Translate this page into:
Therapeutic and prophylactic effects of Punica granatum peel extract versus metronidazole in murine Giardiasis intestinalis
⁎Corresponding author. rabdelgaber.c@ksu.edu.sa (Rewaida Abdel-Gaber)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Giardiasis is considered the most common protozoal infection in humans; it frequently occurs in both developing and developed countries. The present study was designed to investigate the therapeutic and prophylactic effects of Punica granatum peel methanolic extract against experimental giardiasis in comparison to metronidazole (MTZ) in mice infected with Giardia intestinalis. There were five experimental groups, as follows: G1; healthy control group, G2; infected untreated, G3; infected treated with MTZ, G4; Infected treated with pomegranate P. granatum, G5; receiving P. granatum for one week before, during induction of infection than continuous administration for another one week. Giardia cyst per gram stool 7 days and 2 weeks after treatment showed a highly statistically significant difference among the different experimental groups (G3,4,5) versus G2, while the percent reduction of mean Giardia cysts/gm stool 2 weeks after treatment showed a highly significant reduction in G3,4,5 by 92.73, 91.94, and 94.1 %, respectively. The count of Giardia trophozoites in small intestinal content showed a statistically significant difference among G3,4,5 versus G2 respectively with a highly significant reduction in G3,5 and a significant reduction in G4. The percent reduction of mean Giardia trophozoites was approximately 98.69, 58.66, and 95.96 %, respectively. Histopathological examination of small intestines sections of G3 and G4 showed improvement in the pathological lesions including slight restoration of villi/crypt ratio, with partial mucosal ulceration. There was complete healing of intestinal mucosa observable in G5, with a preserved brush border, and no trophozoites were detected. P. granatum peel methanolic extract proved to be valuable in the prevention and treatment of Giardia lamblia infection. Further studies are required to determine the effective therapeutic dose of the extract and its mechanism of action against Giardia lamblia.
Keywords
Giardiasis
Reference drugs
Medicinal plants
Pomegranate
1 Introduction
Giardia lamblia is a human intestinal protozoan flagellate that causes giardiasis, which affects up to 30 % of children in the Eastern Mediterranean and Africa (Hooshyar et al., 2019). According to Ankarklev et al. (2012), roughly 280 million people are infected with Giardia each year. While, Pecková et al. (2018) revealed that a protozoan that affects nearly 200 million people each year, as well as a huge number of animal hosts, lives in the duodenum and jejunum and transmits fecal-oral parasites. It's also an opportunistic parasite that's the leading cause of acute and chronic diarrhea, as well as malnutrition, in low-income populations, with prevalence rates ranging from 10 % to 50 % in developing nations. According to Torgerson et al. (2015), giardiasis causes roughly 200 million gastrointestinal diseases and 171 million disability-adjusted life years per year.
Giardia spp. is a kind of waterborne parasite that has infected humans (Hooshyar et al., 2019). Giardiasis is spread by the consumption of cysts found in food and water, with the latter being simpler to spread due to the cysts' resistance to chlorination. Another infection route is direct transmission from person to person, which occurs through direct fecal-oral contact among members of the same family, particularly in collective institutions like childcare centers and orphanages, as well as sexual behaviors (Escobedo et al., 2014).
Human giardiasis has a wide range of symptoms; some people are asymptomatic, while others experience nausea, stomach discomfort, and chronic or severe diarrhea, which can linger for months, causing malabsorption and weight loss (Halliez and Buret, 2013). Giardiasis is a parasitic infection that has been around for a long time (Berkman et al., 2002).
The therapies of choice for giardiasis are MTZ and nitroimidazole, both of which are administered as a single dosage. Albendazole and nitazoxanide are alternate agents since it is mutagenic and has shown some carcinogenic potential (El-Gendy et al., 2021). However, the majority of the medications utilized have significant adverse side effects and are therefore contraindicated (Petri, 2003). Furthermore, Giardia appears to have a high potential for developing resistance to these medications (Long et al., 2007). Furthermore, the US Food and Drug Administration has refused to certify MTZ because of its terrible adverse effects, which include carcinogenic factors in mice and rats as well as mutagenic microorganisms (Huang and White, 2006). Severe inflammation of the pancreas and brain is also an issue (Lalle, 2010).
As a result, greater research into medicinal plants or herbs for alternate giardiasis treatments is required. Natural products are less harmful than manufactured medications and offer therapeutic potential (Abu ElEzz, 2005). Pomegranate (Punica granatum) belongs to the family Punicaceae considered an ornamental fruit for the Mediterranean people. It is often known as nature's strength fruit, is a plant used in folklore medicine to cure a variety of ailments (Abdel Moneim, 2012) including renal difficulties, diarrhea, dysentery, hyperacidity, piles, cough (Jurenka, 2008), erectile dysfunction, bacterial infections, infertility, Alzheimer's disease, arthritis, and obesity (Abozeid et al., 2020). Ancient Egyptians discovered that pomegranate possesses anti-parasitic, anti-helminthic (intestinal cestodes and trematodes), and anti-coccidial properties. Pomegranate peel is a potential therapy for Cryptosporidium parvum in several investigations (Dkhil, 2013; Al-Megrin, 2016).
This study was designed to investigate the therapeutic and prophylactic effects of P. granatum peel methanol extract against experimental giardiasis in comparison to MTZ in mice infected with Giardia intestinalis.
2 Materials and methods
2.1 Punica granatum peels extract
The peels were used to make a methanolic extract of P. granatum. It was acquired from Giza, Egypt, at a local market. It was kept in the TBRI's Medicinal Chemistry Department. The pomegranate fruit was cleaned, and then the skins were manually peeled and chopped into little pieces before being dried and processed into a powder. In soxhlet extraction equipment, the plant sample powder was extracted for 24 hr with 85 % methanol. The foregoing procedure was repeated three times, after which the P. granatum peel extract was concentrated under vacuum using a rotatory Buchi evaporator and stored in desiccators until needed (Rowayshed et al., 2013).
2.2 Flavonoid and phenolic contents of P. granatum peel extract
The total phenol contents of the extracts were analyzed using a slightly modified version of the Folin–Ciocalteu’s method, according to Lin and Tang, (2007). The absorbance of the samples was measured at 750 nm using a Spekol 11 (Carl Zeiss -Jena) spectrophotometer, and total polyphenolic contents were expressed as milligram gallic acid equivalent (GAE/g) based on the dry weight. Total flavonoid contents were quantified spectrophotometrically at 430 nm using the method of Chang et al. (2002) and displayed as equal milligram quercetin (QE)/g based on the dry weight.
2.3 Experimental animals
The study was carried out in Parasitology Department at Theodor Bilharz Research Institute (TBRI) and Parasitology and Pathology Department at Al-Azhar University during the period from April 2020 to May 2021. The current investigation employed 50 laboratory-bred female Swiss albino mice ranging in age from 9 to 12 weeks and weighing 20 to 25 g (g), which were procured and kept in TBRI's animal house. Before beginning the experiment, feces samples were checked for three days to confirm that the mice were clear of intestinal parasites. After adding D'Antoni's iodine to the smears, they were swabbed immediately (Subbannayya et al., 1989).
2.4 Preparation of Giardia infection
2.4.1 Source of the parasite
Giardia cysts which were used for infection of mice were obtained from patients complaining of diarrhea attending the outpatient clinic of Al Zahraa University Hospital, Al-Azhar University, Cairo, Egypt. Samples were repeatedly sieved and washed using normal saline (0.9 %) to obtain Giardia cysts.
2.4.2 Infection
These cysts were adjusted such that each mouse was given 1 × 103 cysts in 100 µl of physiological saline by oral gavage. Fresh pellets were collected every 24 hr after that to ensure they did not become contaminated. To prevent re-infection, the pellets from each group were weighed and the bedding was replaced.
2.4.3 Experimental design
Animals were divided into five groups, ten animals in each group, as follows:
Group 1: Non-infected-non treated (negative control).
Group 2: Infected-non treated (positive control).
Group 3: Infected and treated with MTZ.
Group 4: Infected and treated with P. granatum peels extract.
Group 5: (prophylactic group) that receive P. granatum peels extract one week before, during, and also one week after induction of infection.
All groups except group 1 will be inoculated with 1 × 103 Giardia cysts. Group 3 was orally treated with the reference drug. Group 4 and 5 were treated orally with the plant extract. The dose of P. granatum peel extract was 3 g/kg body weight which prepared and administered according to Oshiba et al. (2018). Metronidazole (Flagyl, SANOVI Pharmaceutical Industries, Alexandria, Egypt), suspension form 125 mg/ml was purchased from a local pharmacy and was given orally in a dose of 0.3 mg/mouse/day for 7 consecutive days (Brandelli et al., 2009).
2.4.4 Determination of the infection
Starting on the first day of infection and continuing until the Giardia cysts were found and the mice were infected, the stool samples were microscopically inspected regularly. For all mice in each group, the number of Giardia cysts/gm stool was assessed 7 days and 2 weeks following therapy. The number of Giardia trophozoites found in 10 successive power fields (10 × 40) of small intestinal contents, as well as the percent decrease of trophozoites, was determined.
2.5 Sample collection
Sacrification of all mice was done by rapid decapitation 10 days after completing the treatment. Samples of blood were taken from mice after treatment was completed from all groups for hematological studies. Sera were separated for immunological studies. Parts of the duodenum were removed and subjected to parasitological and histopathological studies.
2.6 Histopathological examination
The duodenum portions of mice were removed after they were sacrificed. The gastroduodenal junction was divided into two parts, each measuring 1 cm in length. The following histological examinations were performed on the removed segments; each excised segment was opened lengthwise, orientated on filter paper, and preserved in formaldehyde at a concentration of 10 %. Tissues were treated for paraffin embedding after they had been fixed. Hematoxylin and eosin (H&E) were used to stain 4 µm thick histopathological sections. They were inspected microscopically at low (×200) and high (×400) magnifications to detect giardiasis-related histological alterations and to determine the degree of intestinal mucosa repair following therapy administration (Ross et al., 2016).
2.7 Statistical analysis
Data were collected and statistically analyzed using the statistical package SPSS version 12. It was tabulated as mean and standard deviation (SD) for quantitative variables and percent for qualitative variables. ANOVA and Student t-test were used to detect significance in the quantitative variables, and p-values < 0.001 were considered statistically significant.
3 Results
3.1 Flavonoid and phenolic contents of P. granatum peel extract
Punica granatum peel extract has a total flavonoid and phenolic contents of 13.5 QE/g and 131.04 GAE/mg, respectively.
3.2 Parasitological examination
3.2.1 Giardia cyst count
Quantitative assessment of the intensity of infection of Giardia cyst per gram stool 7 days and 2 weeks after treatment showed a highly statistically significant difference among the different experimental groups (G3,4,5) compared with G2 (Table 1).
Animal groups
Group 1
Group 2
Group 3
Group 4
Group 5
No. of Giardia cysts/gm stool 7 days after treatment *
–
1475 ± 18.95
375.64 ± 2.41
181.69 ± 21.74
139.73 ± 13.75
p-value
P < 0.001**
P < 0.001**
P < 0.001**
No. of Giardia cysts/gm stool 2 weeks after treatment *
–
2189.74 ± 27.94
159.09 ± 24.81
176.40 ± 12.79
129.19 ± 11.27
p-value
P < 0.001**
P < 0.001**
P < 0.001**
No. of Giardia trophozoites/ in 10 successive power fields (10 × 40) small intestine contents *
–
548 ± 189.22
7.16 ± 3.39
226.53 ± 123.52
22.14 ± 9.18
p-value
P < 0.001**
P < 0.001**
P < 0.001**
3.2.2 Giardia trophozoites count
Effect of different treatments regimen on the count of Giardia trophozoites in small intestinal content showed a statistically significant difference among G3,4,5 compared with G2 (Tables 1 and 2).
Animal groups
Group 1
Group 2
Group 3
Group 4
Group 5
Reduction of mean Giardia cysts/gm stool 2 weeks after treatment
–
–
92.73 %
91.94 %
94.10 %
Reduction of mean Giardia trophozoites
–
–
98.69 %
58.66 %
95.96 %
3.3 Histopathological examination
Sections of small intestines of mice included in all groups, G1 revealed normal villous architecture and normal brush border with no histopathological changes. While, G2 showed features of Giardia infection associated with non-specific chronic duodenitis with preserved villus/crypt ratio but lamina propria exhibited marked inflammatory cellular infiltrate (lymphocytes and plasma cells) with edema and shortened, blunted villi. Also, there were Giardia trophozoites with sickle-shaped along the surface of epithelial cells with surface ulceration of villi (Fig. 1 A, B, and C).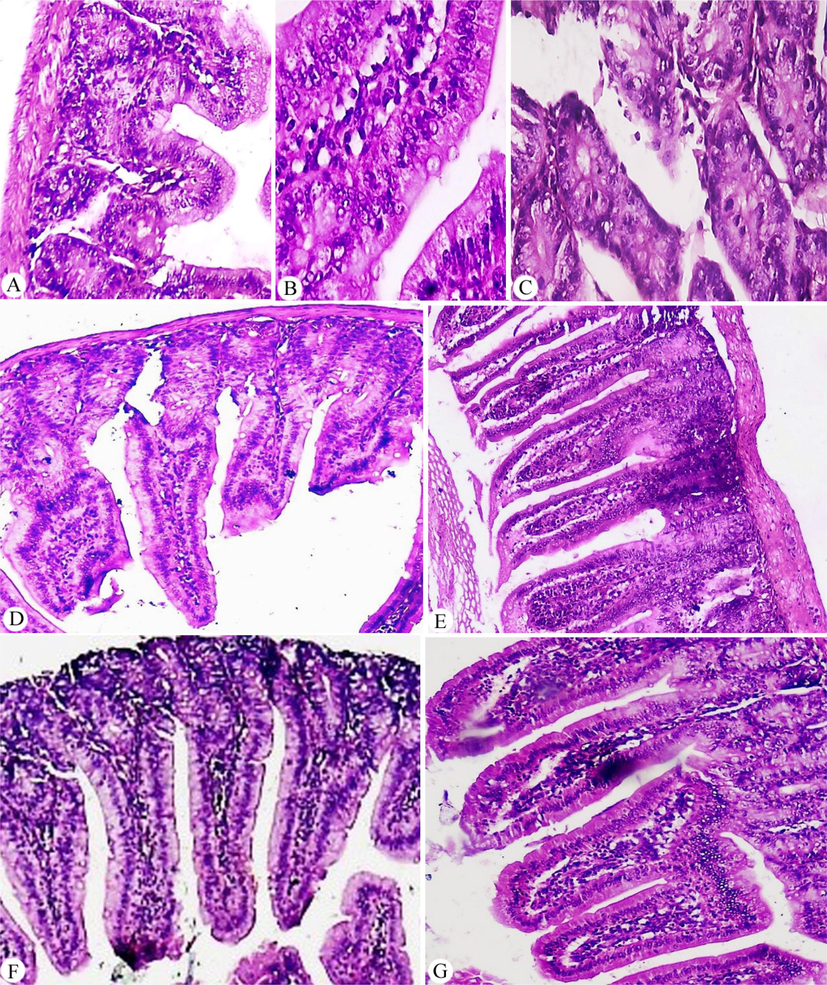
(A, B, and C) G2 with different histological changes showed features of Giardia infection associated with non-specific chronic duodenitis with distorted villus/crypt ratio, lamina propria exhibited marked inflammatory cellular infiltrate (lymphocytes and plasma cells) and edema with trophozoite (A × 100, and B, C × 400). (D) G3 showed a reduction of Giardia trophozoites number with the slight restoration of villi/crypt ratio, moderate inflammatory cellular infiltrate (lymphocytes and plasma cells) and edema of lamina propria (×200). (E) G4 showed villi still blunted with mild regeneration of its lining epithelium and some ulcerated areas, marked inflammatory cellular infiltrate (lymphocytes and plasma cells) and edema of lamina propria also was noticed (×200). (F and G) G5 showed a marked reduction of Giardia trophozoites number with the regeneration of lining epithelium of near-normal villi and mild inflammatory cellular infiltrate (lymphocytes and plasma cells) and edema of lamina propria in the group that received pomegranate as prophylaxis (F × 100 and G × 200).
Sections of small intestines of infected mice in the group treated with MTZ (G3) showed improvement in the pathological lesions showed considering the reduction of Giardia trophozoites number with the slight restoration of villi/crypt ratio. There was moderate inflammatory cellular infiltrate (lymphocytes and plasma cells) and edema of lamina propria (Fig. 1D). The infected group treated with P. granatum peel methanolic extract (G4) revealed an improvement in the form of partial healing of the intestinal mucosa, mild decrease in the ratio between villous heights to crypt length, and focal infiltration with inflammatory cells in the lamina propria. A small number of trophozoites were observed in the lumen (Fig. 1E). In G5 revealed marked improvement in the form of complete healing of intestinal mucosa, with a preserved brush border, absence of mucosal ulceration, and normal villous architecture. There was mild depletion of goblet cells and patchy inflammatory cellular infiltration of lamina propria. The ratio between villous heights to crypt length was more or less preserved and no trophozoites could be detected (Fig. 1 F and G).
4 Discussion
Children, adults, malnourished, and immunocompromised people are all susceptible to giardiasis (Goyal et al., 2011). Giardia infection affects an estimated 280 million individuals each year throughout the world (Ankarklev et al., 2012). Between 2001 and 2017, giardiasis was documented in several locations in Egypt, with prevalence rates ranging from 10 % to 75 %. (Abdel-Moneim and Saeed, 2017). This parasite's cyst stage has been found in surface waterways such as rivers, lakes, and ponds. Food handlers contaminating food and water with Giardia cysts might be one of the major routes of this parasite's transmission to people (Hooshyar et al., 2019).
In giardiasis, a range of chemotherapeutic drugs has been employed, including 5-nitroimidazole compounds, quinacrine, furazolidone, paromomycin, benzimidazole compounds, and nitazoxanide (Lemee et al., 2000). In many human investigations, MTZ was found to be more effective against giardiasis (Busatti et al., 2009). It can also protect against G. lamblia infection by causing DNA damage (Harris et al., 2001). It may also prevent the parasite from consuming oxygen (Gardner and Hill, 2001). MTZ, on the other hand, caused cytotoxicity in gastrointestinal cells, as well as mucosal damage and imbalance (Adil et al., 2018). After treatment for Giardia infection with MTZ, patients still had symptoms and microscopic duodenal inflammation (Hanevik et al., 2007).
Resistance to conventional therapy in G. lamblia is growing by the day, and the number of cases is expected to rise. As a result, significant efforts are being made to discover novel, alternative giardiasis treatments (Long et al., 2007; Müller et al., 2018; El-Gendy et al., 2021). Reinfection, insufficient dosage, poor immunity, drug resistance, sequestration in the biliary system, and the creation of an antioxidant network to protect oxygen-sensitive metalloenzymes are all probable reasons for Giardia therapy failure (Ansell et al., 2015). However, the majority of the medications utilized have significant adverse side effects and are therefore contraindicated (Gardner and Hill, 2001; Petri, 2003). The consumer, and the ever-increasing desire for drug-free food production, is another restraint in the use of anti-Giardia drugs (Harper and Makatouni, 2002).
Alternative natural bio interventions, such as probiotics, that are safe, affordable, and successful in treating intestinal parasitosis are being sought by scientists (Lemee et al., 2000). They may contain novel medicinal compounds to which resistance has not yet arisen, as they are not solely natural products. The use of herbs and natural foods has been proposed by the World Health Organization (WHO) as one approach for eliminating Giardia cysts and trophozoites. The use of medicinal plants has risen in recent years as a result of their minimal side effects (Hezarjaribi et al., 2015). P. granatum peel extract contains phenolic and flavonoid active compounds that capable of suppressing the number of Giardia cysts and trophozoites, which is agreed with Qnais et al. (2007). Through an experimental study on the laboratory mice, the primary goal of this research was to explore P. granatum peel methanolic extract as a preventive and effective drug for treatment and protection against G. lamblia.
The extraction procedure is an important factor that affects the efficacy of the plant extract as a treatment against infections. The present study investigated the effect of P. granatum peel methanol extract on G. lamblia infections as anti-Giardia therapy and prophylaxis. Quantitative assessment of the intensity of infection of Giardia cyst per gram stool 7 days and 2 weeks after treatment showed a highly statistically significant difference among G3,4,5 compared with G2. The percent reduction of mean Giardia cysts/gm stool 2 weeks after treatment was reduced in G3,4,5. The effect of different treatments regimen on the count of Giardia trophozoites in small intestinal content showed a statistically significant difference among G3,4,5 compared with G2 with a highly significant reduction in G3,5 and a significant reduction in G4. While, the percent reduction of mean Giardia trophozoites to approximately 98.69, 58.66, and 95.96 %, respectively, indicate the effectiveness of P. granatum peel methanolic as an anti-Giardia therapy and prophylaxis.
These findings matched those of Aly et al. (2013), who discovered that MTZ treatment reduced the amount of Giardia cysts in hamsters' feces by 90.15 %. When compared to the infected control group, the quantity of Giardia cysts was reduced by 93.7 %. El-Shennawy et al. (2010) found a 72.15 % decrease in Giardia in a group that received pomegranate peel extract compared to an infected control group. Pomegranate peel is an effective anti-protozoan therapy in several additional trials (Dell’Agli et al., 2009). Dkhil (2013) has also observed a considerable increase in antioxidant status and protection of the host tissue against pomegranate extract-induced damage. This might be the major cause of the lower cyst rate in mice given pomegranate peel extract. Furthermore, various researches have shown that P. granatum peel extracts might be potential bioactive natural agents, such as anti-parasitic, that warrant further exploration (Fahmy et al., 2010). Gallic acid, ellagic acid, caffeic acid, p-coumaric acid, quercetin, and vanillic acid were detected in greater concentrations in methanolic pomegranate peel extract than in water extract, according to Mansour et al. (2013), the possible giardicidal efficacy of methanolic P. granatum peel extract studied in this work might be due to the concentration of these components.
Regarding histopathological examination of mice infected and treated with MTZ (G3) showed improvement in the pathological lesions in the form of considered reduction of Giardia trophozoites number with the slight restoration of villi/crypt ratio. In addition, there was mild depletion of goblet cells, focal infiltration with inflammatory cells in the lamina propria, and partial mucosal ulceration. While changes in the infected group treated with P. granatum peel methanolic extract (G4) revealed an improvement in the form of partial healing of the intestinal mucosa, mild shortening, and thickening of villi. Partial mucosal ulceration, mild depletion of goblet cells, mild decrease in the ratio between villous heights to crypt length, and focal infiltration with inflammatory cells in the lamina propria were also detected. A small number of trophozoites were observed in the lumen. In this study, histopathological changes in the prophylactic group (G5), revealed marked improvement in the form of complete healing of intestinal mucosa, with a preserved brush border, absence of mucosal ulceration, and normal villous architecture. There was mild depletion of goblet cells and patchy inflammatory cellular infiltration of lamina propria. The ratio between villous heights to crypt length was more or less preserved and no trophozoites could be detected.
Amer et al. (2015) and Fahmy et al. (2010) both observed partial repair of intestinal villi following MTZ therapy, which is consistent with the current findings. When lauric acid and MTZ were compared in terms of their effect on histopathological lesions, lauric acid seemed to have a better ameliorating effect on the histopathology of the intestinal wall than MTZ. Similarly, Eissa and Amer (2012) found a modest inflammatory infiltration inside the lamina propria with localized shortening of the villi and the presence of few G. lamblia trophozoites in the infected group of mice treated with MTZ. Amer et al. (2015) found that MTZ therapy activated lymphocytes in Peyer's patches, as evidenced by increased cytoplasm thickness. MTZ caused swollen vesicles in the cytoplasm of Giardia trophozoites, according to Campanati and MonteiroLeal (2002).
The therapeutic antibacterial, antioxidant, and anti-inflammatory properties of P. granatum fruit, juice, and peel have been proven in several investigations. Molecules like tannins and polyphenols are primarily responsible for these effects. Furthermore, several recent local studies have found that pomegranate has strong anti-coccidial and anthelminthic action (Dkhil, 2013), making it a prospective anti-coccidial therapeutic therapy (Al-Mathal and Alsalem, 2012). In a mouse model of experimental Cryptosporidium, the peel of P. granatum is beneficial. El-Sherbiny and ElSherbiny (2011), on the other hand, discovered that P. granatum extract had 100 % efficiency against Trichomonas vaginalis in vitro.
Ellagitannins were extracted from the fruit rind, placenta, and barks of the root and stem of P. granatum and tested for their lethal effect on S. mansoni adult worms by Abozeid et al. (2020), who discovered that Ellagitannin-treated worms suffered from erosions, wrinkles, swellings, and losses, as well as degenerations of the surface tubercles and tegument. Furthermore, Ellagitannins caused worms' oral and ventral suckers to distort and degrade, as well as muscle degeneration. Ellagitannins also caused paired worms to separate and reduced their motility.
5 Conclusion
The methanolic extracts of P. granatum peels could be excellently utilized in nutraceuticals because of various functional properties either in the prevention and treatment of G. lamblia infection in the laboratory mice and may be a promising alternative therapy for giardiasis because pomegranate holds great promise as an anti-inflammatory, and will serve as a foundation for further research into pomegranate efficacy against other parasites. Furthermore, it suggests the possibility of discovering novel physiologically active natural chemicals. As a result, more research is needed to discover the active components in pomegranate peel and to conduct toxicity tests to determine acceptable dosage levels.
Acknowledgments
This study was supported by the Researchers Supporting Project (RSP-2021/25), King Saud University, Riyadh, Saudi Arabia.
Ethical considerations
The experimental animal studies were managed following TBRI Research Ethics Committee (TBRI-REC) international valid guidelines and they were maintained under convenient conditions at the animal house in TBRI.
Data availability statement
All the datasets generated or analyzed during this study are included in this published article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro Effects of Punica granatum Ellagitannins on Adult Worms of Schistosoma mansoni. Res. Rep. Trop. Med.. 2020;11:73-80.
- [Google Scholar]
- Effect of Nigella sativa and Alliumcepa oils on Trichinella spiralis in experimentally infected rats. J. Egypt. Soc. Parasitol.. 2005;35(2):511-523.
- [Google Scholar]
- Association of Metronidazole with Cancer: A Potential Risk Factor or Inconsistent Deductions? Curr. Drug Metab.. 2018;19(11):902-909.
- [Google Scholar]
- Pomegranate (Punica granatum) peel is effective in a murine model of experimental Cryptosporidium parvum. Exp. Parasitol.. 2012;131(3):350-357.
- [Google Scholar]
- Efficacy of pomegranate (Punica granatum) peel extract against Hymenolepis nana in infections mice. Biosci. Biotechnol. Res. Asia. 2016;13(1):103-108.
- [Google Scholar]
- Therapeutic Effect of Lauric Acid, a Medium Chain Saturated Fatty Acid on Giardia lamblia in Experimentally Infected Hamsters. Parasitol. United J.. 2013;6(1):89-98.
- [Google Scholar]
- Antioxidant and Anti-Inflammatory Activities of Pomegranate (Punica granatum) on Eimeria papillata -Induced Infection in Mice. Biomed Res. Int.. 2015;2015:1-7.
- [Google Scholar]
- Ankarklev, J., Hestvik, E., Lebbad, M., Lindh, J., Kaddu-Mulindwa, D.H., Andersson, J.O., Tylleskär, T., Tumwine, J.K., Svärd, S.G., 2012. Common coinfections of Giardia intestinalis and Helicobacter pylori in non-symptomatic Ugandan children. PLoS Negl. Trop. Dis. 6, e1780.
- Effects of stunting, diarrheal disease and parasitic infection during infancy on cognition in late childhood: A follow-up study. Lancet. 2002;359:564-571.
- [Google Scholar]
- Indigenous traditional medicine: in vitro anti-giardial activity of plants used in the treatment of diarrhea. Parasitol. Res.. 2009;104(6):1345-1349.
- [Google Scholar]
- The old and new therapeutic approaches to the treatment of giardiasis: Where are we? Biologics. 2009;3:273-287.
- [Google Scholar]
- The effects of the antiprotozoal drugs metronidazole and furazolidone on trophozoites of Giardia lamblia (P1 strain) Parasitol. Rev.. 2002;88(1):80-85.
- [Google Scholar]
- Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal.. 2002;10:178-182.
- [Google Scholar]
- Antiplasmodial activity of Punica granatum L. fruit rind. J. Ethnopharmacol.. 2009;125(2):279-285.
- [Google Scholar]
- Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol. Res.. 2013;112(7):2639-2646.
- [Google Scholar]
- Giardia lamblia: A new target for miltefosine. Int. J. Parasitology. 2012;42(5):443-452.
- [Google Scholar]
- Therapeutic Effect of Chitosan Nanoparticles and Metronidazole in Treatment of Experimentally Giardiasis Infected Hamsters. Iran. J. Parasitol.. 2021;16(1):32-42.
- [Google Scholar]
- Evaluation of ponytail antiparasitic activity of pomegranate juice, peels and leaves against Giardia lamblia. Int. J. Infect. Dis.. 2010;14(Suppl 2):S84.
- [Google Scholar]
- The Effect of Commiphora molmol (Myrrh) in Treatment of Trichomoniasis vaginalis infection. Iran. Red. Crescent. Med. J.. 2011;13(7):480-486.
- [Google Scholar]
- Sexual transmission of giardiasis: a neglected route of spread? Acta Trop.. 2014;132:106-111.
- [Google Scholar]
- Potential antiparasitic activity of pomegranate extracts against schistosomules and mature worms of Schistosoma mansoni: in vitro and in vivo study. Int. J. Infect. Dis.. 2010;14(Suppl 2):S84.
- [Google Scholar]
- Extra-intestinal and long-term consequences of Giardia duodenalis infections. World J. Gastroenterol.. 2013;19(47):8974-8985.
- [Google Scholar]
- Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J. Infect.. 2007;55(6):524-530.
- [Google Scholar]
- Consumer perception of organic food production and farm animal welfare. Br. Food J.. 2002;104:287-299.
- [Google Scholar]
- A systematic review of the effects of Iranian pharmaceutical plant extracts on Giardia lamblia. Asian Pac. J. Trop. Dis.. 2015;5(12):925-929.
- [Google Scholar]
- Giardia lamblia infection: review of current diagnostic strategies. Gastroenterol. Hepatol. Bed Bench. 2019;12(1):3-12.
- [Google Scholar]
- An updated review on Cryptosporidium and Giardia. Gastroenterol. Clin. North Am.. 2006;35(2):291-314.
- [Google Scholar]
- Therapeutic applications of pomegranate (Punica granatum L.): a review. Alter. Med. Rev.. 2008;13:2.
- [Google Scholar]
- Giardiasis in the post genomic era: treatment, drug resistance and novel therapeutic perspectives. Infect. Disord. Drug Targets. 2010;10(4):283-294.
- [Google Scholar]
- Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem.. 2007;101:140-147.
- [Google Scholar]
- Effect of vitamin A and zinc supplementation on gastrointestinal parasitic infections among Mexican children. Pediatrics. 2007;120(4):e846-e855.
- [Google Scholar]
- Phenolic compounds, antioxidant, and antibacterial activities of peel extract from Tunisian pomegranate. J. Agric. Sci. Technol.. 2013;15:1393-1403.
- [Google Scholar]
- Physiological aspects of nitro drug resistance in Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist.. 2018;8(2):271-277.
- [Google Scholar]
- In vivo effect of pomegranate (Punica granatum) extracts versus Nitazoxanide drug on the ileum of experimentally infected mice with Cryptosporidium parvum oocysts. J. Am. Sci.. 2018;14(2):27-39.
- [Google Scholar]
- Effect of piper betle on Giardia intestinalis infection in vivo. Exp. Parasitol.. 2018;184:39-45.
- [Google Scholar]
- Antidiarrheal activity of the aqueous extract of pomegranate (Punica granatum) peels. Pharm. Biol.. 2007;45(9):715-720.
- [Google Scholar]
- Nutritional and Chemical Evaluation for Pomegranate (Punica granatum L.) Fruit Peel and Seeds Powders by Products. Middle East. J. Appl. Sci.. 2013;3(4):169-179.
- [Google Scholar]
- Entamoeba histolytica and other parasitic infections in south Kanara district. Karnataka. J. Commun. Dis.. 1989;21(3):207-213.
- [Google Scholar]
- World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med.. 2015;12(12):e1001920.
- [Google Scholar]







