Translate this page into:
Nanoparticles of thiolated chitosan for controlled delivery of moxifloxacin: In-vitro and in-vivo evaluation
⁎Corresponding authors. shahid.shah@gcuf.edu.pk (Shahid Shah), sulman.shafeeq@ki.se (Sulman Shafeeq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Controlled release drug delivery system of moxifloxacin produces better therapeutic effects and maintain the plasma concentration for prolong period of time. The objective of this work was to formulate nanoparticles of thiolated chitosan (TC) having enhanced mucoadhesion and controlled release behaviour of drug. The chitosan (CS) was thiolated by conjugation with thiourea. Ionic gelation method using TC was used for the preparation of the nanoparticles. FTIR, DSC, TGA and 1H NMR were used to characterize the CS and TC. The measurement of particle size and zeta potential of the developed nanoparticles confirmed that the formulation was stable. The CS, TC and nanoparticles were evaluated for mucoadhesion and swelling. The toxicity of developed nanoparticles was evaluated by Caco-2 cell line. The release of drug from nanoparticles of non-thiolated (NT) and TC was studied using simulated gastric fluid (SGF), simulated intestinal fluid (SIF) and phosphate buffer pH 7.4. The albino rats were used to investigate the moxifloxacin pharmacokinetics from nanoparticles of TC. The thiol group showed peak at 2122 cm−1 which was confirmed by FTIR. The thermal stability of NT and TC was confirmed by DSC and TGA. The thiolation of CS was confirmed by 1H NMR. The nanoparticle of TCN3 showed 105 nm average particle size and zeta potential of TCN1 to TCN5 was range from −18 to + 29. The TC show better mucoadhesion as compare to the CS. The drug, CS, TC and nanoparticles of TCN3 did not exhibit any effect on the viability of Caco-2 cells. In SGF, the drug release from nanoparticles was 5 to 10 %. TCN3 formulation showed 97.84% and 99.43% release of moxifloxacin in SIF and phosphate buffer pH 7.4 within 24 h. The nanoparticles of TCN3 showed 97.13% release of moxifloxacin within 8 h. The TCN3 formulation showed greater bioavailability and improved pharmacokinetic parameters of moxifloxacin as compare to the marketed formulation. Our findings proved that the thiolation of CS with thiourea is an easy and reproducible method to improve the mucoadhesion and the controlled release pattern of the drug over an extended period of time.
Keywords
Nanoparticles
Chitosan
Thiolation
In-vitro release
Pharmacokinetics
1 Introduction
Nanotechnology is a new booming field in the pharmaceutical and medical field. Nanoparticles, as drug delivery systems, confer many advantages in terms of increased efficiency with decreased adverse drug effects. Several materials have been utilized as carrier vehicles for various medicinal products. Because of the outstanding physical as well as biological properties including non-toxicity, low immunogenicity and biocompatibility, polysaccharides are considered as the most suitable carrier vehicles for therapeutic administration (Miao et al., 2018). The addition of thiol groups to these polymers covalently enhances their mucoadhesive properties and penetration characteristics without compromising their biodegradability and improves mucoadhesive strength (Bernkop-Schnürch et al., 2003).
The copolymerization of glucosamine units with N-acetyl glucosamine units results in the formation of CS and is the most abundant natural polysaccharide. The inclusion of a –OH and a free –NH2 group on the backbone of CS gives it a negative charge. Many studies have leveraged this feature to build CS-based particle systems (Ahmed and Aljaeid, 2016). CS is break down in the gut owing to the presence of bacteria that may degrade polysaccharides using various enzymes (McConnell et al., 2008). Many approaches are being used to enhance CS based intestinal delivery methods, together with the use of thermo-responsive, pH-sensitive polymers in formulations (Mourya and Inamdar 2008). A strong disulfide bond is created involving the cysteine-rich domains of mucosal glycoproteins which boosted the mucoadhesive characteristics of modified CS (Sudhakar et al., 2020), particularly TC, in which thiol groups are immobilized on the primary amine group of CS (Sudhakar et al., 2020). It causes the delivery mechanism to adhere to the mucus layer, extending the stomach retention time. Because the medicine spends more time in the gut, it has a higher bioavailability in the blood. Because of its scalability, simplicity of drug integration through physical entrapment, extended shelf life, enhanced absorption, bioavailability, and adequate stability in biological fluids, mucoadhesive nanoparticulate delivery systems have emerged as a potential drug delivery method (Chen et al., 2018). CS was thiolated with L-cysteine and thioglycolic acid (Gök et al., 2017), but no method for thiolation of CS with thiourea was found in the literature. In this study, thiolation of CS with thiourea was accomplished using a one-step microwave oven approach.
Moxifloxacin has been shown to have wide antibacterial action against a number of infections. Researchers have created numerous formulation strategies owing to its potential antibacterial activity, to enhance the efficacy of moxifloxacin against a variety of illnesses. Due to a number of reasons, including inadequate dosage form retention in the gastrointestinal system and low drug bioavailability, current formulations have had little success in attaining satisfactory outcomes (Beg et al., 2020). Our aim was to use TC nanoparticles with enhanced mucoadhesion and a controlled drug release pattern to increase medication retention duration in the gastrointestinal system.
Our aim was to make nanoparticles of TC that was used to deliver moxifloxacin to the colon. In comparison to previous studies, the produced nanoparticles ensure a better controlled drug release pattern and a more homogeneous distribution of multiparticulate systems. 1H NMR was utilized to rectify the structure of produced TC. FTIR, DSC and TGA were used to analyze the drug's chemical compatibility and thermal stability with the TC. The release patterns of moxifloxacin from the manufactured nanoparticle were evaluated using different dissolution medium. In the toxicity study, Caco-2 cells were utilized. Albino rats were used to study medication pharmacokinetics.
2 Methodology
2.1 Materials
Thiourea and CS (40–80 kDa molecular weight and 89 % degree of de-acetylation) was obtained from Merck Darmstadt Germany. Moxifloxacin was gifted by Skims Pharmaceuticals Pvt. Ltd. Faisalabad, Pakistan. Acetonitrile, methanol and ethanol were purchased from Merck Darmstadt, Germany. 5, 5′-dithiobis (2-nitrobenzoic acid) (DTNB), Tripolyphosphate (TPP), KH2PO4 was acquired from Merck Darmstadt Germany.
2.2 Thiolation of CS
The CS was thiolated using thiourea by alteration of previously stated method (Singh et al., 2016). 0.5 g CS was dissolved in 50 mL of 1 % acetic acid solution. 50 mL of 0.1 M thiourea solutions was made in 50 mL phosphate buffer having pH 5.0. Mix the CS and thiourea solution in 1:1 and placed in 640 W oven at 40 °C for 2 min. Add 10 mL of 0.2 N NaOH in the treated solution and again placed in 640 W oven at 40 °C for 90 s. The solution was removed from the oven, cooled, and solution was neutralized with the addition of 10 mL 0.2 M HCl solution. 10 mL ethanol was to the solution for the complete precipitation and precipitate was separated using filtration. The precipitates were dried after washing with acetone.
2.3 Characterization of TC
CS and TC were characterized by FTIR, DSC, TGA and 1H NMR. The FTIR spectrum was recorded in the wavelength range of 400 to 4000 cm−1. The CS and TC were dissolved in heavy water (D2O) for NMR analysis. The samples of DSC and TGA were heated from 50 to 400 °C at a 5 °C/min and 10 °C/min heating rate respectively. The H-NMR spectra of CS and TC were recorded using NMR spectrometer (Bruker Alpha, Germany).
2.4 Preparation of nanoparticles
Solutions of various concentrations and molecular weight of TC were made up by dissolving in 1 % acetic acid through sonication (Gan et al., 2005). The solution of TC was then diluted with deionized water to create CS solution of various concentrations i.e. 0.05, 0.10, 0.15, 0.20, 0.25 and 0.30 % (W/V). At a concentration of 0.7 mg/mL, TPP (Tripolyphosphate) was dissolved in deionized water. The synthesis of TC-TPP nanoparticles began spontaneously after adding 10 mL of TPP solution in 10 mL of TC solution through ionic gelation mechanism. Table 1 shows the weight ratios, 3:1, 4:1, 5:1, 6:1 and 7:1 of TC to TPP used to make the nanoparticles. After that, the nanoparticles were centrifuged and cleaned at 15000 rpm for 15 min. The nanoparticles were then lyophilized in a freeze dryer at – 30 °C for 24 h and then at 25 °C for 48 h. For further investigation and application, the prepared nanoparticles suspension was gently stirred for 1 h at 25 °C.
Formulations
TC and TPP (w/w)
Size of particle (nm)
Zeta-potential
PI
TCN1
3:1
143 ± 11
±29
0.310
TCN2
4:1
113 ± 10
±28
0.330
TCN3
5:1
105 ± 11
±18
0.119
TCN4
6:1
124 ± 12
±26
0.122
TCN5
7:1
157 ± 13
±22
0.315
2.5 Determination of thiol contents
Ellman’s reagent was used to assess the degree of thiol group substitution in TC and developed nanoparticles. For the preparation of Ellman’s reagent, dissolve 0.5 M phosphate buffer in 0.03 % DTNB (w/v) at a pH of 8.0. Separately, take 500 μL solution of TC in 1 % acetic acid and nanoparticles in phosphate buffer pH 7.4, add 500 μL of Ellman’s reagent in both separated baskets and wait for complete dissolution. After that incubate the mixture and evaluate the absorbance by using UV Spectrophotometer at 412 nm. To quantify the thiol groups, a standard solution of thiourea were produced in phosphate buffer saline (0.5 M).
2.6 Mucoadhesion and swelling studies
Direct compression method was used to prepare 30 mg flat discs of CS, TC and nanoparticles. These discs were used to assess the mucoadhesive strength. A newly excised and cleaned mucosal membrane of rabbit stomach was used in the ex vivo mucoadhesion test, after sticking to the petri dish using cynoacacrylate glue. By using a finger press, the compressed tablet bonded to mucosal membrane. At 30 cycles per minute, a modified USP disintegration apparatus having SGF with the pH of 1.2 at 37 °C were used for the evaluation. In pH 1.2 and 7.4 buffer solution, the swelling behavior of tablets of CS and TC were studied. Equation (1) was used to assess the percentage swelling. W1 represents the initial weight of tablet while W2 represents the swelled weight of tablet (Satheeshababu and Mohamed 2015).
2.7 Size of particle and measurement of zeta-potential
Size of particles and zeta-potential of the best selected formulation (TCN3) were determined. The PI presented the equality of particle size and sized distribution of particles at 25 °C. The prepared best nanoparticles were kept at 25 °C in a glass container having pH 7.4 phosphate buffer for a period of 1 month. The zeta potential and average particle size diameter were checked before and after the storage of nanoparticles in order to evaluate the stability of nanoparticles.
2.8 Scanning electron microscopy (SEM)
Surface morphology of prepared nanoparticles of TC (drug unloaded and loaded) were evaluate by scanning electron microscope (JEOL-6700F).
2.9 Nanoparticles stability in the simulated gastrointestinal fluids
Simulated gastrointestinal media were used to estimate the stability of prepared nanoparticles (Shi et al., 2017). The nanoparticles were incubated in SGF at pH of 1.2 and in SIF at pH of 6.8. The SGF consists of NaCl (2 g), pepsin (3.2 g) and HCL (7 mL) and sufficient quantity of sterile water to make 1000 mL. The SIF consists of potassium dihydrogen phosphate (6.8 g) and pancreatin (10 g) with a pH of 6.8. From the prepared formulation of nanoparticles take a small amount of nanoparticles and add in 5 mL of SGF for a period of 120 min or in 5 mL of SIF for a period of 360 min at 37 °C. The uniformity of particles and particle diameter of nanoparticles were assessed at multiple time duration.
2.10 Nanoparticles stability under storage conditions
Accelerated stability studies were performed at a temperature of 40 ± 2 °C and a 75 ± 5 % relative humidity for a period of six months. The sample of TCN3 formulation was withdrawn at 1, 3 and 6 months and assessed for particle size and zeta potential.
2.11 Cell viability assay
To check the toxicity of CS, TC and prepared nanoparticles, MTT assay was performed using Caco-2 cell line. Caco-2 is a human colorectal cell line that has been immortalized. Caco-2 cells are most commonly used as an in vitro model for drug toxicity, as they are immortalized cell lines of human colorectal adenocarcinoma cells. The Caco-2 cell line was the best option in our study since the target of the drug used (moxifloxacin) is also the colon.
The Caco-2 cell line is the best choice for testing the cytotoxicity of moxifloxacin containing nanoparticles that has been designed to target the colon. DMEM along with FBS (10%) was used in 96 well plates for the culture growth of cells. Thirty thousand cells were seeded per each well of a 96-well plate. After culturing the cells for a period of 6 to 24 h in cultured media without FBS with a 0.5 % dispersion of various samples. The cells were thoroughly washed and cleansed one time with phosphate buffer saline. After cleansing the cells were cultured by incorporation of 500 μL MTT solution in FBS free media for 60 min. 500 μL DMSO was introduced into each well, after removing the supernatant, to solubilize the converted dye and the absorbance was measured at 570 nm. The cell viability is calculated using equation (2). This technique is good agreement with the previous studies (Atif et al., 2021, 2012, 2011; Fakhar-e-Alam et al., 2021; Fakhar-E-Alam et al., 2014; .Fakhar-e-Alam et al., 2020; Muhammad Fakhar-e-Alam et al., 2020; Iqbal et al., 2020)(Fakhar-e-Alam et al., 2011) (Iqbal et al., 2019). As is the absorbance of dispersion of samples and Ad is the absorbance of DMEM.
2.12 Loading of drug and entrapment efficiency
10 mg of drug was added in deionized water (4 mL) and then 10 mg nanoparticles were dispersed in it (Gaihre et al., 2009). Moxifloxacin nanoparticles were collected by way of centrifugation at 10000 rpm after slight stirring for 1 day. The collected sample was washed with deionized water (n = 3) and analyzes the sample for its entrapment and loading efficiency of drug. The loading and entrapment efficiency were analyzed by HPLC at a wavelength of 295 nm with a flow rate of 1 mL/min. The following equation was used to calculate the percentage entrapment efficiency and percentage loading.
2.13 Release of moxifloxacin from nanoparticles
Dissolution apparatus (USP type II) was used to examine the release pattern of drug from prepared nanoparticles. The release of moxifloxacin from prepared formulation was performed in 900 mL of SGF, SIF and phosphate buffer with a pH of 7.4. The nanoparticles of NTC containing moxifloxacin were also used to analyze the release pattern of moxifloxacin in phosphate buffer with a pH of 7.4. In a dialysis membrane (Molecular weight cut off value of dialysis membrane was 14000) containing 5 mL of dissolution media (SGF, SIF, pH 7.4 Phosphate buffer), add 100 mg of nanoparticles. Tie up the both ends of dialysis bag and attach with peddle of dissolution apparatus and insert in basket having 900 mL of dissolution media. The rotating speed of paddle was set at 100 rpm with a controlled temperature of 37 ± 0.5 °C for 1 day. At predetermined interval, take 5 mL from the basket and the sink condition was maintained by adding 5 mL dissolution media. The samples thus collected were then passed through 0.2-µm filter and this filtered sample was injected into HPLC for the analysis of pattern of drug release from nanoparticle at 295 nm.
2.14 Release kinetics
Five most common models to evaluate the release study, zero order, first order, higuchi, Hixon Crowell and Korsmeyer peppas, were used and the kinetics behavior and release mechanism of moxifloxacin loaded nanoparticles was evaluated.
2.15 Pharmacokinetic study
12 albino rats weighing 250 to 450 g were included for the pharmacokinetic study of moxifloxacin from the developed nanoparticles under the approval of ethics committee of Government College University Faisalabad, Pakistan. The use and handling of animals in the study was in complete compliance with the guidelines of ICH. Divided the rats into two groups, and prior to dosing, they were fasted for overnight and only given access to water. Group one received TCN3 formulation (test) and the second received the moxifloxacin suspension (Iqbal et al., 2021). In both groups, the dose of drug was 1 mg/kg. The blood samples were withdrawn at predetermined intervals (0, 0.5, 2, 4, 6, 8, 12, 24, 36 and 48 h) and centrifuged the withdrawn samples at 4000 rpm for 20 min. In separated plasma, add acetonitrile and again centrifuged for 20 min at 4000 rpm. After centrifugation, remove the upper clear layer, passed through 0.22 µm filter and injected into HPLC for analysis (Hanif et al., 2020).
3 Results and discussion
3.1 Characterization of TC
TC was effectively synthesized by chemical reaction with thiourea as shown in Fig. 1. The stretching vibration of NH, –OH and C = O of CS was observed at 3342 cm−1, 2962 cm−1 and 1713 cm−1 respectively while C–H bending was at 1432 cm−1 (Mukhtar et al., 2020) as shown in Fig. 2A. FTIR spectrum of TC revealed a characteristic thiol group peak at around 2122 cm−1 substantiating the thiolation of CS (Kaur et al., 2020, Prabahar et al., 2020). The DSC thermogram revealed that the CS exhibits a single endothermic peak at 299 °C given the thermal degradation of amine units in CS. Furthermore, the TC thermogram showed an endothermic peak at 231 °C owing to the occurrence of simply fragile side chains because of the thiolation as shown in Fig. 2B (Laffleur et al., 2015). The TGA thermogram of CS showed a loss of weight starting at 230 °C and continual to 400 °C, and the thermogram of TC exhibited a weight loss starting at 63 °C and a rapid weight loss was spotted at 350 to 400 °C as shown in Fig. 2C. The peaks at 7.00 ppm to 10.5 ppm in the 1H NMR spectrum of CS confirmed the presence of protons of glucosamine units in the structure of CS while the peak appearing at 13.6 ppm could be assigned to the presence of methyl protons of N-acetyl group (Akhtar et al., 2020). The appearance of group of thiol in the structure of TC was corroborated by a new peak emerging at 4.9 ppm as shown in Fig. 2D.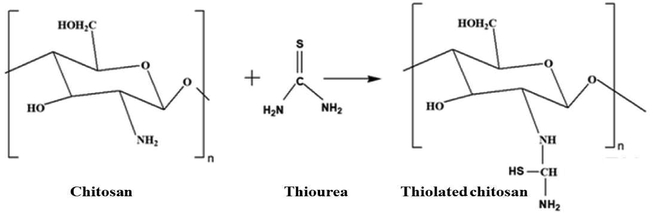
Schematic representation for the formation of TC.
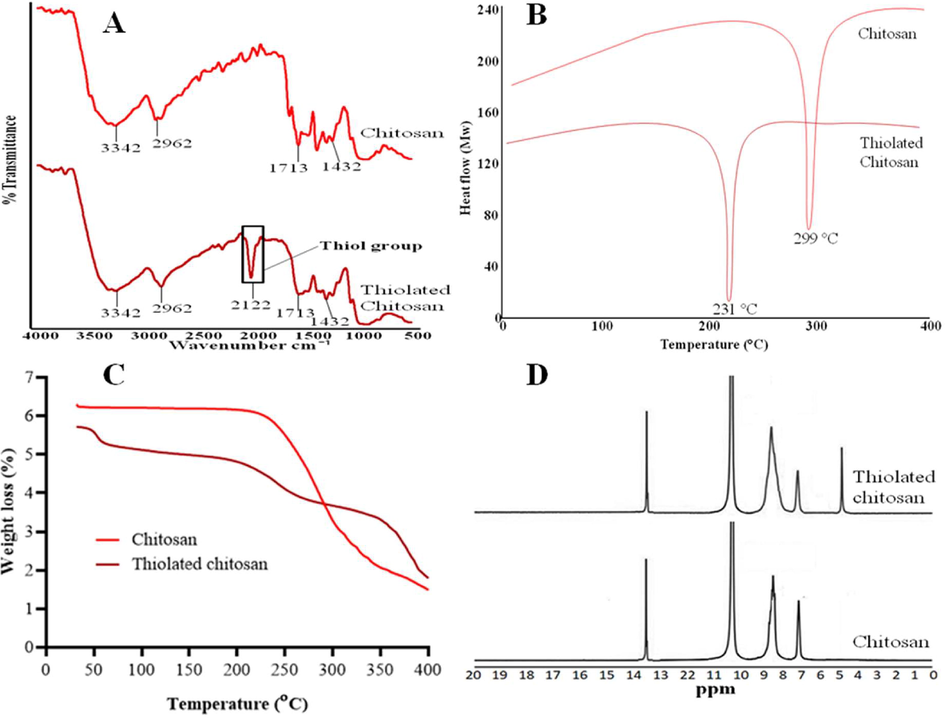
FTIR of TC showed the peak of thiol group at 2122 cm−1 confirmed the thiolation (A), DSC of CS and TC showed endothermic peaks at 299 °C and 231 °C. The endothermic peak of TC confirmed the thiolation due the presence of fragile group (B), TGA of TC showed rapid weight loss as compared to CS (C) and 1H NMR confirmed the thiolation of CS due to occurrence of peak of thiol group at 4.99 ppm (D).
3.2 Preparation of nanoparticles and thiol contents determination
The nanoparticles were successfully prepared by ionic cross-linking method. The free thiol content of TC was 0.07 and 0.19 mmol/mg of polymers. Formulations (TCN1 to TCN5) showed thiol contents in the range 0.05 to 0.14 mmol/mg of prepared formulations.
3.3 Mucoadhesion and swelling studies
Mucoadhesion analysis performed for the tablet containing CS, TC and nanoparticles depicted a noteworthy variation in the time of adhesion with mucosa. The attachment time shown by the CS containing tablets was up to 40 min, while for TC containing tablet, the time was noted to be 2.5 h after which erosion started and the complete detachment was observed in 24 h. the tablets with nanoparticles of CS showed the longest time of adhesion and after 2.45 h the tablets started to erode and a complete removal from the membrane was noticed in 24 h. The longer adhesion time for the tablets containing TC and nanoparticles is due to the fact that these compressed tablets contained some amount of free thiol group on their surfaces which created a S-S linkage (Krauland et al., 2006). Mucoadhesion presented by CS containing tablets attributes to the occurrence of hydroxyl group owing to non covalent interactions. The presence of -SH group, in the TC, is responsible for di-sulphide bond to have all the non-covalent interactions which became the reason for the interaction between modified polymer and the mucus membrane (Sharma and Ahuja 2011). The results have been accredited to previous studies reported by Kast and Bernkop-Schnürch in 2001 (Kast and Bernkop-Schnürch 2001). The dynamic and equilibrium swelling was initially studied by observing changes over specific time intervals. Later the studies were performed for 8 h following the immersion in controlled pH aqueous solutions (both acidic and alkaline pH). The swelling of CS was observed maximum at 4 h in a medium of pH 1.2 following a decline in the swelling while the TC showed equilibrium after 6 h at the aforementioned pH. At pH7.4, both CS and TC reached equilibrium after 6 h (Kast and Bernkop-Schnürch 2001). After maximum swelling, the decrease in swelling can be attributed to the decreased osmotic pressure gradient. Same studies with same results have been reported by Kast and Bernkop-Schnürch in 2001 (Kast and Bernkop-Schnürch 2001).
3.4 Particle size analysis and zeta potential measurement
The Fig. 3 indicated the distribution of size of particle size of TCN3 formulation. The size particle of the TCN3 formulation was found to be 105 nm (Massoudi et al., 2021) as shown in Fig. 3. All the prepared formulations exhibited PI less than 0.3 as mentioned in Table 3. Zeta-potential ± 30 mV is generally sufficient for the stability of prepared system (Masood et al., 2021). The prepared formulation TCN1 to TCN5 showed the zeta potential ranged from −18 to + 29. The result proved that among all the preparations, TCN3 was the most stable one as shown in Fig. 4.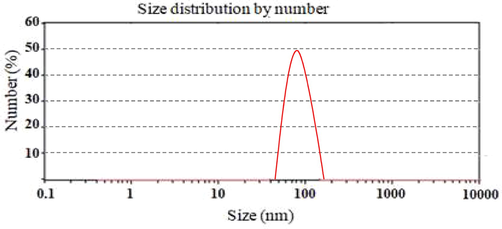
Particle size distribution of TCN3 nanoparticles formulation.
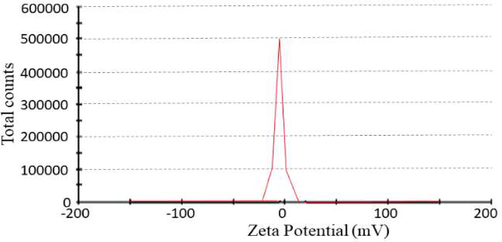
Zeta potential of TCN3 nanoparticles formulation.
3.5 Scanning electron microscopy (SEM)
The drug unloaded nanoparticles of TC showed spherical shape with size of 145 nm and the drug loaded nanoparticles 128 nm with spherical shape as shown in Fig. 5. The spherical shape nanoparticles were appropriate for the delivery of drug.Fig. 6..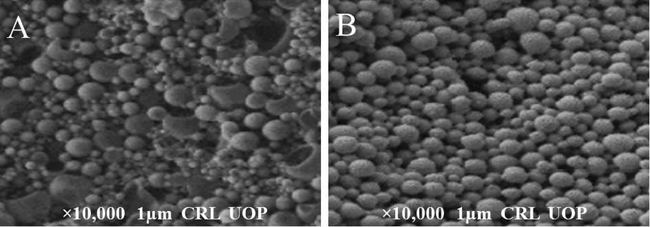
SEM images of (A) drug unloaded and (B) drug loaded nanoparticles of TC.
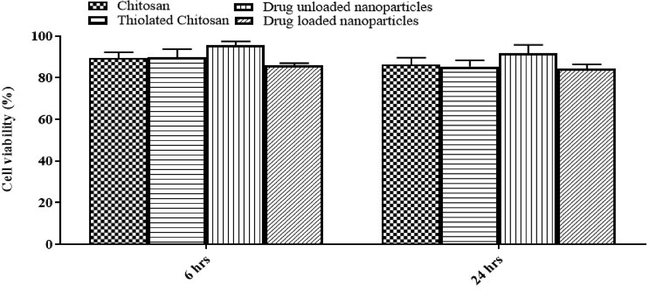
Cell viability study of CS, TC and TCN3 (moxifloxacin loaded and unloaded).
3.6 Stability of nanoparticles in simulated gastrointestinal fluids
The stability of nanoparticles of TCN3 formulation was determined by incubating the nanoparticles in SGF as well as in SIF. The outcomes revealed that the nanoparticles of TCN3 showed same particle size (105 nm) after 2 h in the simulated gastric fluid but for simulated intestinal fluid the particle size was shown to be significantly increased i.e., from 105 nm to 254 nm for the same time interval. At 6 h of incubation in the SIF, the particle size increased up to 623 nm attributing to the efficient swelling of nanoparticles in SIF. Shi et al., in 2017 reported the similar findings of nanoparticles when checked in both SGF and SIF (Shi et al., 2017).
3.7 Nanoparticles stability under storage condition
A slight change in the particle size and zeta potential were observed for a period of six months. The particle size was 106, 108 and 109 µm after 1, 3 and 6 months respectively. The zeta potential values of optimized formulation (TCN3) were ± 20, ±23 and ± 26 after 1, 3 and 6 months respectively.
3.8 Cell viability assay
Cytotoxicity study is an important parameter to be assessed. For cell viability studies, cultures of caco-2 cell were treated with both drug loaded and unloaded formulations. The outcome revealed that the viability of the cells treated with the nanoparticle dose used in study, was not affected. The viability of cells remained above 87% indicating that the formulation is non-toxic and safe after 24 h incubation of TCN3. The cell morphology of cell line observed under microscope was unaltered.
3.9 Release of moxifloxacin
The drug loading capacity of TCN3 was 100.1% and the encapsulation efficiency was revealed to be 88.56%. The in vitro drug release from five different formulations of moxifloxacin loaded nanoparticles was studied in SGF, SIF, and PBS of 7.4 pH at 37 °C. The release of drug form five different preparations of nanoparticles of TC in SGF ranged from 5.34% to 10.34% as shown in Fig. 7A. The release when performed in SIF revelaed a value ranging from 82.56% to 97.84% as depicted by Fig. 7B. The moxifloxacin release from NTC in PBS of pH 7.4 was very high with about 97.13% moxifloxacin released within 8 h as shown in Fig. 7C. The release pattern shown by nanoparticles of NTC was observed as less sustained when compared to the release pattern of nanoparticles of TC. Moxifloxacin released from TCN1 and TCN2 was recoreded to be 83% and 80% respectively. TCN3 was considered to have the best release of 99.43% at pH 7.4 in 24 h of study. TCN4 and TCN5 showed release of moxifloxacin to be 88% and 91% respectively in 24 h. The results of the relese study performed were found to be similar with the already published results (Teaima et al., 2020). The releases of drug from the formulation play an important role when the concern is sustained release or controlled release. It is crucial to investigate the release pattern of the drug from formulation because it governs the efficacy of the formulation. Among the different models used to study the release kinetics, Zero-order kinetics, First-order kinetics, Higuchi model, Korsmeyer Peppas model and Hixon Crowell model are used here. The value of R2 for zero-order ranged from 0.995 to 0.999, indicating that nanoparticles showed the zero-order release. The value of n ranged from 0.18 to 0.39 which is less than 0.5 indicating that the formulation followed Fickian release pattern (Table 2) (Mahor et al., 2016).Fig. 8..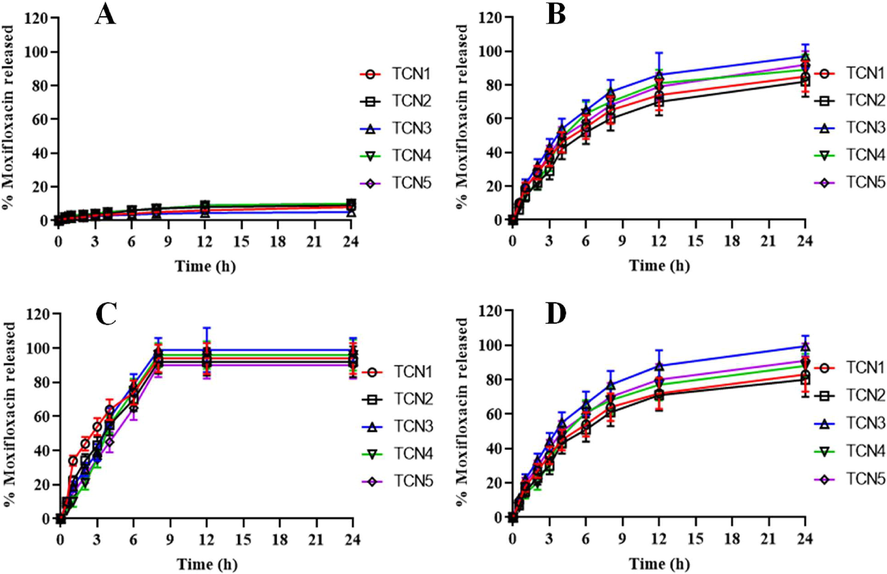
In-vitro release of drug from (A) nanoparticles of TC in SGF, (B) nanoparticles of TC in SIF, (C) nanoparticles of NTC in phosphate buffer pH 7.4 and (D) nanoparticles of TC in phosphate buffer pH 7.4.
Code
Zero-order
First-order
Higuchi
Hixon-crowell
Korsmeyer-peppas
R2
R2
R2
R2
R2
n
TCN1
0.997
0.785
0.991
0.992
0.997
0.21
TCN2
0.998
0.654
0.993
0.988
0.991
0.39
TCN3
0.999
0.865
0.995
0.955
0.997
0.18
TCN4
0.995
0.623
0.993
0.933
0.995
0.28
TCN5
0.998
0.707
0.991
0.937
0.993
0.21
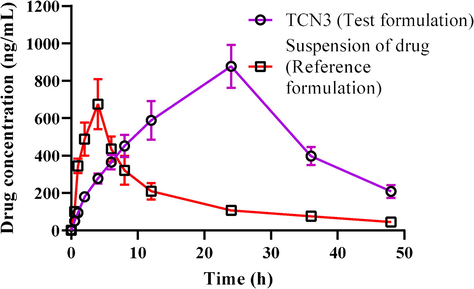
Pharmacokinetic profile of TCN3 (test formulation) and suspension of drug (reference formulation).
3.10 Pharmacokinetics of moxifloxacin
To study the pharmacokinetics of moxifloxacin, the suspension of drug was made in carboxy methyl cellulose and then the suspension and the nanoparticles TCN3 were administered in albino rats via oral route. The pharmacokinetic parameters i.e., Cmax (ng/mL), tmax (hour), AUC0-t (ng/ml.h), AUC0-∞, (ng/mL.h), AUMC (ng/mL.h), t1/2 (h), MRT (hour) and kel (h) were then calculated using non– compartmental approach and the results are organized in Table 3. After the oral administration of TCN3 nanoparticles, the mean ± SEM for Cmax was elevated than the mean ± SEM of the reference formulation (878 ± 4.167 ng/mL (p = 0.002) and 676 ± 2.156 ng/mL respectively) and the difference was attributed to the different composition of formulations. The higher value of Cmax shown by nanoparticles with TC is mainly because of the more controlled and defined release. The mean ± SEM for tmax of TCN3 was calculated as 24 ± 0.014 h (p = 0.001) and was found to be higher than the mean ± SEM of tmax (4 ± 0.003 h (p = 0.001) by reference formulation. The high tmax shown by TCN3 was an indication of controlled release of drug from formulation due to the presence of TC in nanoparticles. The controlled release was also confirmed by the t1/2 of the TCN3 and reference formulations (11.167 ± 3.169 h and 3.59 ± 1.032 h, respectively).
Parameters
Units
TCN3
Suspension of drug
tmax
Hour
24 ± 0.014
4 ± 0.003
Cmax
ng/mL
878 ± 4.167
676 ± 2.156
t1/2
Hour
11.167 ± 3.169
3.59 ± 1.032
AUC0-t
ng/mL.hr
25256.31 ± 4.109
8269.25 ± 4.456
AUC0-∞
ng/mL.hr
27728.15 ± 2.378
11823.37 ± 3.098
AUMC0-∞
ng/mL.hr
782351.58 ± 4.468
31207.56 ± 2.896
MRT
Hour
25.38 ± 1.406
5.77 ± 1.034
Similarly, value of AUC from TCN3 (25256.31 ± 4.109 ng/mL.hr (p = 0.004)) was also greater as compared to the suspension of moxifloxacin (reference formulation) which is an attribution to high value of Cmax. Another study reported that the drug loading in nanoparticles gave high value of AUC (Abdelghany et al., 2019). Value of MRT of TCN3 (25.38 ± 1.406 h) was found to be high than that of reference formulation (Table 3) (Liu et al., 2020). Furthermore, the bioavailability shown by the test formulation TCN3, was greater than the bioavailability of reference formulation.
4 Conclusion
Nanoparticle was successfully synthesized using TC based drug delivery systems. The developed TC showed greater mucoadhesive strength as compared to CS which provide the controlled release pattern of drug. The nanoparticles in culture medium proved to be non-toxic at the dose used in the study. The stability studies of in simulated gastrointestinal fluid and under storage conditions confirmed the stability of developed nanoparticles. The release pattern of drug for 24 h from TCN3 was achieved and showed better bioavailability when compared with the reference formulation. Based on our findings, we will move this project into clinical trials in the near future.
Acknowledgement
Researchers Supporting Project number (RSP-2021/397), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alginate modified-PLGA nanoparticles entrapping amikacin and moxifloxacin as a novel host-directed therapy for multidrug-resistant tuberculosis. J. Drug Deliv. Sci. Technol.. 2019;52:642-651.
- [CrossRef] [Google Scholar]
- Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Develop. Ther.. 2016;10:483-507.
- [CrossRef] [Google Scholar]
- Pharmacokinetic profile of chitosan modified poly lactic co-glycolic acid biodegradable nanoparticles following oral delivery of gentamicin in rabbits. Int. J. Biol. Macromol.. 2020;164:1493-1500.
- [Google Scholar]
- Cytotoxic and photocytotoxic effect of Photofrin® on human laryngeal carcinoma (Hep2c) cell line. Laser Phys.. 2011;21:1235-1242.
- [CrossRef] [Google Scholar]
- In vitro studies of Photofrin® mediated photodynamic therapy on human rhabdomyosarcoma cell line (RD) Laser Phys.. 2012;22:286-293.
- [CrossRef] [Google Scholar]
- Manganese-doped cerium oxide nanocomposite as a therapeutic agent for MCF-7 adenocarcinoma cell line. Saudi J. Biol. Sci.. 2021;28:1233-1238.
- [CrossRef] [Google Scholar]
- Stimuli responsive in situ gelling systems loaded with PLGA nanoparticles of moxifloxacin hydrochloride for effective treatment of periodontitis. AAPS PharmSciTech. 2020;21:1-8.
- [CrossRef] [Google Scholar]
- Thiolated polymers—thiomers: synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int. J. Pharm.. 2003;260:229-237.
- [Google Scholar]
- Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym.. 2018;193:163-172.
- [Google Scholar]
- Tumoricidal effects of nanomaterials in HeLa cell line. Laser Phys.. 2011;21:1978-1988.
- [CrossRef] [Google Scholar]
- Apoptotic effect of TiO2 in HepG2 cellular model. J. Optoelectron. Adv. Mater.. 2014;16:1481-1486.
- [Google Scholar]
- Spectroscopic features of PHOTOGEM® in human Rhabdomyosarcoma (RD) cellular model. J. King Saud Univ. - Sci.. 2020;32:3131-3137.
- [CrossRef] [Google Scholar]
- Assessment of green and chemically synthesized copper oxide nanoparticles against hepatocellular carcinoma. J. King Saud Univ. - Sci.. 2021;33(8)
- [CrossRef] [Google Scholar]
- Synergistic effect of TEMPO-coated TiO2 nanorods for PDT applications in MCF-7 cell line model. Saudi J. Biol. Sci.. 2020;27(12):3199-3207.
- [Google Scholar]
- Gelatin-coated magnetic iron oxide nanoparticles as carrier system: drug loading and in vitro drug release study. Int. J. Pharm.. 2009;365(1-2):180-189.
- [Google Scholar]
- Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B. Biointerfaces. 2005;44(2-3):65-73.
- [Google Scholar]
- The effects of the thiolation with thioglycolic acid and l-cysteine on the mucoadhesion properties of the starch-graft-poly (acrylic acid) Carbohydr. Polym.. 2017;163:129-136.
- [Google Scholar]
- Enhancement of Oral Bioavailability of Ibandronate Through Gastroretentive Raft Forming Drug Delivery System. In Vitro and In Vivo Evaluation. Int. J. Nanomedicine. 2020;15:4847-4858.
- [Google Scholar]
- Structural, morphological, antimicrobial, and in vitro photodynamic therapeutic assessments of novel Zn+2-substituted cobalt ferrite nanoparticles. Results Phys.. 2019;15
- [CrossRef] [Google Scholar]
- Photodynamic therapy, facile synthesis, and effect of sintering temperature on the structure, morphology, optical properties, and anticancer activity of Co3O4 nanocrystalline materials in the HepG2 cell line. J. Photochem. Photobiol. A Chem.. 2020;386:112130.
- [Google Scholar]
- Moxifloxacin loaded nanoparticles of disulfide bridged thiolated chitosan-eudragit RS100 for controlled drug delivery. Int. J. Biol. Macromol.. 2021;182:2087-2096.
- [Google Scholar]
- Thiolated polymers—thiomers: development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials. 2001;22(17):2345-2352.
- [Google Scholar]
- Thiolated chitosan nanoparticles for augmented oral bioavailability of gemcitabine: Preparation, optimization, in vitro and in vivo study. J. Drug Deliv. Sci. Technol.. 2020;61:102169
- [CrossRef] [Google Scholar]
- In vivo evaluation of a nasal insulin delivery system based on thiolated chitosan. J. Pharm. Sci.. 2006;95(11):2463-2472.
- [Google Scholar]
- Evaluation of functional characteristics of preactivated thiolated chitosan as potential therapeutic agent for dry mouth syndrome. Acta Biomater.. 2015;21:123-131.
- [Google Scholar]
- Solubility, antioxidation, and oral bioavailability improvement of mangiferin microparticles prepared using the supercritical antisolvent method. Pharm.. 2020;12:90.
- [CrossRef] [Google Scholar]
- Moxifloxacin loaded gelatin nanoparticles for ocular delivery: Formulation and in-vitro, in-vivo evaluation. J. Colloid Interface Sci.. 2016;483:132-138.
- [Google Scholar]
- Pharmaco-Technical Evaluation of Statistically Formulated and Optimized Dual Drug-Loaded Silica Nanoparticles for Improved Antifungal Efficacy and Wound Healing. ACS Omega. 2021;6(12):8210-8225.
- [Google Scholar]
- Impact of particle size on the structural and magnetic properties of superparamagnetic Li-ferrite nanoparticles. J. Magn. Magn. Mater.. 2021;528:167806
- [Google Scholar]
- An investigation into the digestion of chitosan (noncrosslinked and crosslinked) by human colonic bacteria. J. Pharm. Sci.. 2008;97(9):3820-3829.
- [Google Scholar]
- Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: from bench to bedside. Adv. Sci.. 2018;5:1700513.
- [Google Scholar]
- Chitosan-modifications and applications: opportunities galore. React. Func. Polym.. 2008;68:1013-1051.
- [Google Scholar]
- Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int. J. Biol. Macromol.. 2020;165:3007-3019.
- [Google Scholar]
- Optimization of thiolated chitosan nanoparticles for the enhancement of in vivo hypoglycemic efficacy of sitagliptin in streptozotocin-induced diabetic rats. Pharm.. 2020;12:300.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of sodium alginate conjugate and study of effect of conjugation on drug release from matrix tablet. Indian J. Pharm. Sci.. 2015;77:579-585.
- [Google Scholar]
- Thiolated pectin: Synthesis, characterization and evaluation as a mucoadhesive polymer. Carbohydr. Polym.. 2011;85:658-663.
- [Google Scholar]
- Gastrointestinal stability, physicochemical characterization and oral bioavailability of chitosan or its derivative-modified solid lipid nanoparticles loading docetaxel. Drug Dev. Ind. Pharm.. 2017;43:839-846.
- [Google Scholar]
- A greener approach for impressive removal of As (III)/As (V) from an ultra-low concentration using a highly efficient chitosan thiomer as a new adsorbent. RSC Adv.. 2016;6:64946-64961.
- [Google Scholar]
- Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Bio. Macromol.. 2020;150:281-288.
- [Google Scholar]
- Synthesis of Biocompatible and Environmentally Nanofibrous Mats Loaded with Moxifloxacin as a Model Drug for Biomedical Applications. Pharm.. 2020;12:1029.
- [CrossRef] [Google Scholar]







