted in decreasing of DMH-induced hepatotoxicity and reduction of cancer cells in kidney which correlated with the increase of IVM dose.
7 Conclusion
Our findings showed, IVM significantly reduced the activity of liver enzymes; ALT, ALP, AST, and GGT. The high dose of IVM clearly ameliorated the toxic effect of DMH in male Wistar rats. With respect to inflammatory markers, most of tested genes were upregulated after treatment of IVM. At histopathological level, the protective effect of IVM against DMH was observed as indicated by minimized damage of liver and kidney tissues. Therefore, IVM might be a new potential drug against cytotoxic drugs. This study suggests conducting more investigations on IVM to improve it as an effective cancer treatment in the future.
8 Availability of data and materials
The data generated or analyzed in this article are online publicly available without request.
9 Authors' contributions
Al-Zharani, Alghamdi, and AL-Johani were treated rats, collected samples. Alkeraishan, Alhenaky, and Aljarba were measured liver enzymes. Aldahmash, Alghamdi, and Elnagar, were prepared the parefine section, staining. Elnagar and Alahmari were investigated the histopathological alterations. Al-Zharani, Alhoshani, and Alkahtani were measured the gene expression of inflammatory markers.
Acknowledgment
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R62), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Researchers Supporting Project number (RSP-2021/214), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The biochemical changes in rats' blood serum0 levels exposed to different gamma radiation doses. Afr. J. Pharm. Pharmacol.. 2013;7(15):785-7920.
- [Google Scholar]
- Vitamin E and selenium administration synergistically mitigates ivermectin and doramectin-induced testicular dysfunction in male Wistar albino rats. Biomed. Pharmacother.. 2020;124:109841
- [Google Scholar]
- Effect of ivermectin and albendazole on some liver and kidney function indices in rats. Afr. J. Biochem. Res.. 2009;3(5):190-197.
- [Google Scholar]
- Effects of therapeutic dose of ivermectin on plasma nitric oxide and total antioxidant capacity in rabbits. Eur. Rev. Med. Pharmacol. Sci.. 2009;13:425-429.
- [Google Scholar]
- Bénédicte, S., 2010. Chronic kidney disease and cancer: a troubling connection. J Nephrol. 2010; 23(3): 253–262.
- Protective Effects of Vitamin C on Ivermectin Induced Toxicity on Kidney Functions and Brain Tissue in Rabbits (Oryctolagus cuniculus. Egypt. Acad. J. Biol. Sci., D. Histol. Histochem.. 2021;13(1):63-77.
- [Google Scholar]
- An appraisal of pumpkin seed extract in 1, 2-dimethylhydrazine 1640 induced colon cancer in wistar rats. J. Toxicol. 2018
- [Google Scholar]
- Comparative evaluation of acute toxicity of ivermectin by two methods after single subcutaneous administration in rats. Regul. Toxicol. Pharm.. 2007;47(3):257-260.
- [Google Scholar]
- Ivermectin as an inhibitor of cancer stemlike cells. Mol. Med. Rep.. 2018;17:3397-3403.
- [Google Scholar]
- 14-Day Repeated Intraperitoneal Toxicity Test of Ivermectin Microemulsion Injection in Wistar Rats. Front Vet Sci.. 2020;7:598313
- [Google Scholar]
- Effect of vitamin A and vitamin C on attenuation of ivermectin-induced toxicity in male Wistar rats. Environ. Sci. Pollut. Res.. 2018;25(29):29408-29417.
- [Google Scholar]
- Liver cancer-prevention and early diagnosis. Japan Med. Assoc. J.. 2002;45(3):130-133.
- [Google Scholar]
- Selective targeting of nuclear receptor FXR………………… by ivermectin analogues with therapeutic effects on nonalcoholic fatty liver disease. Sci. Rep.. 2015;5:17288.
- [Google Scholar]
- The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res.. 2018;8:317.
- [Google Scholar]
- Studies on new urease inhibitors by using biochemical, STD-NMR spectroscopy, and molecular docking methods. Med. Chem. Res.. 2017;26(10):2452-2467.
- [Google Scholar]
- Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Complement. Altern. Med.. 2017;22(4):982-995.
- [Google Scholar]
- Experimental infections of baboons (Papio spp.) and vervet monkeys (Cercopithecus aethiops) with Trichinella zimbabwensis and successful treatment with ivermectin. Onderstepoort J. Vet. Res.. 2008;75(2):173-180……….
- [Google Scholar]
- Chronic catheterization using vascular-access-port in rats: Blood sampling with minimalstress for plasma catecholaminedetermination. J. Neurosci. Methods. 1987;22(2):141-146.
- [Google Scholar]
- Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J. Biomed. Biotechnol.. 2011;2011:473964
- [Google Scholar]
- Priyamadhaba Behera, Binod Kumar Patro, Arvind Kumar Singh, Pradnya Dilip Chandanshive, Ravikumar S. R., Somen Kumar Pradhan, Siva Santosh Kumar Pentapati, Gitanjali Batmanabane, Prasanta Raghab Mohapatra, Biswa Mohan Padhy, Shakti Kumar Bal, Sudipta Ranjan Singh, Rashmi Ranjan Mohanty. (2021). Role of ivermectin in the prevention of SARSCoV-2 infection among healthcare workers in India: A matched case-control study. Plos one.
- Protective role of vanillic acid against diethylnitrosamine- and 1,2-dimethylhydrazine-induced hepatocarcinogenesis in rats. Molecules. 2021;26(9):2718.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.. 1957;28(1):56-63.
- [Google Scholar]
- Three pathways of colonic carcinogenesis in rats. Anticancer Res.. 2017;37(1):15-20.
- [Google Scholar]
- Zinc ferrite nanoparticles activate IL-1b, NFKB1, CCL21 and NOS2 signaling to induce mitochondrial dependent intrinsic apoptotic pathway in WISH cells. Toxicol. Appl. Pharmacol.. 2013;273(2):289-297.
- [Google Scholar]
- Dual anti-inflammatory and anti-parasitic action of topical ivermectin 1% in papulopustular rosacea. J. Eur. Acad. Dermatol. Venereol.: JEADV. 2017;31(11):1907-1911.
- [Google Scholar]
- Liver and kidney function trends in a population under mass treatment with ivermectin in The North West Region of Cameroon (Onchocerciasis Endemic Region) Am. J. Biomed. Sci. Res.. 2021;13(3):001875
- [Google Scholar]
- Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res.. 2021;163:105207
- [Google Scholar]
- Evaluation of lipid profile and oxidative stress in STZ induce rats treated with antioxidant vitamin. Brazilian Arch. Bio. Tech.. 2012;55(4):527-536.
- [Google Scholar]
- Protective effect of vitamin C against ivermectin induced nephrotoxicity in different age groups of male wistar rats: bio-histopathological study. Anat. Cell Biol.. 2021;54:501-517.
- [Google Scholar]
- Protective effect of vitamin C against ivermectin induced nephrotoxicity in different age groups of male wistar rats: bio-histopathological study. Anat. Cell Biol.. 2021;54(4):501-517.
- [Google Scholar]
- A reference method for measurement of alkaline phosphatase activity in human serum. Clin. Chem.. 1983;29(5):751-761.
- [Google Scholar]
- Tiffon, C., (2018). The impact of nutrition and environmental epigenetics on human health and disease. Int. J. Mol. Sci. 2018 Nov; 19(11): 3425.
- Topical ivermectin improves allergic skin inflammation. Allergy. 2017;72(8):1212-1221.
- [Google Scholar]
- Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun.. 2018;497(1):241-247.
- [Google Scholar]
- DEAD-box RNA helicase DDX23 modulates glioma malignancy via elevating miR-21 biogenesis. Brain. 2015;138(9):2553-2570.
- [Google Scholar]
- Ivermectin has new application in inhibiting colorectal cancer cell growth. Front. Pharmacol.. 2021;12:717529
- [Google Scholar]
- Effects of avermectin on microsomal cytochrome P450 enzymes in the liver and kidneys of pigeons. Environ. Toxicol. Pharmacol.. 2014;38(2):562-569.
- [Google Scholar]







