Translate this page into:
Comparative study of the anti-diabetic effect of mucilage and seed extract of Abelmoschus esculentus against streptozotocin-induced diabetes in rat model
⁎Corresponding author. msaleissa@imamu.edu.sa (Mohammed S. Aleissa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Diabetes mellitus (DtM) is a collection of recurrent metabolic abnormalities that are generally assessed by persistent hyperglycemia. This condition occurs when the insulin released from the pancreatic cells get destroyed and produce little or no insulin. In traditional Chinese medicine, Abelmoschus esculentus (AS) is used for the treatment for type 1 diabetes. This study aims to mitigate type 1 DtM caused by the administration of streptozotocin (STZ).
Methods
Diabetes was produced in each of the Wistar rats by administering a single shot administration of STZ intraperitoneally. The rats were split into five groups. Group1 and 2 were normal control and diabetic control group. Groups 3, 4, and 5 were received okra mucilage extract and okra seed crude extract at a dosage of 150 mg/kg, 200 mg/kg, and 200 ml/kg. After 4 weeks animals were sacrificed and blood was collected through cardiac puncture and liver and kidney were dissected for further biochemical as well as histopathological examinations.
Result
The FBG, %HbA1c, lipid profile, liver function test, oxidative stress, and histopathology investigation were analyzed. Our result showed a significant difference between different groups in each parameter. Our study revealed that okra mucilage extract has advantage over okra seed crude extract significantly.
Conclusion
The extract of AS has an antidiabetic activity and contributes to the overall reduction in BG levels. Its properties can be a remedy to manage DtM. Based on the results of the tests, it was determined that a solid oral formulation might be made using okra-derived mucilage crude extract and aqueous seed extract as a pharmaceutical excipient.
Keywords
Abelmoschus esculentus
Diabetes
Hyperglycemia
Streptozotocin
Mucilage
Insulin
- STZ
-
Streptozotocin
- GOD/POD
-
Glucose-oxidase-peroxidase
- FBG
-
Fasting BG
- HbA1c
-
Glycated hemoglobin
- PMS
-
Post mitochondrial supernatant
- TC
-
Total cholesterol
- TG
-
Triglycerides
- LDL-C
-
Low-density lipoprotein-cholesterol
- HDL-C
-
High-density lipoprotein-cholesterol
- SGPT
-
Serum Glutamate Pyruvate Transaminase
- SGOT
-
Serum Glutamate Oxaloacetate Transaminase
- ALP
-
Alkaline phosphatase
- PAS
-
Periodic Acid-Schiff
- LPO
-
Lipid peroxidation
- MDA
-
Malondialdehyde
- GSH
-
Reduced glutathione
- SOD
-
Superoxide dismutase
- CAT
-
Catalase
- OSE
-
Okra seed extract
- OME
-
Okra mucilage extract
Abbreviations
1 Introduction
Diabetes mellitus (DtM) is a progressive heterogeneous disturbance of metabolic disorder assessed by hyperglycemia (Inzucchi, 2003; Chaudhury et al., 2017). Diabetes is classified into two types: type 1 and type 2. Diabetes type 1 is caused by the loss of pancreatic -cells, preventing them from producing insulin, a condition known as absolute insulin deficiency. In type-2 diabetes, cells cannot recognize insulin causes hyperglycemia, a condition known as insulin resistance (Seino et al., 2010). A disruption in lipid, protein, and carbohydrate metabolism is typically associated with a decreased amount of insulin action and/or secretion inadequacies. In DtM, a lack of insulin activity on target cells/tissues causes anomalies in glucose, lipid, and protein metabolism. This insufficiency is brought on by inadequate insulin production and/or impaired tissue responsiveness to insulin along with other complicated hormonal action pathways. Alternatively, this deficiency may be brought on by a combination of both of these factors. Diabetes patients often have both insulin secretion and insulin action impaired simultaneously. There is often no consensus on which abnormality, if either, is the most likely cause of hyperglycemia. (The Expert Committee on the Diagnosis and Classification of DtM; 1997). Multiple organs, including the kidneys, blood vessels, eyes, heart, and nerves become dysfunctional and eventually collapse due to DtM. (Tripathi and Srivastava 2006). Furthermore, diabetic complications are associated with morbidity and mortality due to multiple pathophysiological defects (Ivorra et al.,1989).
According to the epidemiologic data, 2.8 % of the global population was diabetic in the year 2000. It may pass more than double by 2030 to 4.4 %, affecting all age and ethnic groups of people (Xing et al., 2009). Diabetic type-1 and type-2 disease progressions are distinct, but they share specific symptoms, such as hyperglycemia and diabetic complications (Chen et al., 2008; Sikarwar and Patil, 2010). In most conditions, raised lipids level leads to increased cardiovascular complications (Yang et al., 2006). Nowadays, diabetes can be managed by using pharmacological and non-pharmacological methods including, diet and exercise monitoring. Most of pharmacological agents have severe side effects, which urges the scientific community to search for alternatives from natural or herbal sources that are possibly less toxic than current drug therapies (Palsamy and Subramanian, 2008). Though, several oral hypoglycemic agents and insulin are present for the treatment of diabetes. A variety of phytoconstituents from medicinal plants have been identified and researched for their anti-diabetic activities.
Abelmoschus esculentus L. (AS), also known as okra, or lady's fingers. It is a blooming shrub of the mallow family that is farmed in tropical and temperate regions for its tasty greenish pods (Badrie, 2016). This plant is known for its mucilaginous properties, as well as its anti-diabetic properties. It has a wide range of active medicinal constituents like vitamins, polyphenolic compounds, polysaccharides, and flavonoids. These phytoconstituents have potent antidiabetic, antihyperlipidemic, neuroprotective, chemoprotective, and radical scavenging properties. This plant's lipophilic and hydrophilic antioxidant components allow it to neutralize free radicals in both lipid and aqueous phases. the flavonoids as well as the vitamin C present in this plant safeguard against the oxidation of low-density lipoproteins in a synergistic way (Xia et al., 2015).
In addition, multiple investigations have demonstrated that okra peel, as well as seed powder, could strengthen the pancreas, kidney, and liver due to its antioxidant effect in diabetic rats. By regulating glucose uptake okra's polysaccharide components are vital for sustaining appropriate blood glucose (BG) levels (Gemede et al., 2015). There are numerous applications for okra mucilage, such as in medicine and food applications (BeMiller et al., 1993), as a tablet binder (Ofoefule et al., 2001), in dose formulation as a suspending agent (Kumar et al., 2009), as a Food additive that protects against chronic conditions that irritate and inflame the stomach (Lengsfeld et al., 2004), as a plasma replacement or blood volume expander. The functional qualities of mucilage extracted from okra vegetables have only been the subject of a limited number of scientific research. On the other hand, the mucilaginous components of okra have not been given nearly as much consideration regarding their potential antioxidant properties (Adetuyi and Dada, 2014; Jideani and Bello, 2009; Noorlaila et al., 2015; Woolfe et al., 1977). This research aims to investigate the effect of AS on liver functions such as oxidative stress, lipid profile, histopathological changes, and per cent of glycated haemoglobin (%HbA1c) level.
2 Methodology
2.1 Plant material and extraction
The unripe and tender okra fruits (pods) were obtained from the local market. The fruits were appropriately washed with water to remove any mud or dust. The fruits were sun-dried for an hour before being shade-dried completely. The Okra seeds were removed since they lack mucilage. Fruits were cleaned and thinly sliced using a knife. To extract the mucilage, the sliced material was let to soak in water that had been distilled for a whole night. Following the soaking process, a white muslin cloth with two layers was employed to filter off the thick gum extract (mucilage). Mucilage was flocculated with ethanol in a 50:50 v/v ratio. Then it was washed with acetone (100 %), and air drying was used for mucilage powder. However, air-tight bottles were used to store the mucilage powder (Fig. 1).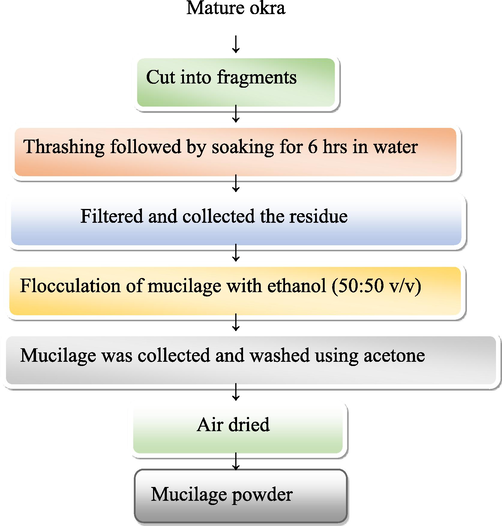
Process and preparation of mucilage from mature okra.
2.2 Effect of extraction conditions on the recovery
In the process of extracting mucilage, the influence of extraction was investigated by repeating the extraction technique under a variety of circumstances, and the amount of mucilage that could be extracted was computed. A number of soaking times including 3, 6, 12, 24, and 48 h were used, and the mucilage production was measured after each of these times. The best dilution was chosen for future investigation since it produced the largest mucilage production.
2.3 Aqueous extraction of okra seed
Seeds were removed and dried under an incubator for two weeks before being ground into a coarse powder and sieved and stored in an airtight container until the completion of the study. Powdered seeds were transferred into a centrifuge tube and extracted with distilled water by centrifugation at 2000 rpm at 4 °C for 10 min. After centrifugation, these extracts were transferred into a Petri plate containing acetone, where they were concentrated to dryness under reduced pressure and at a controlled temperature (50–60 °C), yielding solid masses completely free of solvents (yield: 18.92 % w/w and 12.36 %w/w for aqueous extract, respectively) (Fig. 2). The animals were orally administrated 200 ml/kg body weight of the ethanolic and aqueous extracts.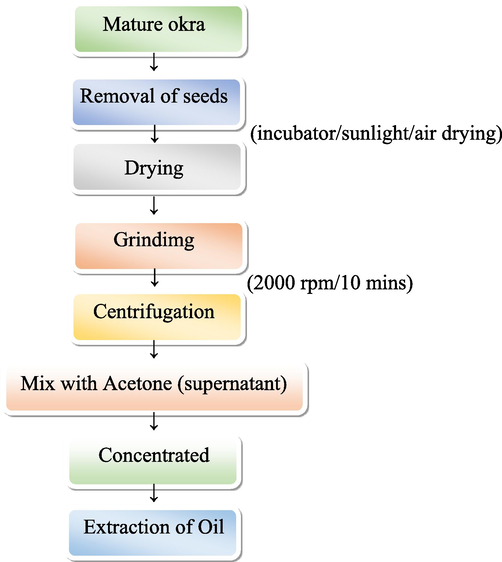
Process and preparation of seed oil from mature okra seeds.
2.4 Animals and experimental design
In the current investigation, an experimental rat model consisting of 30 male Wistar albino rats weighing between 120 and 180 g was used. These rats were obtained from the animal facility at Imam Muhammad Ibn Saud Islamic University in Riyadh, Saudi Arabia. The animals were kept in the animal house, which was having a controlled environment with normal parameters for temperature and humidity. There were also 12 h of light and dark cycle. The animals were separated into 5 groups, and each group had a total of 6 rats. The rats in groups 1 and 2 serve as normal control and diabetic control, respectively. The rats in groups 3, 4, and 5 were given okra mucilage extract and Okra seed aqueous extract by mouth at dosages of 150, 200, and 200 mg/kg of body weight, respectively, for 30 days. Studies on animals were done in accordance with the Imam Muhammad Ibn Saud Islamic University IRB guidelines (IRB No. 106-2021).
2.5 Induction of diabetes
To evoke induction of diabetes in rats, STZ (Sigma-Aldrich) prepared in 0.1 M cold sodium citrate (pH 4.5) was administered intraperitoneally. The dosage of STZ administered was 65 mg/kg body weight (BW), whereas the vehicle was administered to the control normal rats. Following 72 h of injection, blood glucose levels were monitored using an electronic glucometer. (Dr. Morepen, Morepen laboratories, Himachal Pradesh), and the rats with BG above 250 mg/dL were used for the experiments.
2.6 Measurement of fasting BG
This test measures glucose level (a type of sugar) in your blood. Fasting BG was measured by the glucose-oxidase–peroxidase (GOD/POD) method (Braham and Tinder, 1972) using a commercial diagnostic kit obtained from SPAN Diagnostics. Blood was drawn from the retro-orbital plexus under general anaesthesia, and FBG was determined for each group before and after drug treatment.
2.7 Sample collection and tissue preparation
The rats were sacrificed by heart puncture under general anaesthesia. Blood was collected for biochemical analysis and measurement of FBG and HbA1c. The Liver was rapidly removed and homogenized using mortar-pestle in ice-cold phosphate-buffered saline (pH 7.4) to form a 10 % homogenate. Homolysate was centrifuged at 10,000 rpm for 20 mins at 4 °C, and post mitochondrial supernatant was separated for biochemical analysis. For histopathological examination, rat liver from each group was preserved in a 10 % buffered formalin solution.
2.8 Determination of per cent glycated haemoglobin (% HbA1c) level
Glycated hemoglobin (Hb), also known as HbA1c, is produced when glucose residues in the blood bind to Hb molecules, causing the Hb molecules to become glycated. % HbA1c was assayed by cation-exchange method (Nathan et al., 1984) using a diagnosing kit from Crest Biosystem, Goa, India, following manufacture instructions. To determine HbA1c, a hemolysate for each sample was prepared by dispensing 0.5 ml of haemolysing reagent (R3) into a tube and adding 10 µl of well-mixed whole blood, control or calibrator and mixing the reactants and allowing it to stand for 5 min at room temperature for complete lysis. 7.5 µl Hemolysate was incubated with 180 µl HbA1c R1 (latex) for 5 min at 37 °c. After minutes, this sample was mixed with 60 µl HbA1c R2 (consisting of anti-human HbA1c mouse monoclonal antibody, anti-mouse IgG goat antibody) and incubated for 5 min at 37 °C. The absorbance (A) was measured at 630 nm by a spectrophotometer (Shimadzu-1601, Japan).
2.9 Estimation of protein
The BCA technique was used to determine the amount of total protein in both the homogenate and the PMS. A sample volume of 25 ul was mixed with 50 % more distilled water before being combined with 950 ul of BCA solution. After heating the mixture to 37°Celsius for half an hour, the absorbance was determined using a spectrophotometer set at 562 nm (Shimadzu-1601, Japan).
2.10 Lipid profile assays
Lipid profiles (TG, HDL, LDL and TC) were estimated using enzymatic kits provided by SPAN Diagnostics India, ltd. (Surat, India).
2.11 Liver function tests
Liver function (SGOT, SGPT and ALP) tests were determined using commercially available diagnostic test kits (Span diagnostics Limited, Surat, India) following the manufacturer's instructions.
2.12 Histopathological examination of the liver
The liver was preserved in a 10 % buffered formalin solution for histopathological examinations. After fixation in 10 % buffered solution, thin slices of liver tissue with cortex and medulla were dehydrated and embedded in paraffin. At least four cross-sections of 3–4 µm thickness were taken from each liver and stained with Jones periodic Acid-Schiff (PAS). Tissue sections were then washed with xylene for 2 min and mounted with a DPX mountant. The stained slides were then imaged using brightfield microscopy. Microphotographs were taken using an Olympus BX50 microscope system (Olympus, Japan). The PAS stains of the liver were used to assess the extent of mesangial expansion, glomerular lesions, and the presence or absence of any proteinaceous casts in tubules.
3 Assessment of oxidative stress in liver tissue
3.1 Estimation of lipid peroxidation level
Malondialdehyde (MDA), a byproduct of lipid peroxidation, was measured in liver homogenate to detect lipid peroxidation (LPO) (Ohkawa et al., 1979). In a nutshell, 0.1 ml of liver homogenate was mixed with 1.5 ml CH3COOH, 1.5 ml TBA, and 0.2 ml sodium dodecyl sulphate, and the mixture was then kept at 100 °C for 1 h. The solution was then combined with 5 ml n-butanol: pyridine [15:1] and 1 ml de-ionized water, then centrifuged for 10 min at 4000 rpm. Withdrawing the organic layer allowed for the use of a spectrophotometer to measure absorbance at 532 nm.
3.2 Assay for reduced glutathione (GSH) content
GSH content was measured by the standard method (Jollow et al., 1974) with slight modification. In a nutshell, the assay combination was made up of 0.5 ml of cytoplasmic fraction and 0.5 ml of sulfosalicylic acid at a concentration of 4 %. After incubating the solution at 4°Celsius for one hour, it was then processed at 1200 g for fifteen minutes. To make a total volume of 1.0 ml, 1.0 mM DTNB and 0.1 M phosphate buffer were added to 0.1 ml of supernatant. At a wavelength of 412 nm, the absorbance was measured.
3.3 Assay for superoxide dismutase (SOD) activity
SOD activity was assayed by a standard method (Marklund and Marklund, 1974). In a nutshell, 0.1 ml of 10 % PMS was mixed with 2.875 ml of a Tris–HCl buffer that had a concentration of 50 mM and a pH of 8.5, as well as 25 l of pyrogallol that had a concentration of 24 mM in 10 mM HCL. The SOD activity was evaluated by observing the absorbance at 240 nm.
3.4 Assay for catalase (CAT) activity
The assay described by Claiborne (Claiborne, 1985) was used to measure the CAT activity. In a nutshell, 0.1 ml of PMS (10 %) was treated with 1.9 ml of PBS, followed by 1 ml of H2O2. At a wavelength of 240 nm, variations in absorbance were measured. The activity of catalase was measured in terms of the amount of H2O2 that was used per minute per milligram of protein.
3.5 Statistical analysis
The results are shown as the mean ± standard error of the mean (n = 6). When P was<0.05, statistical significance was assumed. The analysis of variance (ANOVA) was used to acquire the statistical analysis of the data, and Tukey's test was then performed on the data. Graph Pad (San Diego, CA) InStat version 3 was utilized to carry out the analyses that needed to be done.
4 Results
4.1 Fasting BG level, insulin content and HbA1c (%)
Comparison of FBG, insulin level, and HbA1c content across all groups, normal control, diabetic control and the three treated groups, are shown in (Fig. 3). A significant increment (p < 0.05) in BG level and HbA1c % content was observed in okra seed extract (OSE) and okra mucilage extract (OME-1 and OME-2) treated STZ induced type-1 diabetic group with compare to normal control. The four-week treatment of STZ induced type-1 diabetic groups with OME-1 (150 mg/kg), OME-2 (200 mg/kg), and OSE (200 ml/kg) resulted in a significant hypoglycemic effect. OME and OSE have shown comparable reductions in %HbA1c levels from 8.475 ± 0.75 to 7.881 ± 0.37 and 8.358 ± 0.25 respectively, compared to diabetic control. Insulin levels are restored to normal in all the three treated groups, with the best effect seen in the OME-2 treated group. The BG level decreases from 392.66 ± 17.50 to 284.83 ± 7.22 and 338.16 ± 5.19 in diabetic control to OME and OSE treated groups, respectively. The total protein concentration increases up to 145.16 ± 5.03 in OME-2 from 107.5 ± 5.16 in the diabetic group.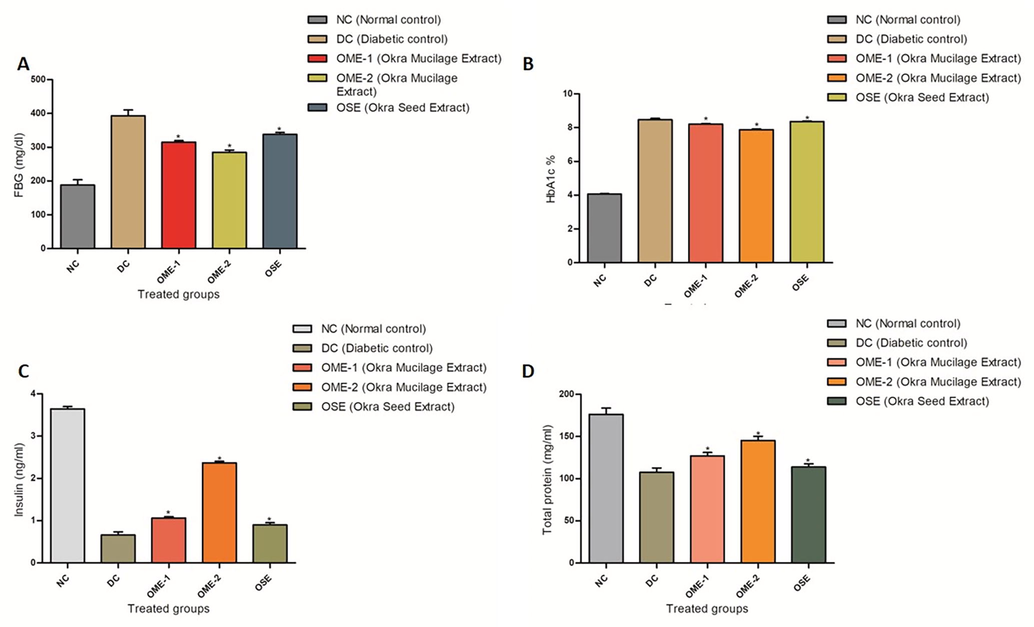
(A) FBG (mg/dl) profile in normal control, diabetic control, OME-1, OME-2, and OSE treated groups. (B) HbA1c% profile of normal control, diabetic control and OME-1, OME-2, and OSE treated groups. A comparative reduction in %HbA1c levels can be seen across all groups. (C) Insulin levels (ng/ml) in normal control, diabetic control, and Okra treated groups, OME-1, OME-2, and OSE. (D) Protein estimation in normal control, diabetic control, and okra treated groups, OME-1, OME-2, and OSE.
4.2 Effect of different doses of OME and OSE on the lipid profile of STZ-induced type-1 diabetic rats
The diabetic control group shows a significant increase (p < 0.05) in the serum levels of total cholesterol (TC), triglycerides (TG), and Low-density lipoprotein cholesterol (LDL-C), while a significant decrease in the serum high-density lipoprotein cholesterol (HDL-C) level, compared to normal control (Fig. 4). Treatment with OME-2 has restored all the changes in lipid profile close to normal control. A significant increase (p < 0.05) in serum HDL-C level is observed across all treated groups, with a decrease in serum TC, TG, and LDL-C levels compared with the diabetic control. OME-2 treated group shows lipid profile closest to normal control compared with OME-1 and OSE treated groups, indicating greater efficiency in restoring normal lipid levels and anti-lipolytic action (Fig. 4).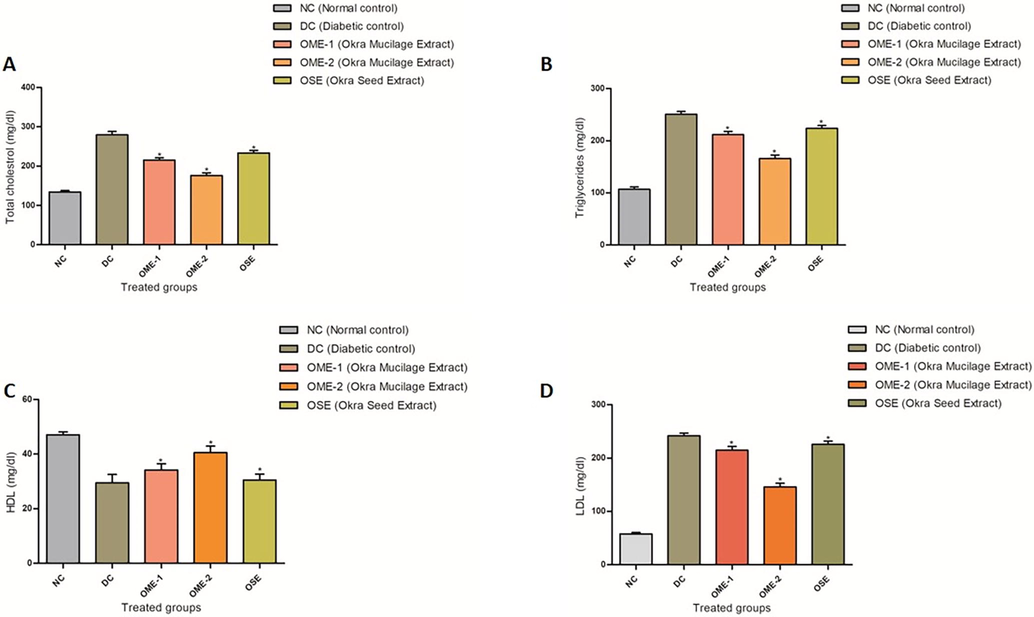
(A) TC profile. A plot of serum level of TC: Total Cholesterol (mg/dl) in normal control (NC), diabetic control (DC), OME-1, OME-2, and OSE treated group. (B) TG profile. A plot of serum level of TG: Triglycerides (mg/dl) in normal control (NC), diabetic control (DC), OME-1, OME-2, and OSE treated group. (C) HDL-C profile. A plot of serum HDL-C (mg/dl) level in normal control (NC), diabetic control (DC), OME-1, OME-2, and OSE treated group. (D) LDL-C profile. A plot of serum LDL-C (mg/dl) level in normal control (NC), diabetic control (DC), OME-1, OME-2, and OSE treated group.
4.3 Evaluation of liver function
Liver health was assessed by performing a liver function test for ALP, SGOT and SGPT activity (Fig. 3). The diabetic control shows a significant increase (p < 0.05) in the serum levels of all three enzymes compared to the normal control group. This result is indicative of liver injury caused as a result of hyperglycemia. Treatment with all the three dosages of okra seed crude extract and okra mucilage crude extract shows a decrease in serum levels of SGPT, SGOT, and ALP, indicating an improvement in liver health with treatment. OME-2 treated groups show the highest reduction in SGOT, SGPT, and ALP activity (Fig. 5).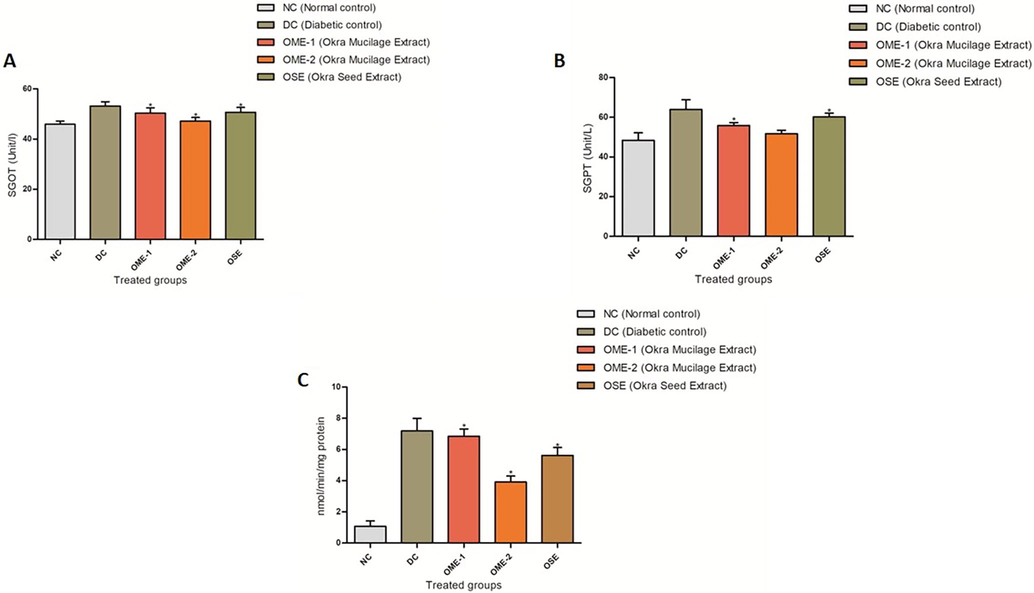
(A) SGOT profile. A plot of serum SGOT activity (U/L) in normal control (NC), diabetic control (DC), OME-1, OME-2, and OSE treated group. (B) SGPT profile. A plot of serum SGPT activity (U/L) in normal control (NC), diabetic control (DC), OME-1, OME-2, and OSE treated group. (C) ALP profile. A plot of serum ALP activity (U/L) in normal control (NC), diabetic control (DC), OME-1, OME-2, and OSE treated group.
4.4 Evaluation of LPO, SOD, GST, and CAT activity in diabetic and treated groups
The SOD, GST, and CAT activity in all the three treated groups have elevated significantly (p < 0.05), with the highest elevation in all the three enzymes levels in OME and OSE treated groups (Fig. 6). A depletion in SOD (34.5 ± 3.27), GST (81.33 ± 4.3), and CAT (18.16 ± 2.9) activity are seen in the case of diabetic control, indicating extensive oxidative stress. OME-2 increased CAT, SOD GST levels to 40.16 ± 4.62, 55.66 ± 6.02, and 122.16 ± 10.40, respectively (Fig. 6).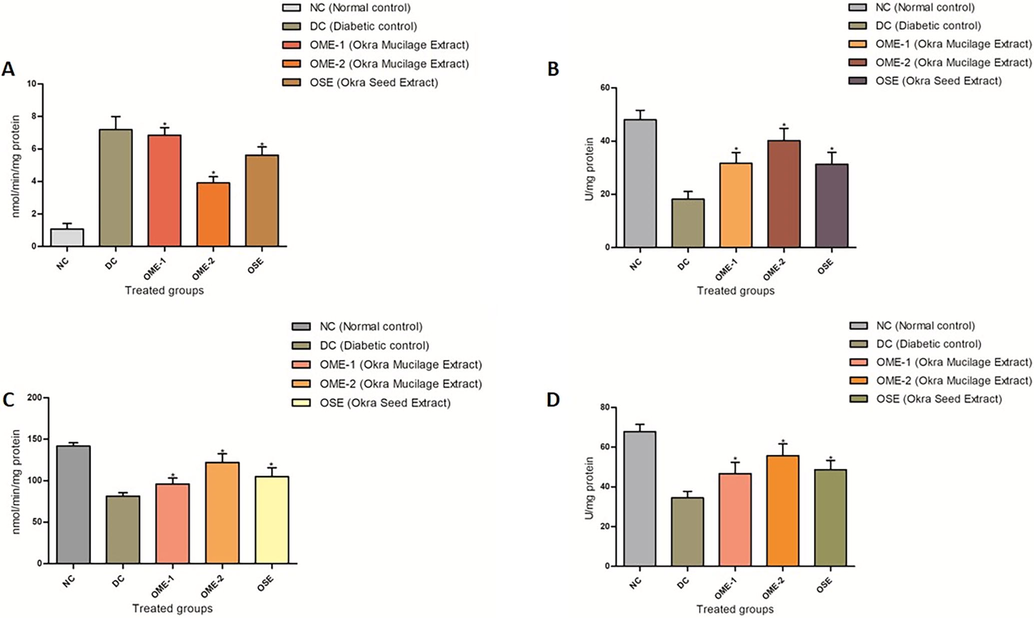
(A) Lipid peroxidation assay. A plot of comparison of MDA content (nmol/min/mg) in normal control, diabetic control, and treated groups (OME-1, OME-2, and OSE). (B) Catalase activity. A plot of comparison of Catalase levels (U/mg) in normal control, diabetic control, and treated groups (OME-1, OME-2, and OSE). (C) GST activity. A plot of comparison of GST levels (U/mg) in normal control, diabetic control, and treated groups (OME-1, OME-2, and OSE). (D) SOD activity. A plot of comparison of SOD levels (U/mg) in normal control, diabetic control, and treated groups (OME-1, OME-2, and OSE).
4.5 Histopathology of liver
In normal control rat liver, hepatocytes appeared to be distributed normally in radial patterns around hepatic cords (Fig. 7). Hepatocytes contain single or sometimes double central nuclei. Diabetic rats show an upsurge in vacuolation in the cytoplasm, which looks like a scattering of indistinct vacuoles and indicates glycogen infiltration, has been observed. The vacuolation of hepatocytes is minimal in diabetic rat livers that have been treated with OME. The diabetic rat liver that was medicated with OSE demonstrates normal liver architecture, including normal hepatocyte distribution in the cords that surround the major vein. Normal hepatocytes with less vacuolation can be seen in diabetic rat livers that have been treated with OME (Fig. 7).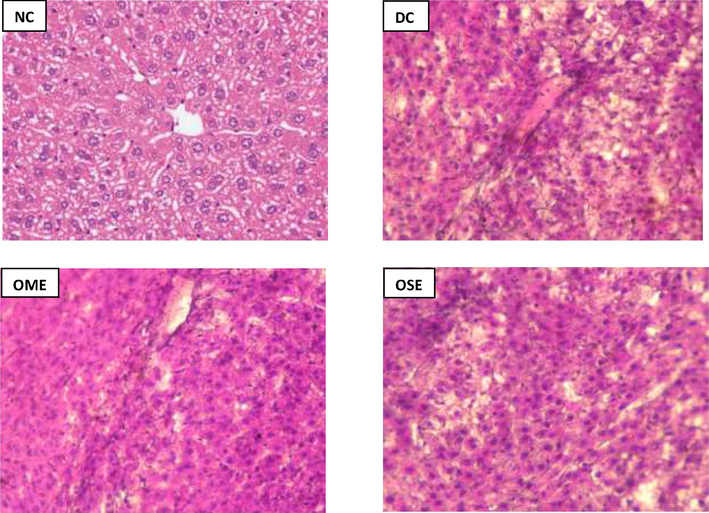
Histopathology of Liver. NC: Normal control, DC: Diabetic Control, OME: Okra mucilage extract, and OSE: Okra seed extract. Images were acquired using a magnification of 40X.
5 Discussion
This study demonstrated that STZ causes hyperglycemia in rats accompanied by oxidative damage in hepatic and renal tissues. The objective of this research was to evaluate the efficacy of treatment with AS seed and mucilage in restoring normal metabolic function following exposure to STZ. Even though neither of the herbal preparations was able to prevent diabetes from developing in rats after being injected with STZ, the rats that were already diabetic were able to benefit from their antidiabetic activity. This study evaluated the hypolipidemic and glucose-lowering potential of aqueous seed extracts and mucilage crude extract from AS in STZ-induced type-1 diabetic rats. Oral administration of mucilage, crude mucilage oil, and seed powder extract of okra at 150, 200, and 200 mg/kg doses in diabetic rats showed a significant reduction in BG level and increased body weight than diabetic control rats.
Furthermore, aqueous crude seed extracts and mucilage crude extract of AS fruits on experimentally induced hyperglycemia were investigated. The okra extracts at a dose of 150 mg/kg significantly reduced BG levels. There is a reduction of %HbA1c 7.881 ± 0.37 and 8.358 ± 0.25 respectively in OME-2 AND OSE compared with diabetic control 8.475 ± 0.75. The lipid profile has also increased in all three treatment groups. The OME group shows the most effective lipid profile, 3.91 ± 0.37, compared with 7.18 ± 0.80 maximum decrement in TC, TG, and LDL-C levels have been observed in the case of OME-2 with equally increment in HDL-C level compared with diabetic control. Serum levels of SGPT, SGOT, and ALP also decreased in all three treated groups; here highest reduction can be seen in the case of OME-2. The total protein content of the treated groups has also increased (145.16 ± 5.03), indicating protein synthesis in the liver near to normal levels (176.16 ± 7.57). Other studies showed that the polysaccharide extracted from okra injected into diabetic mice significantly reduced the BG by decreasing the adsorption of cholesterol and sugar from the diet. Rhamnogalacturonan, a carbohydrate found in okra was found to be responsible for this hypoglycemic activity (Egharevba and Gamaniel, 2018). In another experiment, alloxan-induced diabetic mice showed the administration of AS considerable reduction in the level of BG, TC, TG and LDL (Uddin Zim et al., 2021).
At doses of 150, 200, and 200 mg/kg, aq. extract from seed and mucilage caused a substantial reduction in total cholesterol and TG levels, respectively. Because the AS extract was able to lower TC, the LDL portion of the blood also reduced, which was helpful for the hypolipidemic medicines. This finding points to the quick degradation of LDL-C through its hepatic receptors, which allows for its final removal in the form of bile acids. As a result, the hypolipidemic effect of the extract is likely related to this process. In terms of the effects described above, the effectiveness of the plant seeds was greater compared to the mucilage. STZ is digested inside the beta cells of the pancreas, which results in the generation of NO and inhibits the manufacture and secretion of insulin. In addition to this, STZ causes an elevation in hba1c and has a negative impact on various other indicators of glycemic control (Rafieian-Kopaei et al., 2014).
The levels of GSH, SOD, and catalase in diabetic rats' livers, as well as the levels of TBA reactive substances, were substantially enhanced after administration of varying doses of mucilage and seed crude extract. This was in comparison to the levels of these enzymes and substances in diabetic control rats. (Sabitha et al., 2012). The liver tissues from all three instances have shown complete regression of disease upon histopathological examination.
Polyphenolic phytochemicals have anti-diabetic properties. Elevated quantities of quercetin and phenolic compounds are found in okra seeds. Quercetin may have vasodilator and anti-oxidant properties (Nasri et al., 2013). It may also shield and improves the release of insulin from the beta cells of the pancreas (Rafieian-Kopaei et al., 2014). Flavonoids regulate glucose homeostasis and insulin activity at the cellular level. Myricetin, a flavonoid has been demonstrated to have hypoglycemic effects in STZ-induced diabetic rats (Gomes et al., 2010). STZ causes hyperglycemia, which is followed by a considerable reduction in antioxidant capacity as well as an increased level of reactive oxygen species (ROS) formation and lipid peroxidation. Antioxidants can be found in significant quantities in AS. In STZ-induced diabetic rats, the flavonoid antioxidant known as quercetin offers protection against the destruction to pancreatic beta cells. In STZ-induced diabetic rats, quercetin was found to limit the generation of nitric oxide and lower CRP concentrations, both of which are qualities that are associated with anti-inflammatory activity. There are a lot of medicinal herbs that are quite similar to AS that play important roles in scavenging different kinds of ROS. The current findings demonstrated a substantial impact of AS seed as well as mucilage in reducing blood glucose levels. The abundance of polysaccharides in AS is primarily responsible for its ability to lower blood sugar levels. AS seeds contain polysaccharides that can bind to bile acids in the intestine, which in turn inhibits the recycling of bile acids and speeds up the excretion of these acids. The subsequent impact could be the transfer of hepatic lipid storage to the bile acid production pathway, which would ultimately result in lower levels of both serum and hepatic cholesterol. In addition, AS includes several other biologically active elements, including flavonoids, phytosterols, and phenolic compounds, all of these are documented to exhibit cardioprotective characteristics. The outcomes achieved by this study disclose that feeding the rats orally with 150, 200 mg of AS seed aqueous extract and mucilage reduced BG levels in STZ induced type1 diabetic rats and resulted in a lower percentage increase in average serum LDL and average body weightless when compared to rats fed with food supplemented. Our study report that OME-2 compared with OME-1 and OSE is nutritious, safe, and effective in treating type-1 DtM with a low risk of hypoglycemia.
5.1 Research limitation
The current investigation is restricted to STZ-induced type 1 diabetic rats and their BG level, in addition to a number of different biochemical markers and histological examinations. It is necessary to conduct additional study on the various forms of diabetes in a variety of other rodent models, which will then be followed by the isolation and characterisation of lead compounds and their respective modes of action. In addition, prior to conducting clinical trials on humans, it is recommended to do studies on toxic effects, pharmacokinetics, pharmacodynamics, as well as cellular and molecular research.
6 Conclusion
AS is defined in folk medicine for the therapy for DtM. The monarch seeds of AS could be used to develop a drug for diabetes due to their anti-diabetic activity. Mucilages are acidic polysaccharides that are commonly found in pod walls and are associated with minerals and proteins. It has an antidiabetic property against STZ-induced Type-1 diabetic rats. AS extract has a hypoglycemic effect and helps lower BG levels, as indicated in the above results. Its properties can be a remedy to manage DtM. The evaluated parameters showed that okra-derived mucilage crude extract and aqueous seed extract could be used as a pharmaceutical excipient to formulate a solid oral dosage form. It has an acceptable pH value and organoleptic properties, so it can easily be used to formulate various dosage forms. More scientific research can be conducted to uncover more nutritional properties of the AS fruit based on these findings.
7 Authors’ contributions
Mohammed Aleissa, Daoud Ali and Lina M Alneghery were involved in plant extraction, induction of diabetes and statistical analysis. Mohammed AL-Zharani and Saad Alkahtani were estimated liver profile and lipid profile. Md Saquib Hasnain and Saud Alarifi were performed the histopathological study. Bader Almutairi was evaluated the oxidative stress markers. Saud Alarifi and Saad Alkahtani contributed to the overall conception and planning of the study, as well as the analysis of the collected data and the thorough revision of the paper. The final text was reviewed and finalised by all of the authors.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work through Research Group no. RG-21-09-88.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nutritional, phytoconstituent, and antioxidant potential of mucilage extract of Okra (Abelmoschus esculentus), water leaf (Talinum triangulare) and Jews mallow (Corchorus olitorius) Int. Food Res. J.. 2014;21(6):2345.
- [Google Scholar]
- Badrie, N. 2016. Nutrient profile, bioactive components, and functional properties of okra (Abelmoschus esculentus (L.) Moench). Fruits, Vegetables, and Herbs, pp.365-409.
- Aloea, chia, flax seed, okra, psyllium seed, quince seed, and tamarin gums. In: Whistler R.L., BeMiller J.N., eds. Industrial Gums. New York: Academic Press; 1993. p. :227-256.
- [Google Scholar]
- Clinical review of antidiabetic drugs: implications for type-2 DtM management. Front. Endocrinol.. 2017;8:6.
- [Google Scholar]
- Hypoglycemic effects of a sesquiterpene glycoside isolated from leaves of loquat (Eriobotrya japonica (Thunb.) Lindl.) Phytomedicine. 2008;15(1-2):98-102.
- [Google Scholar]
- Handbook of Methods for Oxygen Radical Research. CRC Press Inc; 1985. p. :283-284.
- Potentials of some Nigerian herbs and spice as source of pharmaceutical raw materials: opportunity for global market competitiveness. Int. J. Pharmacog. Phytochem. Res.. 2018;9(12):1435-1441.
- [Google Scholar]
- Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. Food Process. Technol. 2015
- [Google Scholar]
- Classification and diagnosis of DtM. In: Porte D., Sherwin R., Baron A., eds. Elenberg & Rifkin’s DtM. New York: McGraw Hill; 2003. p. :274.
- [Google Scholar]
- A review of natural products and plants as potential antidiabetic drugs. J. Ethnopharmacol.. 1989;27(3):243-275.
- [Google Scholar]
- Functional properties of okra protein products containing different levels of mucilage. J. Food Agric. Environ.. 2009;7(2):252-255.
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-169.
- [Google Scholar]
- Evaluation of Abelmoschus esculentus mucilage as paracetamol suspension. Intl. J. Pharm. Tech. Res.. 2009;1:658-665.
- [Google Scholar]
- Glycosylated compounds from okra inhibit adhesion of Helicobacter pyroli to human gastric mucosa. J. Agric. Food Chem.. 2004;52(1495):503.
- [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47(3):469-474.
- [Google Scholar]
- The clinical information value of the glycosylated hemoglobin assay. N. Engl. J. Med.. 1984;310(6):341-346.
- [Google Scholar]
- Emulsifying properties of extracted Okra (Abelmoschus esculentus L.) mucilage of different maturity index and its application in coconut milk emulsion. Int. Food Res. J.. 2015;22(2):782.
- [Google Scholar]
- Application of Abelmoschus esculentus in solid dosage forms 1: use as binder for poorly water soluble drug. Indian J. Pharm. Sci.. 2001;63:234-238.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed. Pharmacother.. 2008;62(9):598-605.
- [Google Scholar]
- Comment on: effect of pomegranate flower extract on cisplatin-induced nephrotoxicity in rats. J. Nephropathol.. 2014;3:121-123.
- [Google Scholar]
- Investigation of in vivo antioxidant property of Abelmoschus esculentus (L) moench. fruit seed and peel powders in streptozotocin-induced diabetic rats. J. Ayurveda Integrative Med.. 2012;3(4):188.
- [Google Scholar]
- Seino, Y., Nanjo, K., Tajima, N., Kadowaki, T., Kashiwagi, A., Araki, E., Ito, C., Inagaki, N., Iwamoto, Y., Kasuga, M., Hanafusa, T., 2010. Report of the committee on the classification and diagnostic criteria of DtM.
- Sikarwar, M.S., Patil, M.B., 2010. Antidiabetic activity of Crateva nurvala stem bark extracts in alloxan-induced diabetic rats. J. Pharmacy Bioallied Sci. Jan;2(1):18.
- Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Sci. Nutrit.. 2021;9(12):6854-6865.
- [Google Scholar]
- Studies on the mucilages extracted from okra fruits (Hibiscus esculentus L.) and baobab leaves (Adansonia digitata L.) J. Sci. Food Agric.. 1977;28(6):519-529.
- [Google Scholar]
- Antioxidant and anti-fatigue constituents of okra. Nutrients. 2015;7(10):8846-8858.
- [Google Scholar]
- Antidiabetic effects of Artemisia sphaerocephala Krasch. gum, a novel food additive in China, on streptozotocin-induced type-2 diabetic rats. J. Ethnopharmacol.. 2009;125(3):410-416.
- [Google Scholar]
- Antidiabetic and hypolipidemic effects of Collybia confluens mycelia produced by submerged culture in streptozotocin-diabetic rats. Arch. Pharmacal Res.. 2006;29(1):73-79.
- [Google Scholar]







