Translate this page into:
Biological control of Root-knot nematode (Meloidogyne javanica) by potential antagonism of endophytic fungi isolated from Taify roses
⁎Corresponding author. aabaazeem@tu.edu.sa (Alaa Baazeem),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Plant-parasitic nematodes are a serious threat to global agricultural production worldwide. To avoid synthetic chemistries toxicity there is a dire need of the hour to develop innovative nematode control strategies. Biological control using the antagonistic fungi of the plants is considered very economical and environment friendly.
Methods
Efficacy of different fungal filtrate concentrations was estimated towards the mortality and clutching of Meloidogyne javanica. To determine the antagonistic potential of Penicillium citrinum (MN518391), time of disclosure was assessed in the laboratory with the result “exposure to 8% filtrate for a clock (24 hrs.), expressively reduced the viable juveniles and hatched eggs.
Results
Exposition to aforementioned filtrate of P. citrinum for a clock (24 hrs.) revealed that juvenile body turned into straight stature leading to reduction in viability of nematodes. Contrary to it most of nematodes exhibited bent body when exposed to Aspergillusniger (MK713445), or (MN513383) its filtrates. Among the various isolated antagonistic fungi, GC–MS analysis revealed 22 unique compounds e.g., quinazoline and its derivatives as the most prevalent and squalene as least occurring possess significant nematicidal properties against the rootknot nematodes.
Conclusion
Hence, using antagonistic fungus from the described plans as BCAs in agriculture against plant-parasitic nematodes is a viable long-term biocontrol technique and provide future avenues in this area of research.
Keywords
Obligate parasites
Small animals
Bio-control
Meloidogyne javanica
Bioactive compounds
1 Introduction
To date, about 4100 species of plant-parasitic nematodes have been identified, of which a small number of genera are regarded major plant diseases, while others are specialized to a smaller number of crops, both of which have a significant influence on economically important crops. Plant nematodes are anticipated to cause a global yield loss of 12.3 % ($157 billion) which is higher than invasive insects ($70 billion) (Bradshaw et al., 2016). Growers are typically ignorant of the existence of nematodes due to non-specific symptoms in plants, making it difficult to attribute crop losses to worm damage (Siddique and Grundler, 2018). Food quality and visual defects linked to illness symptoms could result in additional losses (Palomares-Rius et al., 2017). The most important nematodes in terms of agricultural losses are sedentary endoparasites, root knot (Meloidogyne spp.), and cyst nematodes (Heterodera and Globodera spp.) (Jones et al., 2013). Plant-parasitic nematode control is becoming a growing global demand as a result of the increasing need to feed a growing population (FAO, 2017). Because parasitic nematodes have such a large economic impact in agriculture, a variety of nematode-control techniques, including the use of chemical nematodes, have been developed. The loss of pesticides as a result of EU rules (EC No 1107/2009), which indicate that they are detrimental to human health and a pollutant to the environment, has heightened the demand for robust nematode resistance (Zhang et al., 2014; Zhang et al., 2017). Among the organisms reported to act as biocontrol agents (BCAs) against plant-parasitic nematodes include fungi, bacteria, viruses, protists, nematode antagonists, and other invertebrates. When used in conjunction with reduced chemical dosages in an integrated pest management scenario, BCAs, physical measures such as solarization and fallowing, and cultural tactics such as crop rotation have all been found to be effective (D’Addabbo et al., 2019). Despite the fact that predatory nematodes have been utilised to combat plant-parasitic nematodes since the early twentieth century, their full potential has only lately been discovered. These nematodes are vital in improving plant nutrition cycle, which allows plants to better protect themselves against illnesses, in addition to acting as BCAs against plant-parasitic nematodes. Meloidogyne spp. is one of the most dangerous pests of vegetables in the world, causing massive losses on a variety of agricultural plants, particularly vulnerable vegetable plant species (Kaskavalci, 2007). In KSA (Kingdom of Saudi Arabia) RKNs commonly distributed as widespread but M. incognita and M. javanica are known to be the most commonly occurring and parasitizing species on roses (Nour El-Deen et al., 2015). RKNs infestation leads to reduction of normal root system with extreme galls formation with entirely chocked vascular system (Lopez-Llorca and Jansson, 2006). This chocked vascular system of reduced rootlets leads to obstruction in normal transport and uptake of nutrients minerals and water (Nour El-Deen et al., 2015). Many nematicidal compounds has been standardized from various fungi but nothing is commercialized as industrial product with these compounds from natural fungal for extensive usage (Li et al., 2007; Anke, 2010). Use of mycoendophytes against phyto parasitizing nematodes has been studied by Zabalgogeazcoa (2008). There are possibilities related to mode of action of MEs (Mycoendophytes) on nematodes either by preparing nematicidal compounds, paralysis or killing of nematodes or by activating plant defense mechanism against nematodes (Tian et al., 2014). Acremonium implicatum obtained from tomato root galls possesses exceptional potential for control of M. incognita (Schouten, 2016). The scientists have reported that Ch1001 “Chaetomium” mycoendophyte possesses huge potential for controlling infection of M. incognita if utilized in seed treatment (Yan et al., 2011). A number of compounds has been synthesized from Fusarium oxysporum strain “Fo162” possessing the ability to control M. incognita (Hawranik and Sorensen, 2010). Until now management of phyto parasitizing nematodes using MEs is still rare. Hence, the research experiment intended to assess the impact of three MEs obtained from Taify rose against Meloidogyne javanica under in vitro conditions.
2 Materials and methods
Current study was performed at Department of Biology, Collage of Science., Taif University, Kingdom of Saudi Arabia.
2.1 Mycoculture filtrate preparation
Three MEs’ already identified strains viz., Penicillium citrinum (MN518391), Aspergillus niger (MK713445, A), and A. fumigatus (MN513383) obtained from Taify roses were cultured on general purpose media (Potato Dextrose Agar). Fungal discs from PDA plates were obtained 10 mm and inoculated in flasks having 150 ml Potato Dextrose Broth followed by aggressive shaking at 100 rpm for 10 days at 28 °C. Filtration of culture was done through sterilized filter paper and to separate the liquid from fungal masses micropore filter was utilized. Filtrate was kept at 4 °C till its use.
2.2 Nematode culture
Nematode culture was obtained from the roots of the Taify roses infested with M. javanica egg masses and characterized by prineal pattern analysis. The nematode culture was maintained and proliferated on most susceptible plants of tomato cultivar as specific host in greenhouse. Eggs were obtained from infested roots of tomato by 0.5 % sodium hypochlorite solution followed by shaking for 120 s contrary to it juveniles J2s which were gathered by hatching methodology for approximately-seven days (Hussey, 1973). Final inoculum was maintained to 50 juveniles or 100 eggs/ml.
2.3 Biological assay
To test the mycofilterate’s toxicity, juveniles and eggs of M. javanica were poured into 1 ml filtrates into twenty-four welled tissue culture plate. Culture filtrates were divided into three concentrations i.e., 2, 4 and 8 % over 50 juveniles and 100 eggs solution in distilled water while PDA broth 2 ml and Nematode culture suspension was kept as control. Five diverse replications were designed for treatments and control experiment was reiterated once. Mortalized larvae were calculated after periodic duration of 6, 12, and 24 hrs wherein death of larvae was confirmed by touching with fine needle. Rate of Mortality was derived using Abbott’s formula (Abbott, 1925).
2.4 Gas chromatography based analysis of mycofilterates
GC–MS analysis was utilized for profiling of chemical constituents of Mycofilterates by using Agilent (7890A-5975B) model (DB 5 ms, columnar formation, with dimensions (30 m × 250 µm × 0.25 µm). Column was adjusted at 40 °C for 120 s followed by rise to 50 °C with respective rate 4 °C/minute and kept for 3 min, followed by another rise in temperature to 150 °C with rate of 10 °C/minute, held for 3 min, followed by rise to 220 °C with the rate of 10 °C/minutes and kept for 6 min, finally upregulation of temperature until 280 °C with rate of 10 °C/minute and kept for 10 min. The carrier gas used in this column was pure Helium (99.999 %) for ∼ 10 min with 0.5 ml/minute flow rate, then 1 ml/minute for 30 min. Chromatographic analysis was done without the use of inter or intra standards of chemical. Interpretation for mass spectrum data based chromatographic peaks was done using National Institute of Standards and Technology for the identification of chemical constituents of samples.
2.5 Statistical analysis
The collected datasets were subjected to two-way ANOVA and Duncan's multi-range test (p < 0.05) for comparing the treatments means using the CoStat software package (version 6.45).
3 Results
3.1 Mortality of larvae
Three mycofilterates viz. Penicillium citrinum (MN518391), Aspergillus niger (MK713445) and A. fumigatus (MN513383) with 8, 4 and 2 % concentrations ammended with PDA broth were evaluated for percent mortality of newly borne juveniles of M. javanica after periodic exposure of 6, 12, and 24 hrs. (Fig. 1). Data obviously demonstrating the direct relationship between the percent larval mortality and exposure time that rising with the concentration of filtrate rises with the exposure time. P. citrinum based filtrate gave remarkable reduction in viable juveniles J2s (51.67), followed by Aspergillus niger MN513383 at the concentration of 8 %. A. niger (MK713445) gave larval mortality percentage as 34.17 % upon exposure to various concentrations at different timings (Fig. 1c). Viable juvenile J2s counting did not shifted significantly when exposed to P. citrinum’s 8 % filtrate as compared to exposure of A. niger (MN513383) at exposure for 6 to 12 hrs. as illustrated in (Fig. 1a & b). Two ANOVA yield values upon 6 and 12 hrs. exposure are 35.42 %, 32.5 % and 41.25 %, 40 % respectively. J2s exposure to A. niger(MN513383) gave similar mortality (27.57 %).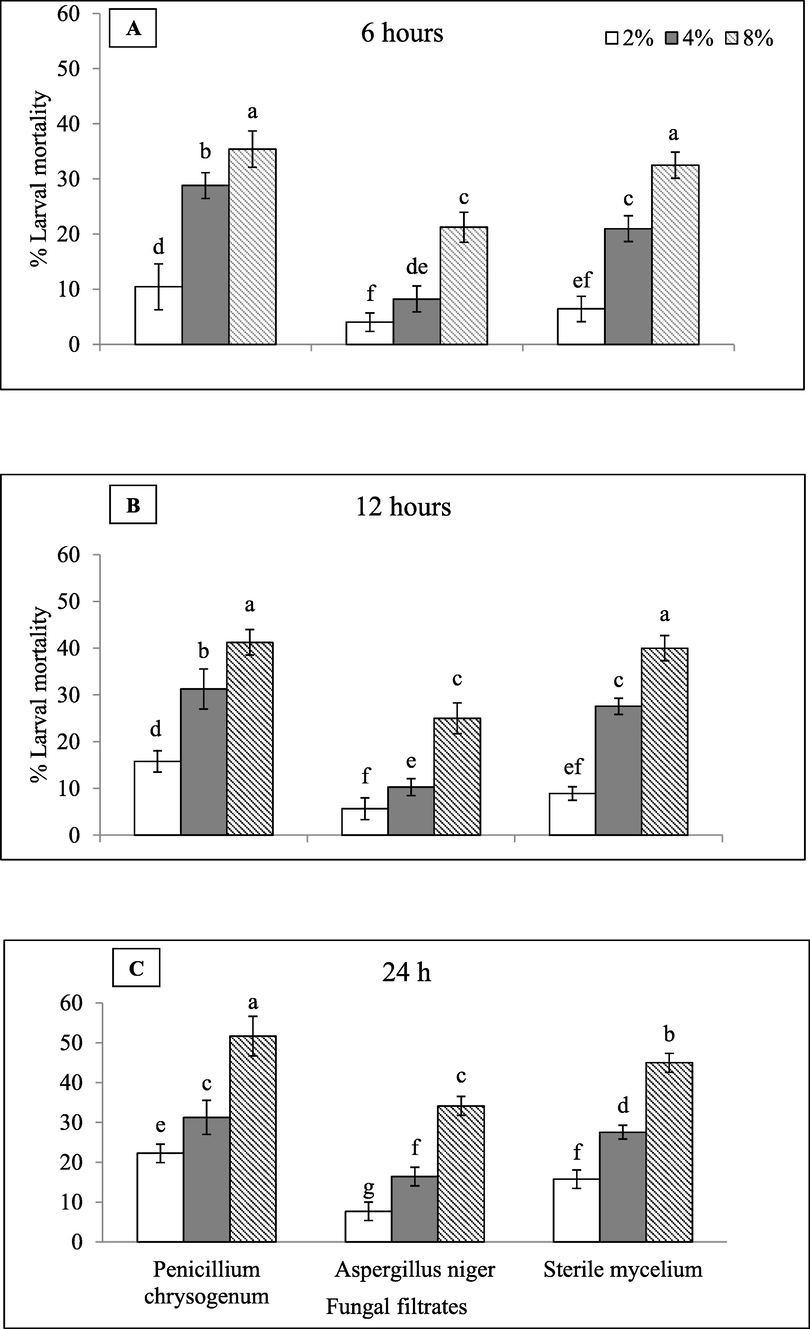
Mortality of M. javanicaJ2s effected by endophytic fungi culture filtrates after 6, 12 and 24 h.
3.2 Percentage of eggs hatch
The hatching inhibition of M. javanica eggs as result of exposure to mycofilterates, shown in (Fig. 2). High concentration of mycofilterates inhibited egg hatching as compared to lower concentrations. P. citrinum exhibited 89.27 and 86.49 % hatchability inhibition at 8 and 4 % concentrations respectively. However, A. niger(MN513383) filtrate lead to lessening of hatching at similar concentration to 84.41 and 81.05 % respectively. There was not significant difference in hatched eggs between A. niger (MN513383) and P. citrinum at 2 % filtrate concentration. The percentage of hatching inhibition of M. javanicawhen exposed to A. niger (MK713445) at 4, 8, and 12 % was significantly low as compared to P. citrinum and A. niger (MN513383) with (73.28, 69.35, and 53.8 %).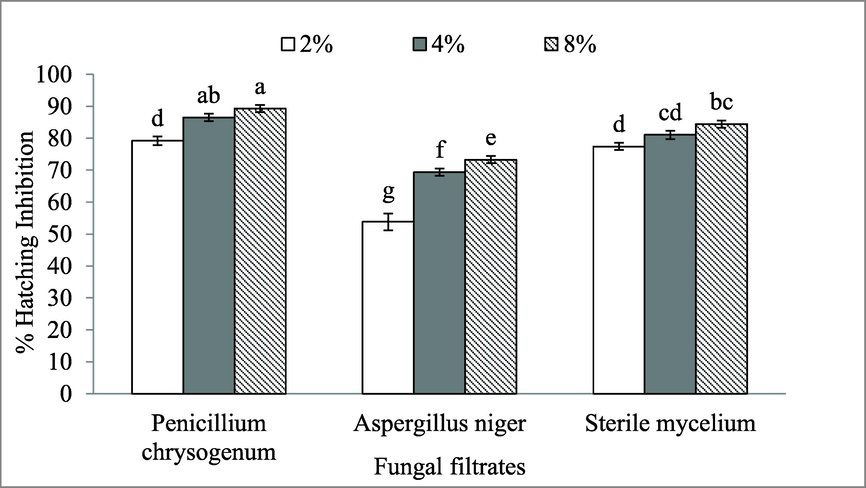
Egg hatching of M. javanica effected by endophytic fungi culture filtrates.
3.3 J2s characteristics
In control, nematodes were visually mobile and sigmoidal which is known as typically alive nematode’s character. In contrary, there was significant reduction in nematodes viability after exposure to mycofilterates appearing as straight non-motile body shape (Fig. 3B). Post exposure to A. niger(MK713445) or A. fumigatus (MN513383), nematodes were looking like banana shaped curved body like crescent hinting and showing a significant reduction in its viability (Fig. 3c and d).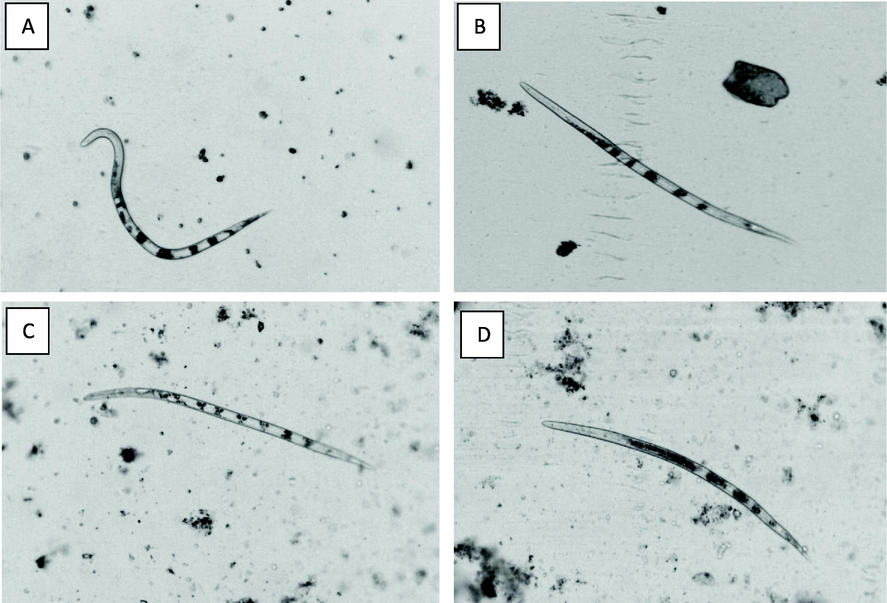
Effect of exposure to: A. Potato dextrose broth (Control)-sigmoid, B.P. citrinum MN518391-straight, C. and D.A. niger MK713445 and A. niger MN513383-bent on characteristic shapes of M. javanica J2s, A.(∑-shape),B.(I shape), and C. and D. are(banana-shape).
3.4 GC–MS profiling of dynamic mycofilterates
Three mycofilterates were imperiled for GC–MS analysis where 22 chemical compounds were determined from filtrates analysis. Chemicals were varying in their characteristics from fungi to fungi with similar count of compounds in all testified isolates i.e., 10 compounds, few of them were primarily important (Table 1). Results depicted that quinazoline was extensively found chemical followed by octadecane and Palmitic acid with concentrations of 64.8, 6.1 and 7.4 % respectively. In P. citrinum (MN518391) majorly quinazoline was reported 65.3 % followed by palmitic acid 7.9 % and pentadecane 6.3 %. Likewise, A. niger(MK713445) filtrate exhibited abundantly quinazoline, acetic acid, piperidide and triacontane with concentrations (58.8, 15.03, and 6.08 %) respectively. The commonly found minor components were octadecane, docosane, phytane and squalene with varying concentrations viz., 1.8, 1.5, 1.3 and 1.0 % respectively. In P. citrinum (MN518391) tetracosane was detected in small amount as (1.8 %). Further, 1,1,2,2-Tetrachloroethane (1.654 %) was assessed solitarily in A. niger(MK713445) (Table 1).
Active compounds
A. niger MN513383
P. citrinum MN518391
A. niger MK713445
Octadecane
6.108
1.875
–
11-Butyldocosane
4.333
–
–
Heptacosane
3.933
–
–
Squalane
1.088
4.921
–
Dodecanoicacid
4.704
–
–
Hexadecanoicacid (Palmiticacid)
7.437
7.973
–
10-Methoxy-nb-alpha-methylcorynantheol
1.548
–
–
3-Methyl-2-butenoic acid,
2,7-dimethyloct-7-en-5-yn-4-yl ester2.702
–
–
Docosane
3.251
–
1.593
6,8-dibromo-2-(3-pyridyl)-4-phenyl-quinazoline
64.895
65.334
58.889
Boricacid,ethyl-,didecylester
–
2.494
–
Dotriacontane
–
2.715
–
Eicosane
–
5.065
3.442
Pentadecane
–
6.370
–
Phytane
–
1.397
4.152
Tetracosane
–
1.856
–
Triacontane
–
–
6.081
Aceticacid,piperidide
–
–
15.037
Palmitinicacid
–
–
4.609
2-Methyl-,2-ethyl-2-[[(2-methyl-1-oxo-2-propenyl)oxy]methyl]-2-Propenoicacid
–
–
2.462
1,1,2,2-Tetrachloroethane
–
–
1.654
4-Oxopentanoicacid,
p-tolylsulfonylhydrazone,ethylester–
–
2.081
4 Discussion
Plant-parasitic nematode biocontrol techniques are a viable alternative to toxic chemical nematodes (Baazeem et al., 2021a; Baazeem et al., 2021b). MEs were isolated from leaves of Taify rose and screened to assess the potential to be a biocontrol agent against RKNs (Meloidogyne javanica). Mycoflora were founded to be very effective agent for regulation of nematodes count in rhizosphere (Nour El-Deen et al., 2015). In the investigation, it is evident that with the rise in concentration of mycofilterates and exposure time juvenile mortality significantly upraised with reference to control. Among all testified mycoflora P. citrinum significantly decreased the viable juveniles and eggs count indicating the sufficient production happened in PD Broth. These results are in line with Gotlieb et al. (2003) who were the depicter of cucumber and tomato growth reducing the gall formation upon inoculation of mycelial mass of P. chrysogenumin soil. Mycoflora can be good option to regulate nematode count in soil by exhibiting range of fighting activity like nematotoxic chemicals (Suwannarach et al., 2013). Fusarium oxysporum obtained from coffee rhizosphere was an excellent nematicide to control the M. incognita (Freire et al., 2012). There was no significant difference between high and medium concentration of A. niger (MK713445) and A. fumigatus (MN513383) in reducing the juvenile’s viability upon exposure of 6 and 12 hrs. respectively. Previous studies have reported the Aspergillus sp. to produce toxins which were very effective to control Meloidogyne while Penicillium sp. produced toxins that were reported very effective to control Aphelenchoidescomposticola (Cayrol et al., 1989; Grewal et al., 1989). A. niger were not significantly effective at higher and medium concentrations even at all exposures for lessening the juvenile viability contrary to the results of the authors of the reference (Jang et al., 2016) wherein he demonstrated that mycofilterates of A. nigerwere highly effective for inhibition of hatching and juvenile mortality of M. incognita.
GC–MS analysis depicted the presence of multiple effective nematicidal compounds in mycofilterates which were classified as alkane (C---- C) hydrocarbons, Alkaloids, fatty acids, aromatic hydrocarbons, aliphatic hydrocarbons, carboxylic acids, chlorinated hydrocarbons, heterocyclic amine, and keto groups. Nitrogen containing compounds (quinazolinone derivatives) exhibit effective biological activity against the plant parasitic nematodes. In this experiment, quinazoline was found to produce abundantly in all testified isolates with high percentage from P. citrinum (MN518391) exhibited good nematicidal activity. Results depicted that Palmitic acid (Fatty acid) obtained from P. citrinum (MN518391), A. niger (MK713445) and A. fumigatus (MN513383) filtrates, dodecanoic acid isolated from A. niger (MN513383). Bardhan et al. (2019) mentioned that isolated fatty acids from P. citrinum (PKB20) were oleic acid, Linoleic acid and hexadecenoic acid with percentage of (30 %, 33.1 %, and 20.2 %). Likely, in Pseudomonas sp. survives in rhizosphere yielded hexadecenoic acid and hexadecenoic acid from the ethyl acetate and hexane fractions. Elsewhere, it was reported previously (Oliveira et al., 2009) that Palmitic acid is very toxic to various nematode species. In mycofilterates of A. niger (MK713445) and P. citrinum (MN518391) long chain alkanes e.g., triacontane and dotriacontane were in traces. Triacontane compounds are known as good antibacterial and antimycologic agent. When both compounds were standardized with 50 mg mL−1 of water eggs hatching of M. incognita reduced to 94 % and following 6–4 days exposure lead to immobilize entire juvenile 2 stage. Hu et al. (2013) have isolated 1,3-dimethyl citrate chemical from isolates of A. niger known to be component of developed plants. According to the obtained results, apparently, nematodes were dead and paralyzed just like in case of pyrethroid application that has direct effect on central nervous system of nematode and transformed into different type of shapes such as bent or straight shapes when they were exposed to the fungal filtrate, however, nematodes maintained their sigmoid shape and remained feasible when they grew into potato dextrose broth as a control media (Strobel et al., 2011). Evidently, the result of this study is similar with those who described that dead pattern of nematodes was different as well as groups of pesticides or bio-agents can distinctly be differentiated based on this particular phenomenon.
5 Conclusion
This finding depicted the highly effective biocontrol agents (endophytic fungi) for nematodes that consisted of trust-worthy mode of action and nematocidal properties. For the isolation and detection of endophytic fungi from the new host plant in field and greenhouse condition to examine their nematocidal properties, additional studies are inevitable.
Acknowledgement
The author extend his appreciation to Taif University for funding current work by Taif University Researchers Supporting project number (TURSP-2020/295), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A method of computing the effectiveness of and insecticide. J. Am. Mosq. Control Assoc.. 1925;3:302-303. PMID: 3333059
- [Google Scholar]
- Anke, H., 2010. Insecticidal and nematicidal metabolites from fungi. In: The Mycota: Industrial Applications.10: 2nd (Ed.): M. Hotrichter. Springer- Verlag, Berlin.
- Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House. J. Fungi. 2021;7(8):632.
- [Google Scholar]
- In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi. 2021;7(5):331.
- [Google Scholar]
- Microbial lipids from cellulolytic oleaginous fungus Penicillium citrinum PKB20 as a potential feedstock for biodiesel production. Ann. Microbiol.. 2019;69(11):1135-1146.
- [Google Scholar]
- Massive yet grossly underestimated global costs of invasive insects. Nat. Commun.. 2016;7:1-8.
- [Google Scholar]
- Study of the nematicidal properties of the culture filtrate of the nematophagous fungus. Paecilomyces lilacinus. Revue-de-Nematol.. 1989;12(4):331-336.
- [Google Scholar]
- Biostimulants for plant growth promotion and sustainable management of phytoparasitic nematodes in vegetable crops. Agronomy. 2019;9:616.
- [CrossRef] [Google Scholar]
- The Future of Food and Agriculture. Rome: Trends and Challenges; 2017.
- Volatile substances produced by Fusarium oxysporum from coffee rhizosphere and other microbes affect Meloidogyne incognita and Arthrobotrys conoides. J. Nematol.. 2012;44(4):321.
- [Google Scholar]
- Dry mycelium of Penicillium chrysogenum protects cucumber and tomato plants against the root-knot nematode Meloidogyne javanica. Phytoparasitica. 2003;31(3):217-225.
- [Google Scholar]
- Grewal, P.S., Sohi, H.S., Grabbe, K., Hilber, O., 1989. Effect of various fungal metabolites on Aphelenchoides composticola Franklin and its multiplication on some fungi. pp. 813-820. MushroomScience. Proc. 12thInt. Cong. Science & Cultivation of Edible Fungi. Vol.2. Braunschweig, Germany.
- The isolation of citric acid derivatives from Aspergillus niger. FEMS Microbiol. Lett.. 2010;306(2):122-126.
- [Google Scholar]
- Nematicidal activity of chaetoglobosin A producedby Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric Food Chem.. 2013;61:41-46.
- [Google Scholar]
- A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep.. 1973;57:1025-1028.
- [Google Scholar]
- Biological control of Meloidogyne incognita by Aspergillus niger F22 producing oxalic acid. PLoS One. 2016;11(6):e0156230.
- [Google Scholar]
- Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol.. 2013;14:946-961.
- [Google Scholar]
- Effect of soil solarization and organic amendments treatment for controlling Meloidogyne incognita in tomato cultivars in Western Anatolia. Turk. J. Agric. For.. 2007;31:159-167.
- [Google Scholar]
- Lopez-Llorca L.V., Jansson H.B., 2006. Fungal parasites of invertebrates: multimodal biocontrol agents. pp. 310-335. In: Exploitation of f Fungi. (Eds.): G.D. Robson, P. vanWest and G.M. Gadd. Cambridge University Press, Cambridge.
- Evaluating the pathogenicity of nematodes infecting roses at Taif Governorate, KSA. Res. J. Pharmaceut. Biol. Chem. Sci.. 2015;6(2):1562-1570.
- [Google Scholar]
- The activity of amino acids produced by Paenibacillus macerans and from commercial sources against the root-knot nematode Meloidogyne exigua. Eur. J. Plant Pathol.. 2009;124:57-63.
- [Google Scholar]
- Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front. Plant Sci.. 2017;8:1987.
- [Google Scholar]
- Mechanisms involved in nematode control by endophytic fungi. Ann. Rev. Phytopathol.. 2016;54
- [Google Scholar]
- Parasitic nematodes manipulate plant development to establish feeding sites. Curr. Opin. Microbiol.. 2018;46:102-108.
- [Google Scholar]
- An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol. Lett.. 2011;320(2):87-94.
- [Google Scholar]
- Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Protect.. 2013;45:63-70.
- [Google Scholar]
- Suppression of Meloidogyne incognita by the endophytic fungus Acremonium implicatum from tomato root galls. Int. J. Pest Manage.. 2014;60(4):239-245.
- [Google Scholar]
- Potential use of cucumber (Cucumis sativus L.) endophytic fungi as seed treatment agents against root-knot nematode Meloidogyne incognita. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.). 2011;12(3):219-225.
- [Google Scholar]
- Fungal endophytes and their interaction with plant pathogens. Span. J. Agric. Res.. 2008;6:138-146.
- [Google Scholar]
- The parasitic and lethal effects of Trichoderma longibrachiatum against Heterodera avenae. Biol. Control. 2014;72:1-8.
- [CrossRef] [Google Scholar]
- Mechanisms and characterization of Trichoderma longibrachiatum T6 in suppressing nematodes (Heterodera avenae) in wheat. Front. Plant Sci.. 2017;8:1491.
- [CrossRef] [Google Scholar]







