Translate this page into:
Morphogenetic characterization of Xanthomonas citri pv. citri and its management
⁎Corresponding authors. dr.ak.mnsuam@gmail.com (Akhtar Hameed), msaqlainzaheer@gmail.com (Muhammad Saqlain Zaheer), smanoharadas@ksu.edu.sa (Salim Manoharadas)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Background
Pakistan’s economy largely depends on citrus cultivation, and citrus fruits generate significant foreign exchange. Xanthomonas citri pv. citri (Xcc) is the primary cause of citrus canker (CC), which poses a significant threat to the industry. The management of disease is made more difficult by the lack of resistant variants against different Xcc races. Understanding and addressing Xcc are critical for maintaining the industry given the economic reliance on citrus.
Methods
In order to isolate Xcc in a lab, a thorough survey was conducted in the districts of Bahawalpur, Multan, and Dera Ghazi Khan to collect samples showing canker symptoms. Gram-negative bacteria were identified in the isolates by biochemical analysis, and Koch’s postulates confirmed Xcc as the CC causing agent. DNA extraction and sequencing were used in the molecular characterization, which confirmed the phylogenetic relationship with Xcc. Using the disc sensitivity method, nine antibiotics were tested at 300, 500, and 700 ppm concentrations to evaluate CC management. Amoxicillin was found to be highly effective in inhibiting the growth of Xcc colonies.
Results
The study provided morphogenetic insights and established Xcc as the causal agent of CC. All strains matched Xcc in molecular characterization, but antibiotic sensitivity testing revealed inconsistent efficacy. Amoxicillin proved to be very effective at stopping the growth of Xcc colonies at every tested dosage.
Conclusion
This study makes an important contribution to our understanding of XCC and helps the citrus industry develop better disease control plans. Future interventions against citrus canker can benefit greatly from the understanding provided by morphogenetic characterization and antibiotic sensitivity profiles. The discovery of amoxicillin’s high efficacy highlights the drug’s potential for treating diseases linked to Xcc. These results help to protect citrus growing, maintaining the viability of an important industry in Pakistan’s agricultural landscape.
Keywords
Antibiotics
Characterization
Citrus canker
Management
Xanthomonas citri pv. citri
1 Introduction
Citrus belongs to a genus of flowering tree and shrubs in the group Aurantioideae of the rue family Rutaceae, which is a large group of shrubs and trees (Liu et al., 2012). The genus produces citrus fruits, including important crops such as oranges, lemons, grapefruits, pomelos and limes. Citrus fruits are grown in over 140 countries and are native to South Asia, East Asia, Southeast Asia, Melanesia and Australia. China, United States, Mexico, Brazil, India, Spain, and Argentina are known as prominent contributors to the global citrus production (Faostat, 2016). In 2021, citrus fruits accounted for 161.8 million tons, making them the world's second most produced fruit (Gonzatto and Santos, 2023). Citrus fruits are known for their refreshing flavour and high nutritional value. These possess a high content of essential nutrients, including vitamins, minerals, dietary fiber and various bioactive compounds such as carotenoids, flavonoids, and limonoids, which have been associated with various health benefits. Citrus fruits are considered functional foods that can contribute to the prevention of chronic diseases (Kumar et al., 2023; Meng et al., 2024).
Citrus production faces several challenges that can decrease both yield and fruit quality. These challenges include insect infestations, lack of proper nutrition, and sudden changes in overall climate. Pathogens such as bacteria, fungi, viruses and nematodes can also attack citrus fruit, directly reducing plant production and lowering farmers' earnings associated with citrus production (Khan et al., 2023). Pakistan has reported several diseases affecting citrus crops, including citrus canker, citrus gummosis, citrus decline, CTV and HLB, caused by various microorganisms. Canker disease poses a significant challenge for citrus cultivation on a global scale and results in substantial annual losses. This disease is primarily responsible to various strains of the Xcc bacterium that can significantly reduce the market and nutritional value of citrus crops. The disease is found in all regions of the world where citrus is grown and can have negative impacts on both yield and fruit quality (Ali et al., 2023).
Xanthomonas citri pv. citri (Xcc) is the primary inducer of CC, causing significant economic losses for citrus-producing countries worldwide by reducing export market value and fruit production (Ali et al., 2023). The epidemiology of citrus disease affected by Xcc appears at 29–29.5 °C with 80–90 % humidity and rainfall of 8.9–9.97 mm (Thind, 2012). The Xcc bacteria are classified into five different pathovars: citri (Pathotype A), aurantifolii (Pathotype B, C, D), and citrumelo (also known as type E). These pathovars contain a variety of bacterial strains as well (Ali et al., 2023; Yao et al., 2023).
The primary symptom of CC is the emergence of lesions of varying sizes on the surface of leaves, initially appearing as pinpoint spots that are typically found on the abaxial side (Du et al., 2023; Hu et al., 2022). After a few days, the lesions become corky and the lesions on the leaves or fruits typically take on a sunken appearance at the center with an elevated margin that is encircled by a yellow halo. The size of lesions on infected fruits can also vary and fruits are more susceptible to infection compared to leaves because they can undergo multiple infection cycles on their surface (Derso et al., 2007). In severe cases of infection, the disease can cause premature fruit drop, twigs to dieback, defoliation of the tree, and result in blemished fruit. Of these, premature and non-marketable fruits are the most economically significant damages caused by the disease. Bacteria may endure in infected leaves for up to 6 months and infected twigs for up to 76 months (Dunger et al., 2005).
The rise in international travel and trade has considerably heightened the chances of introducing invasive plant pests and diseases to crops, including citrus canker (Gottwald et al., 2002). Different strategies are being implemented to prevent the introduction of invasive plant pests and diseases like citrus canker, limit their spread, and eliminate them. Significant amounts of money are allocated each year towards the implementation of quarantines, prevention measures, eradication initiatives and management programs on a global scale. In areas where canker is prevalent, effective management strategies necessitate the integration of many cultural practices, such as the implementation of windbreaks, sanitation measures, the use of pesticides to suppress the leaf miner and the use of antibiotics (Gottwald and Graham, 2014). The timing of antibiotic treatment and the duration between successive sprays are crucial factors to consider. The initial three-month period following the shedding of petals is of utmost importance, as it is at this time that the fruit is particularly vulnerable to the development of canker. Therefore, it is advisable to spray antibiotics onto the surface of the fruit during this particular time period (Ibrahim et al., 2017). Therefore, conducting an assessment of the effectiveness of several commercially available antibiotics would greatly contribute to the efficient treatment of the condition (Almalki and Varghese, 2020).
The present study was aimed to analyse the morphogenetic characterization of the Xcc causal organism of CC in the regions of District Bahawalpur, Multan and Dera Ghazi Khan of Punjab Province of Pakistan. Moreover, the present investigation was conducted with the aim of assessing the comparative effectiveness of commercially accessible antibiotics in combating the pathogen in in-vitro conditions.
2 Material and methods
2.1 Survey plan for the collection of samples
A systematic survey was conducted for collection of leaves samples showing citrus canker disease symptoms in areas of District Bahawalpur, Multan and Dera Ghazi Khan of Punjab Province. A total of 72 leaves samples affected with citrus canker disease were collected from the 12 sampling sites and put in brown paper bags (Fig. 1). These samples were brought to the Diagnostic laboratory of MNSUAM for the purpose of bacterial isolation and stored in the refrigerator at 4 °C until further processing.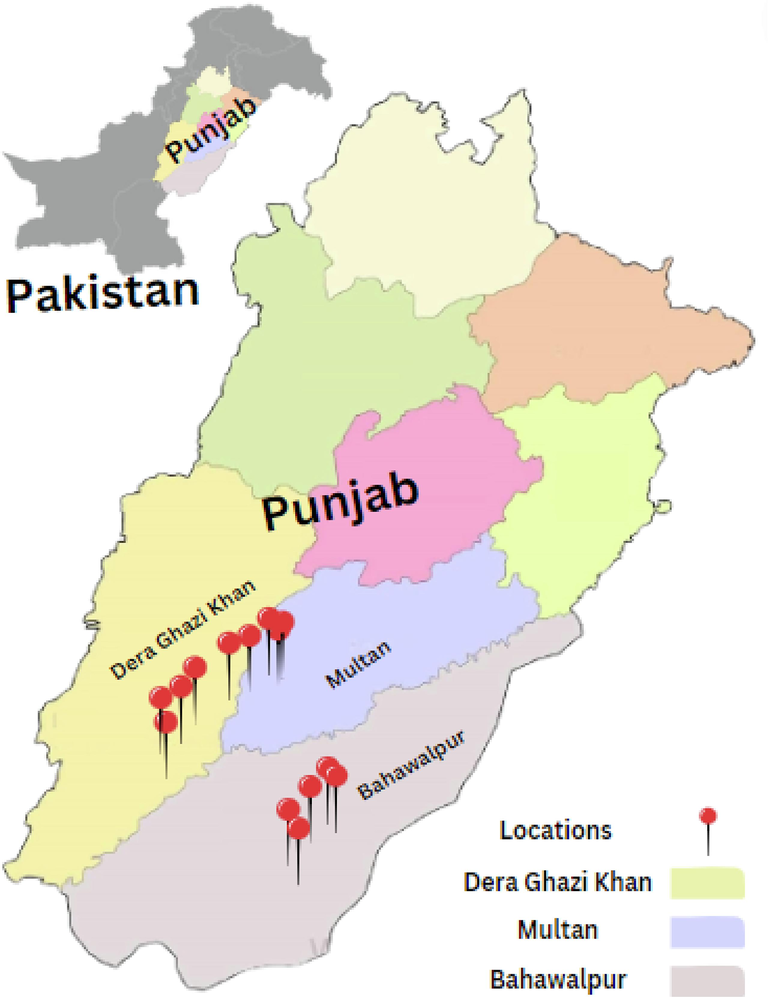
Location of survey areas for sampling of citrus canker infected leaves.
2.2 Isolation and purification and multiplication of the pathogen
A nutrient agar media was prepared to isolate, multiply and purify the pathogen. A sterilized beaker with capacity of 1L was taken and 14 g of nutrient agar and 500 mL of distilled water were added to it. Autoclaved the media at 121 °C and 15 psi for sterilization (Riaz et al., 2008). After that infected leaves were carefully cut from infected portion around with small healthy portion. Surface sterilization was done by using distilled water follow by 0.1 % HgCl2 and distilled water. After surface sterilization moisture was removed by putting the samples on blotter paper. These samples were put on poured plates containing NA media and wrapped with wrapping tape to protect them and the experiment's integrity. After proper tagging, the plates were kept in the incubator at 28 °C for 24 h. After 24 h, a light yellowish ooze was appeared beneath the infected samples and streaked in to new plates containing NA media by using streaking method for purification and preserved (50 % glycerin solution) 4 °C at for further study.
2.3 Biochemical and physiological tests
All the isolates that grew on the NA media culture plate was identified morphologically by observing its colour, type of colony and conducting biochemical tests viz., Gram staining and 3 % KOH test (Mubeen et al., 2015).
2.4 Pathogenicity test
Confirmation of the bacterial isolates was done by fulfilling Koch’s postulates (Juhasz et al., 2013). For pathogenicity, the bacterial culture was grown overnight in 45 ml nutrient broth at 28 °C following Hoque and Mansfield's (2005) protocol and placed on a shaking incubator (NB-205LF) at 250 rpm. The resulting bacterial suspension was centrifuged at 104 rpm at 4 °C for ten minutes. The pellet of bacteria was resuspended in sterile 10 mM MgCl2 solution and centrifuged again. The washed pellet was suspended in 10 mM MgCl2 solution more than once and adjusted to 620 nm, which was equal to 108 colony-forming units/mL and 0.1 OD with the help of a spectrophotometer (Hoque and Mansfield, 2005). For pathogenicity, one and half year-old citrus plants were obtained from a nursery. Prior to inoculation, all plants were watered properly and covered with polythene bags for 2 h and placed in sunlight to create high humidity conditions and maximum opening of stomata. Inoculation was done in the morning using the syringe method, with approximately 2 μl of bacterial suspension injected into the plant leaves (Francis et al., 2010). Plants that were treated as control were injected with distilled water.
2.5 DNA isolation and PCR
The process of isolating cellular DNA from a freshly grown aqueous bacterial culture was conducted using the modified CTAB method (Iqbal et al., 2021). The quantification of the extracted DNA was conducted, followed by its utilization in polymerase chain reaction (PCR) for the purpose of molecular identification of the pathogen. For the optimization of the PCR-based identification process, Xcc specific primers were used (Golmohammadi et al., 2007). The PCR protocol included a total of 40 amplification cycles. Each cycle consisted of denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min, and extension at 72 °C for 1 min. The PCR reaction completed with a final extension step at 72 °C for 10 min. After staining with ethidium bromide, the amplified product was separated on a 1.5 % agarose gel and subsequently observed using a UV gel documenting system (Photonyx Ultra, NYXTecnhnik).
2.6 In-vitro evaluation of different antibiotics
Relative efficacy of nine antibiotics (Amoxcillin, Kanamycin, Erythromycin, Azithromycin, Vibramycin, Ampicillin, Clindamycin, Streptomycin and Clarithromycin) against colony growth of Xcc was evaluated at 300, 500 and 700 ppm by disc sensitivity method (Fairbrother and Martyn, 1951). Sterilized distilled water was put to use as the control treatment. The Petri plates were enveloped with parafilm and subjected to incubation at 28 °C. Inhibition zones data were recorded by measuring after 24, 48 and 72 h of incubation with the help of digital Vernier caliper (500–196, Mitutoyo). The experimental design employed in this study was a Completely Randomized Design (CRD) with three replications.
2.7 Statistical analysis
The analysis of variance was performed on the recorded data with a significance level of 5 %. In order to compare the effectiveness of various treatments of antibiotics, the Fisher's Least Significant Difference (LSD) test was employed (Steel et al., 1997). The “Microsoft Office-2016″ was used for analysing and presenting data.
3 Results
3.1 Biochemical and physiological tests
Twelve isolates of bacteria isolated from infected citrus plants formed yellow pigmented, less convex, round and smooth colonies on NA media. The bacterium was determined to be Gram-negative based on the positive result obtained from the KOH test. After Gram's staining, microscopic examination revealed the presence of bacterial cells exhibiting a slight-pink coloration.
3.2 Pathogenicity test
The typical symptoms of CC disease, such as corky water-soaked spots, necrotic lesions with a yellow halo and defoliation were observed on the citrus leaves two weeks after they were inoculated. These symptoms closely resembled those observed in the citrus orchards during our surveys. The bacterial pathogen's ability to cause disease of CC were confirmed by re-isolating and re-purification from the inoculation citrus plants and confirmed the Koch's postulates.
3.3 Molecular characterization
DNA was extracted and PCR was carried out using 16 s rDNA, resulting in a 581 base pairs DNA product. This product was purified and visualized using gel electrophoresis under fluorescent light. The identified DNA was commercially sequenced (Figs. 2, 3).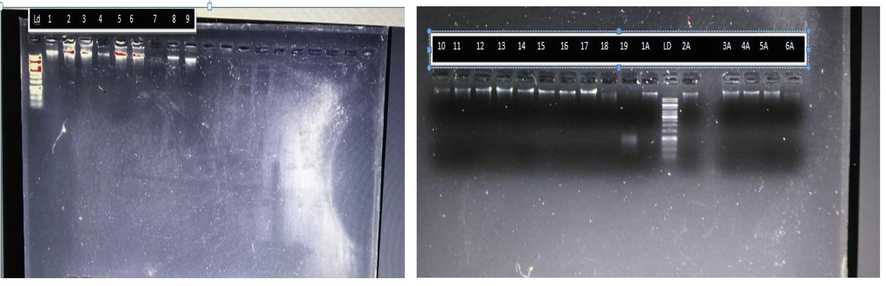
Molecular identification of X. citri using PCR technology.
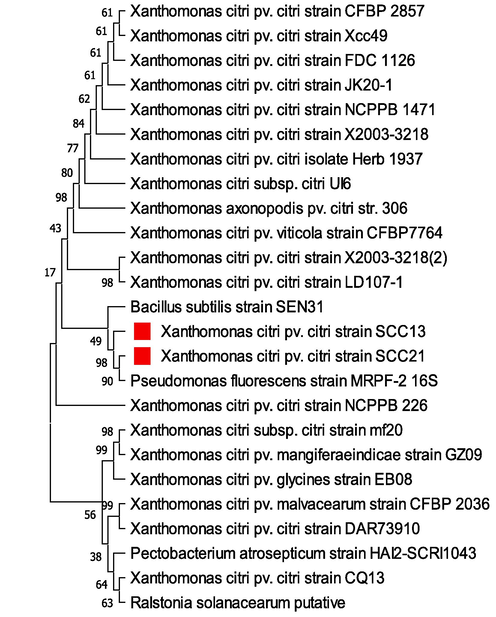
Dendrogram representing genetic relationships between isolated Scc13 and Scc21 with the strains of X. citri pv. Citri.
3.4 In-vitro evaluation of different antibiotics
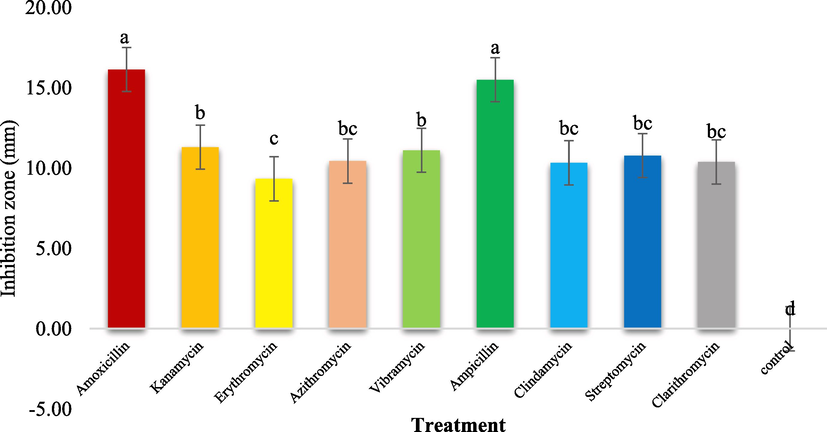
Different amounts of antibiotics showed substantial differences in Xcc colony growth efficacy (Table 1). No inhibitory zone was seen where sterilized distilled water was utilized as the control. There were the maximum inhibition zones (mm) produced by Amoxicillin (16.14) followed by Ampicillin (15.50), Kanamycin (11.31), Vibramycin (11.11), Streptomycin (10.78), Azithromycin (10.44) Clarithromycin (10.39), Clindamycin (10.33), Erythromycin (9.33) mm respectively as compared to control by different antibiotics (Fig. 4, Fig. 5).
Sr No.
Antibiotics
Active ingredients
Mode of action
Molecular formula
Molecular Weight
1
Amoxicillin
Amoxicillin Trihydrate
inhibition of cell wall synthesis
C16H19N3O5S
365.4 g/mol
2
Kanamycin
Kanamycin sulfate
misreading of mRNA
C18H36N4O1
484.499 g/mol
3
Erythromycin
Erythromycin BP
inhibit protein synthesis
C37H67NO13
733.9 g/mol
4
Azithromycin
Zithromax
inhibit protein synthesis and proinflammatory cytokine production
C38H72N2O12
749 g/mol
5
Vibramycin
Doxycycline Hyclate
inhibit growth of bacteria
C22H24N2O8·H2O
462.46 g/mol
6
Ampicillin
Ampicillin Trihydrate
inhibit cell wall synthesis
C16H19N3O4S
349.4 g/mol
7
Clindamycin
Clindamycin Hydrochloride
inhibit protein synthesis
C18H33ClN2O5S
424.98 g/mol
8
Streptomycin
Streptomycin Sulfate
blocking the ability of 30S ribosomal subunits to make proteins
C21H39N7O12
581.6 g/mol
9
Clarithromycin
Clarithromycin
inhibit protein synthesis
C38H69NO13
747.953 g/mol
10
Water
Control
−
H2O
18.01528 g/mol

Pictorial view of inhibition zones formed by the antibiotics (a) Amoxicillin, (b) Kanamycin, (c) Erythromycin, (d) Azithromycin, (e) Vibramycin, (f) Ampicillin, (g) Clindamycin, (h) Streptomycin and (i) Clarithromycin with control in-vitro application for the management of X. citri pv citri.
In-vitro evaluation of various antibiotics on the development of Xcc.
The interaction between treatments and concentrations (T × C) showed that maximum inhibition zones (20.66) mm were produced by Ampicillin at 700 ppm, (10.66) at 500 ppm and (15.16) mm at 300 ppm respectively while Erythromycin exhibited minimum inhibition zones of (7.00 mm) while Amoxicillin expressed 16.00, 16.00, 16.41, Kanamycin 11.58, 11.33, 11.00, Vibramycin 8.00, 10.33, 15.00, Streptomycin 7.66, 9.00, 15.66, Azithromycin 9.33, 9.33, 12.66, Clarithromycin 8.83, 10.00, 12.33, Clindamycin 8.66, 10.33, 12.00 mm inhibition zone at 300, 500 and 700 ppm concentration respectively as compared to control (Fig. 6).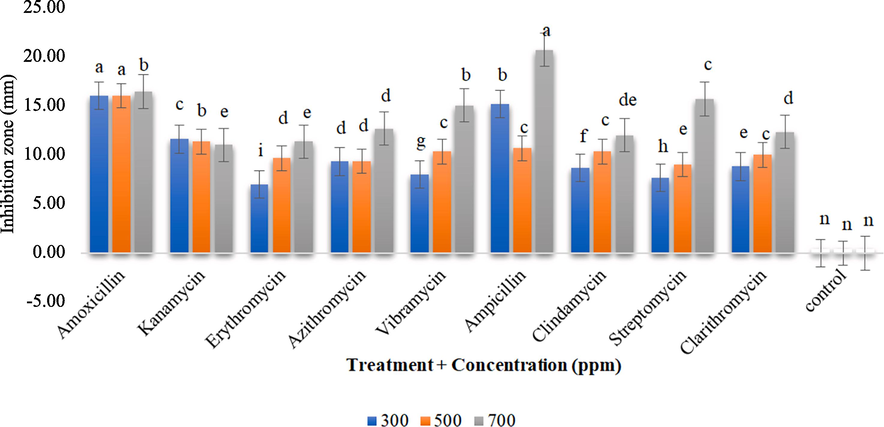
Impact of interaction b/w treatments and concentrations (TxC) on the development of citrus canker.
4 Discussion
Citrus canker disease poses a significant threat to citrus production worldwide and results in substantial economic losses by affecting the physical appearance of the fruit, rendering it unsuitable for the market (Ali et al., 2023). This disease is primarily responsible to various strains of the X. citri pv. citri bacterium. The bacterium primarily affects the leaves of trees and leads to defoliation and reduced fruit production in affected plants. In severe cases, it can even prevent fruit formation altogether. Through stomatal openings, injuries and wounds, the bacteria can penetrate the plant tissues where they multiply and colonize the apoplast pathway by breaking through the epidermis of leaves through hyperplasia in the cell (Thind, 2012). Bacteria may endure in infected leaves for up to 6 months and infected twigs for up to 76 months (Dunger et al., 2005). Some citrus resistance varieties have shown tolerance to the CC disease. The major strategy for controlling citrus canker disease on a commercial scale is thought by most to be chemical control (Gottwald et al., 2002). Chemical management strategies, such as preventing or disrupting metabolic processes, have been shown to be successful in inhibiting the growth and spread of diseases (Ali et al., 2024), but they also have serious limitations (Mahmud and Chong, 2021). Environmental and health dangers from these practices are considerable. On the other hand, using antibiotics has shown to be useful in both controlling plant disease and increasing the plants' resistance to prospective diseases (Islam et al., 2014).
X. citri pv. citri was isolated from citrus with canker symptoms in areas of District Bahawalpur, Multan and Dera Ghazi Khan of Punjab Province of Pakistan. The pathogen displayed some heterogeneity in physiological and biochemical tests. Similar results were observed during the KOH and Gram staining test performed by the Iqbal et al. (2021) that confirmed the Xcc bacterium were Gram-negative and exhibit pinkish color of under the microscope respectively. This study used the pin-prick method to inoculate the bacterial suspension on healthy citrus plant leaves for a pathogenicity test. After 13–15 days, small, moist patches first developed on the bottom surface of the leaves, and then they spread to the top surface. The lesions developed corky, crust-like symptoms as they became older, along with a depressed yellow symptom. The pathogenicity findings were in line with the results from previous research conducted by Mustafa et al. (2015), who also observed same way symptoms after the pathogenicity of Xcc by spray inoculating 10 citrus seedlings that were two years old
The DNA of the two most aggressive isolate culture was extracted and analyzed using PCR targeting the 16 s rDNA gene. Gel electrophoresis with fluorescent light was used to observe a DNA fragment of 1500 base pairs. After identification, the DNA was commercially sequenced. To perform a phylogenetic analysis of the Xcc genome sequence, the BLAST tool on NCBI was employed. The BLAST results confirmed the correspondence between the genome sequence and Xcc, confirming that this pathogen is the causal agent of CC. Iqbal et al. (2021) analyzed rep-PCR fingerprinting on two isolates and sequencing revealed 100 % similarity with 40 nucleotide sequences of Xcc indicating the presence of pathogen in Sargodha districts of the Punjab, Pakistan. Izadiyan et al. (2018) also confirmed similar results after sequence that all their six irani isolated Xcc strains were closely related to the other Xcc strains found in the gene sequence data of NCBI. Arshadi et al. (2013) conducted rep-PCR fingerprinting analysis on a total of 25 isolates of Xcc. A significant amount of genetic variation was identified among the isolates, suggesting the potential existence of multiple pathotypes of the bacterium within the Malaysia.
The efficacy of nine antibiotics in managing the canker disease was examined in in-vitro condition. The most effective in preventing the development of Xcc among them was ampicillin at a concentration of 700 (ppm). The present study was in line with research done in by Naqvi et al. (2014) who studied at the manner in which various antibiotics affected bacterial blight of rice. They came to the conclusion that Ampicillin Trihydrate had the second best results in reducing X. oryzae pv. oryzae. By attaching to the bacteria act as inhibition of cell wall peptidoglycan synthesis and inactivation of inhibitors to autolytic enzymes. Ampicillin trihydrate has been proven to have antibacterial ability against Xanthomonas spp. with inhibitory zones in in-vitro conditions (Islam et al., 2014). Hameed et al. (2022) also studied at the manner in which various antibiotics against Xcc were evaluated. They came to the conclusion that oxytetracycline had the best results in reducing Xcc. By attaching to the bacterial chromosomes, oxytetracycline inhibits bacterial reproduction and functions as a bacteriostatic drug. Islam et al. (2014) conducted an evaluation of various antibiotics, including cefotaxime, chloramphenicol, bacitracin, gentamycin and streptomycin, in order to assess their effectiveness against Xcc. The efficacy of chloramphenicol was determined to be the highest.
The results of this study highlight how important it is to manage citrus canker brought on by Xanthomonas citri pv. citri (Xcc) by using morphogenetic characterization and antibiotic sensitivity profiling. Because of its high effectiveness in preventing Xcc growth, amoxicillin is a good option for managing diseases in citrus crops. In comparison to conventional antifungals and antibacterials, amoxicillin is more effective against Xcc. Conventional therapies, like compounds based on copper, have drawbacks because of the possibility of resistance growing and environmental issues. Nanoscaled oxides like zinc oxide and titanium dioxide have antimicrobial properties but can be cost-prohibitive and require further research for long-term impacts (Madubuonu et al., 2020). Natural extracts offer an eco-friendly alternative but often need higher concentrations for comparable results (Maaza et al., 2014). The use of amoxicillin provides a robust, cost-effective, and highly effective means of controlling Xcc, making it a practical solution for the citrus industry (Mbonyiryivuze et al., 2015). By comparing amoxicillin with other treatments, this study emphasizes the potential for tailored antibiotic use in agricultural disease management and advocates for further research to optimize its application in the field.
5 Conclusions and recommendations
This study was focused on morphogenetic characterization of Xcc, that evaluated the aggressiveness of bacteria that isolated from Districts Multan, Bahawalpur and Dera Ghazi khan and to explore effective management strategies using various antibiotics. The findings will contribute to the development of appropriate management strategies against this pathogen in citrus. Furthermore, antibiotics (Amoxcillin) show significant results against citrus canker alone and in various combinations under lab conditions. These less toxic and eco-friendly strategies should be including in the management programs against citrus canker.
CRediT authorship contribution statement
Subhan Ali: Conceptualization. Akhtar Hameed: Investigation, Conceptualization. Rana Binyamin: Supervision, Methodology. Muhammad Waqar Alam: Writing – original draft. Hafiz Muhammad Usman Aslam: Software. Hasan Riaz: Validation. Muhammad Saqlain Zaheer: Writing – review & editing, Resources, Methodology, Formal analysis, Conceptualization. Salim Manoharadas: Writing – review & editing. Hafiz Haider Ali: Writing – review & editing. Yasir Niaz: Visualization, Data curation. Nurettin Baran: Writing – review & editing. Kamran Ikram: Visualization, Validation, Project administration, Data curation.
Acknowledgement
The authors thank the Researchers Supporting Project for funding this work through Research Supporting Project number (RSPD2024R708), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ali, N., Rafiq, R., Zaib-un-Nisa, Wijaya, L., Ahmad, A., & Kaushik, P. 2024. Exogenous citric acid improves growth and yield by concerted modulation of antioxidant defense system in brinjal (Solanum melongena L.) under salt-stress. J. King Saud Univ. Sci. 36, 103012. DOI: 10.1016/j.jksus.2023.103012.
- Citrus canker: A persistent threat to the worldwide citrus industry—An analysis. Agronomy. 2023;13:1112.
- [Google Scholar]
- Prevalence of catheter associated biofilm producing bacteria and their antibiotic sensitivity pattern. J. King Saud Univ. Sci.. 2020;32:1427-1433.
- [CrossRef] [Google Scholar]
- Genetic diversity of Xanthomonas citri subsp. citri, causal agent of citrus canker. J. Plant Prot. Res.. 2013;53:312-316.
- [Google Scholar]
- Status of citrus canker caused by Xanthomonas axonopodis pv. citri in Peninsular Malaysia. Int. J. Agric. Biol.. 2007;9:54-58.
- [Google Scholar]
- Biodegradation of sulfametoxydiazine by Alcaligenes aquatillis FA: Performance, degradation pathways, and mechanisms. J. Hazard. Mater.. 2023;452:131186
- [CrossRef] [Google Scholar]
- Participation of Xanthomonas axonopodis pv. citri hrp cluster in citrus canker and nonhost plant responses. Plant. Pathol.. 2005;54:781-788.
- [Google Scholar]
- The disc technique for determining sensitivity to the antibiotics. J. Clin. Pathol.. 1951;4:374.
- [Google Scholar]
- Faostat, F., 2016. FAOSTAT statistical database. Publisher: FAO (Food and Agriculture Organization of the United Nations), Rome, Italy.
- Detached leaf inoculation of germplasm for rapid screening of resistance to citrus canker and citrus bacterial spot. Eur. J. Plant Pathol.. 2010;127:571-578.
- [Google Scholar]
- Diagnosis of Xanthomonas axonopodis pv. citri, causal agent of citrus canker, in commercial fruits by isolation and PCR-based methods. J. Appl. Microbiol.. 2007;103:2309-2315.
- [Google Scholar]
- Gonzatto, M.P., Santos, J.S., 2023. Introductory Chapter: World Citrus Production and Research. Citrus Research-Horticultural and Human Health Aspects, hlm. IntechOpen.: DOI: 10.5772/intechopen.100891.
- Gottwald, T.R., Graham, J.H., 2014. Citrus diseases with global ramifications including citrus canker and huanglongbing. CABI Reviews. 1–11. DOI: 10.1079/PAVSNNR20149016.
- In-vitro antibacterial potential of antibiotics against Xanthomonas axonopodis PV. citri. Fresenius. Environ. Bull.. 2022;31:3886-3892.
- [Google Scholar]
- A simple and reliable method for pathogenicity tests of bacterial blight disease of rice. Bangladesh J. Bot.. 2005;34:11-16.
- [Google Scholar]
- Hu, B., Das, P., Lv, X., Shi, M., Aa, J., Wang, K., et al., 2022. Effects of 'Healthy' Fecal Microbiota Transplantation against the Deterioration of Depression in Fawn-Hooded Rats. mSystems. 7, e21822. DOI: 10.1128/msystems.00218-22.
- Management of asiatic citrus canker under field conditions in Saudi Arabia using bacteriophages and acibenzolar-S-methyl. Plant Dis.. 2017;101:761-765.
- [Google Scholar]
- Iqbal, M., ul Haq, M.E., Kamran, M., Idrees, M., Nazir, S., Ullah, I., Naz, S., Ali, S., Iqbal, M.Z., 2021. Morpho-molecular characterization of Xanthomonas axonopodis Pv. Citri associated with kinnow (Mandarin) and its management. Pakistan J. Agri. Res. 34, 8.
- Isolation, identification and in vitro antibiotic sensitivity pattern of citrus canker causing organism Xanthomonas axonopodis. Adv. Life Sci.. 2014;1:215-222.
- [Google Scholar]
- Characterization of Xanthomonas citri subsp. CITRI isolated from grapefruit in Iran. J. Plant Pathol.. 2018;100:257-267.
- [Google Scholar]
- First report of Xanthomonas citri pv. citri causing Asiatic citrus canker in Burkina Faso. Plant Dis.. 2013;97:1653.
- [Google Scholar]
- Khan, A. M., Khan, M., Salman, H. M., Zia Ullah Ghazali, H. M., Imtiaz Ali, R., Hussain, M., et al., 2023. Detection of seed-borne fungal pathogens associated with wheat (Triticum aestivum L.) seeds collected from farmer fields and grain market. J. King Saud Univ. Sci. 35, 102590. Elsevier BV. DOI: 10.1016/j.jksus.2023.102590.
- Effect of heat treatment on the quality of citrus juices. J. King Saud Univ. Sci.. 2023;35:102819
- [CrossRef] [Google Scholar]
- History, global distribution, and nutritional importance of citrus fruits. CRFSFS. 2012;11:530-545.
- [Google Scholar]
- Maaza, M., 2014. Natural Dyes for Photonics Applications. Novel Plant Bioresources. Wiley. DOI: 10.1002/9781118460566.ch35.
- Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A. 2020;126:72.
- [CrossRef] [Google Scholar]
- Formulation of biofertilizers from oil palm empty fruit bunches and plant growth-promoting microbes: A comprehensive and novel approach towards plant health. J. King Saud Univ. Sci.. 2021;33:101647
- [CrossRef] [Google Scholar]
- Fourier transform infrared spectroscopy for sepia melanin. Mater. Chem. Phys.. 2015;3:25-29.
- [Google Scholar]
- Monaspin B, a novel Cyclohexyl-furan from cocultivation of Monascus purpureus and Aspergillus oryzae, exhibits potent antileukemic activity. J. Agric. Food Chem.. 2024;72:1114-1123.
- [CrossRef] [Google Scholar]
- In-vitro efficacy of antibiotics against Xanthomonas axonopodis pv. citri through inhabitation zone techniques. Int. J. Appl. Agric. Sci.. 2015;7:69-71.
- [Google Scholar]
- Commercial citrus cultivars resistance evaluation and management to canker disease. Int. J. Agril. Res. 2015;6:1-9.
- [Google Scholar]
- Determination of antibacterial activity of various broad spectrum antibiotics against Xanthomonas oryzae pv. oryzae, a cause of bacterial leaf blight of rice. Int. J. Microbiol. Mycol.. 2014;2:12-19.
- [Google Scholar]
- Metagenomics revealed a quorum quenching lactonase QlcA from yet unculturable soil bacteria. Commun. Agric. Appl. Biol. Sci.. 2008;73:3-6.
- [Google Scholar]
- Steel, R.G., Torrie J.H., Deekey, D.A., 1997. Principles and Procedures of Statistics. A biometrical approach 3rd edition. S.T. McGraw Hill book Co. Inc. New York, U.S.A.
- A novel image encryption scheme for DNA storage systems based on DNA hybridization and gene mutation. Interdiscip. Sci.. 2023;15:419-432.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103339.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







