Translate this page into:
Phytochemical screening and characterization of the antioxidant, anti-proliferative and antibacterial effects of different extracts of Opuntia ficus-indica peel
⁎Corresponding author. moustafa.abdelmoneim@agr.bsu.edu.eg (Moustafa A. Aboel-Ainin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Opuntia ficus-indica peels need further investigations as they could contain multiple active molecules. Bioactive secondary metabolites are important for health maintenance and play a significant role in treating some common diseases. Hence, the present study analyzed the chemical composition and biological activities of the plant extract using in vitro models.
Methods
The antioxidant assays were estimated in terms of DPPH radical scavenging; the chemical characterization is done in terms of total polyphenol and flavonoids, as well as HPLC characterization. The SRB assay was used for anticancer analysis whereas disc diffusion and minimum inhibitory concentration was determined to assess antimicrobial activity.
Results
The content of total polyphenol (TPC) and flavonoids (TFC) differed significantly in Opuntia ficus-indica peel cyclohexanone extract (OFICE), Opuntia ficus-indica peel 70 % ethanol in water extract (OFI70EE), and Opuntia ficus-indica peel 70 % ethanol in water extract (OFI100EE)), with varying values of TPC and TFC. In HPLC analysis, there observed the presence of 8 phenolic and flavonoids compounds. Antioxidant analysis showed that OFICE has higher antioxidant activity. Bioassay based on MCF-7 showed that O. ficus-indica extracts significantly (p < 0.05) reduced cell viability of cancer cells at 400 µg/mL after 72 h of incubation time. Concerning the antibacterial activity, the cyclohexanone extract gave the highest antibacterial inhibition as compared to the other extracts, and the highest activity was recorded against Listeria monocytogenes. Also, the Opuntia ficus-indica peel extracts showed potential antiviral activity with the mean inhibition rates (39, 19, and 67.66 %) and (35, 16.66, and 54.66 %) against H5N1 and rotavirus respectively.
Conclusion
Overall, the study confirms the antioxidant, antimicrobial and anticancer properties of the extract and further studies in animal models are necessary to validate their pharmacological applications.
Keywords
Opuntia ficus-indica
Total phenols
Total flavonoids
MTT
Antioxidant
Antitumor
Antibacterial & Antiviral
1 Introduction
Plant-derived natural chemicals play an important role in disease prevention and health benefits (Kesba and El-Beltagi, 2012; Yabalak et al., 2020b). Among, these the major ones include functional foods and nutraceutical compounds (Indu et al., 2021; Narayanankutty, 2021a). There is a high demand and need for novel antimicrobial and anticancer agents; the emerging drug resistance and chemotherapy resistance in cancer cells and microbials strains is the major reason for such increased demand for novel drug candidates (Aslam et al., 2018; Housman et al., 2014). Various natural products and plant extracts are promising drug candidates against bacterial communities and virus (Sagaya Jansi et al., 2021; Narayanankutty et al., 2021a). Similarly, natural products are important sources of anticancer agents; several natural products including curcumin, turmeron, resveratrol, and sulforaphane (Narayanankutty, 2020). Apart from these numerous plants and extracts are emerging as drug candidates from various parts of the world.
Cactaceae plants and fruits contain glycosylated flavonols, dihydroflavonols, flavanones, and flavanols (Kuti, 1992). Many studies have found a strong connection between the phenol content of extractable polyphenol extracts from Opuntia spp. and their antibacterial and antioxidant properties (Anwar and Sallam, 2016). Furthermore, the majority of researchers have found that the extraction solvents and processing procedures utilized can alter the biological activity, yield, and phenolic component profile of the extracts examined (Abou-Elella and Ali, 2014). The prickly pear or nopal cactus Opuntia ficus-indica (L.) Mill., belongs to the dicotyledonous angiosperm Cactaceae family (Chougui et al., 2013). Opuntia fruits have a great medical potential and include several bioactive chemicals such as phenolic compounds and flavonoids, which are antioxidant compounds (Chahdoura et al., 2015). Opuntia ficus-indica (L.) has a chemical composition of (12–15 % sugars, 0.6 % protein, and 0.1 % lipids; calcium 2200 ppm, potassium 490 ppm, and magnesium 850 ppm) (Kim et al., 2006). Prickly pear cactus peels are produced in Egypt in about 58,344 tons per year (Benattia et al., 2019). We can extract a large number of bioactive chemicals from PPCP, which accounts for 36–48 % of the entire fruit and is regarded a plant waste. Furthermore, phenolic substances, flavonoids such as kaempferol and quercetin, which have antioxidant capabilities, are found in the prickly pear (Saxena et al., 2013).
Hence, the present study analyzed the phytochemical composition of Opuntia ficus-indica using different chromatographic techniques and the antioxidant, antimicrobial, anticancer, and antiviral activities of different extracts from the plant is also analyzed.
2 Materials and methods
2.1 Plant materials
Prickly pear fruits were obtained from the Department of Tropical Fruits, Agricultural Research Center, Giza, Egypt, in May 2019, Fig. 1. Peels were separated, washed, and dried at room temperature and ground into fine powder for extraction.
Prickly pear (Opuntia ficus-indica).
2.2 Preparation of Opuntia ficus-indica extracts
The peels of ripe prickly pear fruits were chopped into little pieces and dried at 80 °C overnight. The dry pieces were milled as a mechanical preparation and stored in the freezer until they were needed. The dried materials were extracted progressively with 500 ml of cyclohexanone, 70 %, and 100 % ethanol. At 45 °C, each fraction was evaporated separately using a rotating vacuum evaporator. The extracts were stored at −25 °C in light-protected containers until they were used.
2.3 Phytochemical analysis
2.3.1 Estimation of total polyphenol content (TPC)
The total polyphenol content of the extract was determined using the reaction with Folin-Ciocalteu reagent using the gallic acid as internal standard. The TPC of samples were expressed in mg gallic acid equivalence (GAE) according to the standard methods described previously (Kim et al., 2022).
2.3.2 Estimation of total flavonoid content (TFC)
The flavonoid content was quantitatively estimated in terms of the rutin equivalent and expressed as mg rutin equivalent (RE) (Anusmitha et al., 2021). The reaction was based on the spectrophotometric changes induced by the AlCl3 with flavonoids in the extract.
2.4 Analysis of phenols by HPLC
The phytochemical composition of the extracts was analyzed using HPLC method using appropriate standards. The column, solvent system and chromatographic conditions were set according to the methods described by Skendi et al. (2017). Individual compound identities were validated by comparing the retention times of corresponding standards to the peaks in Opuntia ficus-indica extracts.
2.5 Antioxidant activity: DPPH radical scavenging activity assay
The antioxidant activity was measured by DPPH radical scavenging activity (Anusmitha et al., 2021; Yabalak et al., 2020a). The stock solution was prepared using 10 mg / 1 ml DMSO. Serial dilutions for each extract were prepared. Readings were recorded at λ = 540 nm. The percentage of DPPH inhibition = [1-(A sample -A background)/ (A DMSO -A background)] X 100.A calibration curve was constructed using the inhibition rate values of the standard Trolox solution.
2.6 In vitro antitumor activity: Analysis of cell viability by SRB assay
Antitumor activity was performed by SRB assay is described as the preferred method according to quantitative estimation of the number of cell viability in a culture protocol (Kim et al., 2022; Koottasseri et al., 2021). MCF-7 was purchased from the NCI, Cairo, Egypt.
2.7 Antimicrobial activity assay
2.7.1 Test bacteria
Clinical isolates of Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus, and Listeria monocytogenes) and Gram-negative bacteria (Escherichia coli, Salmonella typhi, and Shigella boydii) were cultured in nutritional broth at 37 °C for 24 h before being tested for antibacterial activity.
The diameters of the inhibition zones were measured in millimeters for determining the antibacterial activity of the investigated substances using a modified Kirby-Bauer disc diffusion method (Narayanankutty et al., 2021a; Narayanankutty et al., 2021b). Filter discs impregnated with 10 > l of solvent (cyclohexanone, 100 % ethanol in water, and 70 % ethanol in water) served as a negative control for antimicrobial activity, while standard discs of Gentamycin (antibacterial agent) served as a positive control.
2.7.2 Determination of MICs
In antimicrobial susceptibility testing, the Opuntia ficus-indica extracts demonstrated substantial antibacterial activity. The condensed extract was used to make a stock solution of 10 mg/mL using dimethyl sulfoxide (DMSO) as solvent. This was serially diluted to obtain various concentrations ranging from 3.12 mg/mL to 50 mg/mL.
2.8 Antiviral assay
2.8.1 Virus stocks
Both characterized Avian Influenza Virus and Rotavirus were kindly provided by the Center of Excellence for Influenza viruses, National Research Centre, Egypt.
2.8.2 Determination of cytotoxicity of Opuntia ficus-indica extracts by MTT assay
The O. ficus-indica extracts were tenfold serially diluted with Dulbecco’s modified eagle medium (DMEM). The cytotoxicity effect of the three O. ficus-indica extracts was determined in MDCK and African Rhesus monkey kidney MA-104 cell line for H5N1 and Rotavirus respectively, (using MTT assay), according to the previous methods (Anusmitha et al., 2021). The percentage of cytotoxicity compared to the untreated control cells was determined using the following equation:
Cytotoxicity (%) = (Absorbance of cells without treatment – Absorbance of cells with treatment) × 100/ Absorbance of cells without treatment. % Cytotoxicity versus sample concentration was used to estimate the concentration which exhibited 50 % cytotoxicity (IC50).
2.8.3 Plaque titration assay for viruses
The Plaque titration assay was implemented for counting the plaque-forming units (PFUs), as previously described (Tobita, 1975). The virus titer was calculated through the following equation according to:
Plaque forming unit (PFU)/ml = No. of plaques × Reciprocal virus dilution × multiplicand number to complete the volume of the inoculums to 1 ml.
2.8.4 Antiviral activity for O. ficus-indica extracts
The plaque reduction assay was done according to (Hayden et al., 1980) in a six-well plate, where MDCK cells and MA-104 cell line for H5N1 and Rotavirus respectively with 105 cells/ mL. The plaques were counted and the reduction percentage in plaques formation in contrast to the control wells was recorded as the following equation:
2.9 Statistical analysis
All experiments and analyses were carried out in triplicate, with mean values and standard deviations calculated accordingly. The differences between them and values of multiple groups were analyzed by IBM® SPSS® Statistics software, significance was defined at P ≤ 0.05 (IBM® SPSS®, 2011).
3 Results
3.1 Total phenolic acid and flavonoid contents
The amount of total phenolic acids and flavonoids in Opuntia ficus-indica peel cyclohexanone extract (OFICE), Opuntia ficus-indica peel 70 % ethanol extract (OFI70EE), and Opuntia ficus-indica peel 100 % ethanol (OFI100EE) differed significantly (Table 1). The amounts of phenolic acids in the dry weight of plant material was 51.11, 40.49, and 28.27 mg GAE/100 g, respectively as measured by the Folin-Ciocalteau method. The aluminium chloride (AlCl3) method was used to determine the flavonoid concentration in the extracts, and the levels were 2.24, 0.10, and 1.30 mg RE/100 g dry weight. *TPC expressed in mg GAE/100 g dry weight of extract; TFC expressed in mg RE/100 g dry weight of extract; Each value is the mean ± SD of triplicate measurements. The data are presented as the mean ± SD of technical replicates (n = 9). a- indicate significant difference with OFI70EE, b-indicate significant difference between OFI100EE.
O. ficus-indica extracts
TPC (mg/g)
TFC (mg/g)
OFICE
51.11 ± 2.73a,b
2.23 ± 0.260 a,b,c
OFI70EE
28.27 ± 0.85
0.10 ± 0.062
OFI100EE
40.49 ± 2.50 a
1.30 ± 0.23 a
3.2 HPLC analysis
Data in Figs. 2 and 3 showed that OFICE, OFI70EE, and OFI100EE contain 8 phenolic and flavonoids compounds. From the results obtained, it is proved that the best solvent for extraction is OFICE, OFI70EE, and OFI100EE, respectively. The main constituent is catechin followed by gallic acid, caffeic acid, chlorogenic acid, and ellagic acid (phenolic acids). In addition, O. ficus-indica extracts contain many flavonoids compounds such as quercetin, kaempferol, and rutin. The O. ficus-indica extracts contain high concentrations of quercetin. Peel the prickly pear extract contains the main constituent being ferulic acid (34.8 %), caffeic acid (2.6 %) and vanillic acid occurring in small quantities (0.1 %).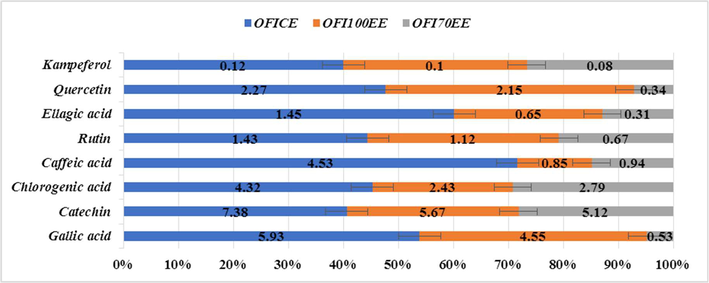
The Phenolic acids and flavonoids in O. ficus-indica extracts by HPLC analysis.
Bioactive compounds of OFIPs involve simultaneously chemical reduction of Pt NPs and the antioxidant role for these compounds (Ishak et al., 2020).
3.3 Antioxidant activity
Antioxidant activity for OFICE, OFI70EE and OFI100EE was evaluated at two doses 10 and 100 μg/ml. The results showed that OFICE has higher antioxidant activity > 50 % inhibition at both doses than OFI70EE, and OFI100EE, which presented a high positive correlation (p < 0.05) of DPPH and IC50 was 843.2 ± 11.37 μg/ml. Data obtained for antioxidant activities of OFI70EE and OFI100EE reveals that both extracts have approximately the same value for antioxidant activity < 50 % inhibition at two doses 10 and 100 μg/ml. On the other hand, IC50 were 346.9 ± 8.97 and 551.7 ± 14.66 µg/ml for OFI70EE and OFI100EE, respectively.
3.4 In vitro antitumor activity by SRB assay
The assay is based on mammalian cells involving antioxidant and upregulation of some cellular self-defense mechanisms, which are related to prevention as well as treatment of cancer. The cytotoxicity of different concentrations (1, 10, 100, 200, 300, 400 μg/ml) for OFICE, OFI70EE, and OFI100EE of OFIPs, against MCF-7 cell line detected by SRB assay. Examination of the cultures under a phase-contrast inverted microscope showed significant effects on cell viability depending on concentrations of O. ficus-indica extracts.
A high dose of OFICE, OFI70EE, and OFI100EE highly significantly (p < 0.05) reduced cell viability of MCF-7 cancer cells at concentration 400 µg/ml after 72 h. incubation time. Thus, a high dose was the best compared to other concentrations, where it’s the percent of cell viability were 47 %, 66 %, and 73. Moreover, the IC50 was 1506 µg/mL, 1972.7 µg/mL, and 2236 µg/mL for OFICE, OFI70EE, and OFI100EE, respectively. In other words, an increasing concentration decreased cell viability for cancer cells. Showing all treated tumor cells were inhibited with all tested concentrations beginning from 1 to 400 µg/ml. Respecting the effect of three O. ficus-indica extracts on cells exhibits dose-dependent toxicity. At low dose 1 µg/ml OFICE, OFI70EE and, OFI100EE, the cells viability of cancer cell was 91 %, 97 % & 99 %, respectively. Moreover, the treated cancer cell at 10, 100, 200 and 300 µg/ml of OFICE gives 87 %, 72 %, 68 %, 53 %, respectively, whereas, OFI70EE and OFI100EE give 93 %, 96 %, 84 %, 90 %, 79 %, 85 %, 71 % and 78 % viability, respectively.
As over the above results of in vitro cytotoxicity of three O. ficus-indica extracts for biocompatibility evaluations on MCF-7 cell line after 72 h incubation, with different concentrations, ranging from 1 to 400 µg/ml, OFICE at different concentrations showed significantly reduced cell viability than the ethanol extracts and the former has a greater effect as antitumor.
3.5 Antibacterial activity
The intensive use of antibiotics is usually followed by the presence of resistant strains of microorganisms. The sight of the resistance of bacteria to drugs, the search for natural compounds having antibacterial activity is an urgent demand to deal with the harmful effects of those pathogenic microorganisms. Accordingly, during this study-three O. ficus-indica extracts i.e. OFICE, OFI70EE, and OFI100EE were tested against different microorganism's Gram-positive B. cereus, L. monocytogenes and S. aureus, and Gram-negative bacteria E. coli, S. boydii, and S. typhi.
The OFICE displayed that the highest antibacterial activity by preventing the growth of the tested isolates compared with the other extracts (OFI70EE and OFI100EE). The strongest antibacterial activity of the OFICE has recorded against L. monocytogenes strains with inhibition zone 34 mm, whereas the lower activities were against the S. boydii and S. typhi strains with inhibition zone 17 mm.
The antibacterial data are recorded (Table 2, 3) and illustrated in Fig. 4. The inhibitory effect of OFICE started at 6.25 mg/ml with inhibition zones of 6.4 mm against B. cereus, while extract concentration at 12.5 mg/ml suppressed L. monocytogenes, S. aureus, E. coli, S. boydii, and S. typhi growth at 12.5 mg/ml with inhibition zones of 8, 9, 9, 5 and 9 mm, respectively.
Microorganisms
Concentrations (mg\mL)
100
50
25
12.5
6.25
3.12
0
Bacillus cereus
25
20
15
9
6.4
0
Listeria monocytogenes
35
30
20
8
0
0
Staphylococcus aureus
23
19
12
9
0
0
E. coli
24
19
17
9
0
0
Shigella boydii
17
9
6
5
0
0
Salmonella typhi
17
15
11
9
0
Microorganisms
Cyclohexanone
Ethanol 90 %
Ethanol 70 %
Bacillus cereus
25
8
6
Listeria monocytogenes
34
6
6
Staphylococcus aureus
19
6
6
E. coli
24
6
6
Shigella boydii
17
7
6
Salmonella typhi
17
9
6
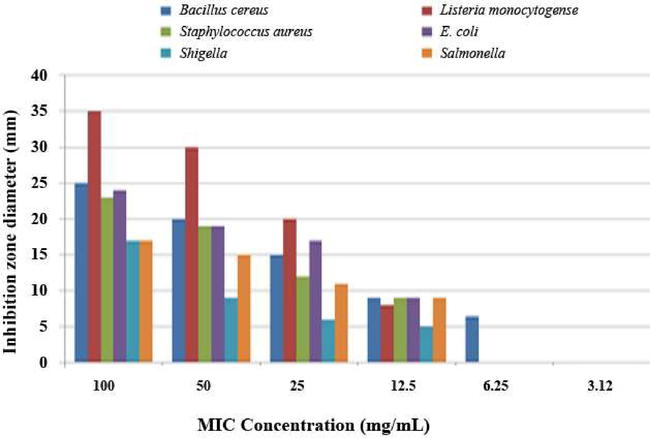
Effective MICs of OFICE against different pathogenic bacteria.
3.6 Cytotoxicity assays
In the obtained data the MDCK and MA-104 cell lines were treated with different concentrations of the three O. ficus-indica extracts for 24 h, and then the MTT compound was added on the next day to determine cell viability. Data revealed that all three Opuntia ficus-indica extracts showed no cytotoxicity on the tested cell lines on both H5N1 and Rotavirus. At least three different concentrations of each tested peel extract were prepared in triplicates. The calculated IC50 for each peel extract is presented in Table 1. The Opuntia ficus-indica extracts have a wide range of 50 % toxicity to the cell lines that ranged from 450 μg/μl to > 1000 μg/ μl and from 367 μg/μl to > 800 μg/ μl for H5N1 and Rotavirus respectively (Table 4). *IC50: The concentration of O. ficus-indica extracts, which exhibited 50% cytotoxicity.
No.
Extracts
IC50 (μg/ μl) for H5N1
IC50 (μg/ μl) for Rotavirus
1
OFI70EE
630
700
2
OFI100EE
>1000
>800
3
OFICE
450
367
3.7 Inhibitory potential of the Opuntia ficus-indica extracts acts against viruses
The antiviral activity of the three Opuntia ficus-indica extracts against rotavirus and H5N1 in vitro was investigated in this study. These findings (Table 5) demonstrated that all of the Opuntia ficus-indica extracts tested had potential action against rotavirus and H5N1 and should be favored for further research. Few investigations on the antiviral properties of our tested Opuntia ficus-indica extracts on animal and human viruses have been published, with the majority of studies focusing on plant viruses. The antiviral activity of cyclohexanone peel plant extract against rotaviruses and H5N1 has never been reported before. The plaque infectivity titer of H5N1 and Rotavirus was initially computed and proved to be 2107 and 2106 PFUs/ml for H5N1 and Rotavirus, respectively, according to the collected data. Opuntia ficus-indica extracts in various concentrations of 25 g/l, 50 g/l, and 100 g/l were produced and tested. The percentages of H5N1 and Rotavirus inhibition for each peel extract are shown on the graph (Table 5).
No.
Treatments
Conc. (μg/ μl)
(%) Viral inhibition
% Mean value of viral inhibition
H5N1
Rotavirus
H5N1
Rotavirus
1.
OFI70EE
32
23
17
39
35
8
41
39
4
53
49
2.
OFI100EE
32
7
8
19
16.66
8
23
21
4
27
21
3.
OFICE
32
53
47
67.66
54.66
8
72
51
4
78
66
OFI70EE suppressed the H5N1 virus by 23, 41, and 53 %, respectively. For concentrations of 25 g/L, 50 g/L, and 100 g/L, respectively, OFI100EE inhibited the H5N1 virus by 7, 23, and 27 %, while OFICE inhibited the H5N1 virus by 53, 72, and 78 %. OFI70EE, on the other hand, inhibited rotavirus by 17, 39, and 49 %, respectively. For concentrations of 25 g/l, 50 g/l, and 100 g/l, OFI100EE inhibited rotavirus by 8, 21, and 21 %, whereas OFICE inhibited rotavirus by 47, 51, and 67 %. For H5N1 and rotavirus, the mean inhibition rates were 39, 19, 67.66 % and 35, 16.66, 54.66 %, respectively. The results showed that OFICE, at a concentration of 100 g/L, had the greatest inhibitory impact on H5N1 and rotavirus, followed by OFI70EE.
4 Discussion
Medicinal plants are important sources of various bioactive compounds and also the nutrient molecules for the well-being of various organisms (Narayanankutty, 2021b; Narayanankutty et al., 2021c). Plants are rich suppliers of various chemicals, including phenols, flavonoids, alkaloids, phenolic acids, tannins, saponins, anthocyanins, lignans, carbohydrates, carotenoids, and isoflavones, according to a previous study (Rasoulpour et al., 2020). Opuntia ficus-indica is one among the widely utilized plants in Arabian subcontinent. The present study analyzed the antioxidant, antimicrobial, and anticancer activities and phytochemical composition analysis of the plant. There observed high polyphenol content in the plant; corroborating with our results, previous studies also reported the total phenolic content of Opuntia ficus-indica extracts varied from 221.3 to 1501.7 g GAE/100 g dry weight, while the phenolic compounds values of water extracts were 612.10 g GAE/ 100 g (Abou-Elella and Ali, 2014). In addition, the identified compounds such as quercetin, kaempferol, and rutin are reported to possess antiproliferative, anticarcinogenic, and antioxidant activities (Chougui et al., 2013; Illam et al., 2017; Job et al., 2021; Parathodi Illam et al., 2019).
Studies from different authors attributed the inhibitory effect of plant extracts against microbial pathogens to their phenolic composition (Fiad et al., 2020). The inhibitory effect of those phenolics might be explained by adsorption to cell membranes, interaction with enzymes, or deprivation of substrate and metal ions (Fiad et al., 2020; Illam et al., 2017). A great variation in MIC of OFICE as demonstrated in several investigations could be because of the variation in their method of extraction (Moosazadeh et al., 2014). The study observed significant antimicrobial properties of the plant against Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, E. coli, Shigella boydii, and Salmonella typhi; it is therefore possible that the plant could offer strong defense against various bacterial diseases.
Flavonoids are also the most abundant phenolic chemicals in nature, with a wide range of actions, including antiviral activity (Ozçelik et al., 2011). The possible mechanism of antiviral effect could either the interference with viral adsorption or by the inhibition of viral replication. Human rotavirus is a potentially fatal virus that causes severe diarrhea in children under the age of five years, primarily in impoverished countries (Knipping et al., 2012). Influenza virus, particularly H5N1, is also regarded as a major global public health issue. The use of vaccines to combat rotavirus and H5N1 has some drawbacks because they are costly and may be out of reach for developing countries (Cecílio et al., 2012). As a result, new chemicals must be found to be used as alternative strategies to control H5N1 and rotavirus infection. In comparison to natural substances, either as pure compounds or as extracts obtained from plants utilized in conventional medicine, artificial antiviral agents were not superior. Many plants have a diverse range of biological activity, including antiviral properties (Palombo, 2006). To study the antiviral effect of three Opuntia ficus-indica extracts, the cytotoxicity of the tested Opuntia ficus-indica extracts should be determined.
Numerous plant extracts against rotavirus are reported as viral inhibitors (Chingwaru et al., 2011). By limiting viral adsorption, pre-treatment of the cells may provide protective benefits against some viruses (Schnitzler et al., 2008). It's also worth noting that flavonoids can prevent viruses from infecting cells by inhibiting a variety of pathways, similar to how DASM inhibits cell fusion. Meanwhile, flavonoids have been shown to impede virus replication by blocking enzymes like reverse transcriptase and cellular DNA or RNA polymerase (Tang et al., 2012). Evan though many publications have focused on the identification of bioactive compounds, it is important to remember that plants are complex, and a single compound may not be responsible for the observed activity, but rather a combination of minor and major compounds interacting in a preservative or synergistic manner (van Vuuren, 2008). To summarize, Opuntia ficus-indica peel extracts s may have an inhibitory effect on H5N1 and rotavirus, but more research is needed to identify the most potent compounds, their mechanisms of action in animal models, and their application in vivo investigations.
Results of the anticancer activity were consistent with some studies, that flavonoids of OFI possessed many notable biological activities; such as inhibition of lipid peroxidation, oxidation of low-density lipoproteins, anti-carcinogenic activities (Park et al., 2007). The antioxidant effects are due to the major flavonoids encountered in OFI (Kuti, 1992) and flavonoids are more efficient antioxidants than vitamins and OFI have been shown to induce hyperpolarization of the plasma membrane and to raise the intracellular pool of calcium in human Jurkat T-cell lines (Aires et al., 2004), also, the highest anticancer activity of extract (Wu et al., 2004).
Overall, the present study confirms the antimicrobial, antioxidant and anticancer potential of Opuntia ficus-indica. However, further studies using animal models and clinical trials are necessary to validate the application of the plant and bioactive compounds in medicine.
5 Conclusions
The bioactive compounds were extracted from the prickly pear samples by using three solvents: cyclohexanone, 70 % ethanol and100% ethanol, and these compounds were identified by HPLC. These extracts showed antimicrobial effect against pathogenic virus and bacteria, which indicates the possible application as a antimicrobial agent. There also observed significant selective cytotoxicity towards the cancer cells; however, more studies using different cell lines are necessary to use it as an anticancer agent. Optimal exploitation of the prickly pear peel may thus yield various bioactive compounds which can exhibit antioxidant, antimicrobial and antiviral activity. However, further studies using other in vitro and in vivo model experiments are necessary to validate the results of the study and it is also recommended to conduct a thorough biosafety analysis using animal models.
Acknowledgement
The authors acknowledge the support from Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant and anticancer activities of different constituents extracted from Egyptian prickly pear Cactus (Opuntia ficus-indica) Peel. Biochem. Anal Biochem. 2014;3(158)
- [Google Scholar]
- Modulation of intracellular calcium concentrations and T cell activation by prickly pear polyphenols. Mol Cell Biochem. 2004;260(1–2):103-110.
- [Google Scholar]
- Phytochemical analysis, antioxidant, anti-inflammatory, anti-genotoxic and anticancer activities of different ocimum plant extracts prepared by ultrasound-assisted method. Physiol. Mol. Plant Pathol.. 2021;101746
- [CrossRef] [Google Scholar]
- Utilization of prickly pear peels to improve quality of pan bread. Arab. J. Nucl. Sci. Appl.. 2016;49(2):151-163.
- [Google Scholar]
- Antibiotic resistance: a rundown of a global crisis. Infection and drug resistance. 2018;11:1645-1658.
- [CrossRef] [Google Scholar]
- Chemical Composition and Nutritional Analysis of Seeds Cactus (Opuntia ficus-indica.L) Current Nutrition & Food Science. 2019;15(4):394-400.
- [CrossRef] [Google Scholar]
- Screening of Brazilian medicinal plants for antiviral activity against rotavirus. J Ethnopharmacol. 2012;141(3):975-981.
- [Google Scholar]
- Dietary fiber, mineral elements profile and macronutrients composition in different edible parts of Opuntia microdasys (Lehm.) Pfeiff and Opuntia macrorhiza (Engelm.) LWT - Food Science and Technology. 2015;64(1):446-451.
- [CrossRef] [Google Scholar]
- <i>Tylosema esculentum</i> (Marama) Tuber and Bean Extracts Are Strong Antiviral Agents against Rotavirus Infection. Evidence-Based Complementary and Alternative Medicine. 2011;2011:284795
- [CrossRef] [Google Scholar]
- Oil composition and characterisation of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 2013;139(1–4):796-803.
- [Google Scholar]
- Evaluation of antioxidant and antimicrobial properties of Opuntia ficus-indica, seeds and peels extracts. Zagazig Journal of Agricultural Research. 2020;47(2):587-596.
- [Google Scholar]
- Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob. Agents Chemother.. 1980;17(5):865-870.
- [CrossRef] [Google Scholar]
- Polyphenols of virgin coconut oil prevent pro-oxidant mediated cell death. Toxicol Mech Methods. 2017;27(6):442-450.
- [CrossRef] [Google Scholar]
- Desmodium gyrans dc modulates lipid trafficking in cultured macrophages and improves functional high-density lipoprotein in male wistar rats. Indian Journal of Pharmacology. 2021;53(4):286-293.
- [CrossRef] [Google Scholar]
- Biogenic platinum from agricultural wastes extract for improved methanol oxidation reaction in direct methanol fuel cell. J Adv Res. 2020;28:63-75.
- [Google Scholar]
- Borassus flabellifer Linn haustorium methanol extract mitigates fluoride-induced apoptosis by enhancing Nrf2/Haeme oxygenase 1 -dependent glutathione metabolism in intestinal epithelial cells. Drug Chem Toxicol. 2021;1–7
- [CrossRef] [Google Scholar]
- Biochemical changes in grape rootstocks resulted from humic acid treatments in relation to nematode infection. Asian Pac J Trop Biomed. 2012;2(4):287-293.
- [Google Scholar]
- Azima tetracantha Leaf Methanol Extract Inhibits Gastric Cancer Cell Proliferation through Induction of Redox Imbalance and Cytochrome C Release. Applied Sciences. 2022;12(1):120.
- [Google Scholar]
- Opuntia ficus-indica attenuates neuronal injury in in vitro and in vivo models of cerebral ischemia. J Ethnopharmacol. 2006;104(1–2):257-262.
- [Google Scholar]
- An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol J. 2012;9(137):9-137.
- [Google Scholar]
- Antioxidant, Anti-Inflammatory and Anticancer Activities of the Methanolic Extract of Thottea siliquosa: An In Vitro and In Silico Study. Recent Pat Anticancer Drug Discov. 2021;16(3):436-444.
- [CrossRef] [Google Scholar]
- Growth and compositional changes during the development of prickly pear fruit. Journal of Horticultural Science. 1992;67(6):861-868.
- [CrossRef] [Google Scholar]
- Chemical composition and antimicrobial activity of Opuntia stricta F. essential oil. Journal of Biodiversity and Environmental Sciences (JBES). 2014;4(5):94-101.
- [Google Scholar]
- Natural Products as PI3K/ Akt Inhibitors: Implications in Preventing Hepatocellular Carcinoma. Curr Mol Pharmacol 2021
- [CrossRef] [Google Scholar]
- Pharmacological potentials and Nutritional values of Tropical and Sub-tropical Fruits of India: Emphasis on their anticancer bioactive components. Recent Pat Anticancer Drug Discov 2021
- [CrossRef] [Google Scholar]
- Targeting Toll like Receptors in Cancer: Role of TLR natural and synthetic modulators. In: Curr Pharm Des In Press. 2020.
- [Google Scholar]
- Chemical Composition of Cinnamomum verum Leaf and Flower Essential Oils and Analysis of Their Antibacterial, Insecticidal, and Larvicidal Properties. Molecules. 2021;26(20)
- [CrossRef] [Google Scholar]
- Mango ginger (Curcuma amada Roxb.) rhizome essential oils as source of environmental friendly biocides: Comparison of the chemical composition, antibacterial, insecticidal and larvicidal properties of essential oils extracted by different methods. Environ Res. 2021;202:111718
- [CrossRef] [Google Scholar]
- Analysis of the chemical composition of root essential oil from Indian Sarsaparilla (Hemidesmus indicus) and its application as an ecofriendly insecticide and pharmacological agent. Saudi journal of biological sciences 2021
- [CrossRef] [Google Scholar]
- Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm Biol. 2011;49(4):396-402.
- [Google Scholar]
- Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res. 2006;20(9):717-724.
- [Google Scholar]
- Natural combination of phenolic glycosides from fruits resists pro-oxidant insults to colon cells and enhances intrinsic antioxidant status in mice. Toxicol. Rep.. 2019;6:703-711.
- [CrossRef] [Google Scholar]
- Flavonoids from the stems of eastern picklypear Opuntia humifusa. Cactaceae. Journal of Applied Biological Chemistry. 2007;50(4):254-258.
- [Google Scholar]
- Opuntin B, the antiviral protein isolated from prickly pear (Opuntia ficus-indica (L.) Miller) cladode exhibits ribonuclease activity. Microb Pathog. 2020;140(103929):14.
- [Google Scholar]
- A comparative study on quantitative estimation of tannins in Terminalia chebula, Terminalia belerica, Terminalia arjuna and Saraca indica using spectrophotometer. Asian J. Pharm. Clin. Res.. 2013;6(3):148-149.
- [Google Scholar]
- Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Phytomedicine. 2008;15(12):1108-1116.
- [Google Scholar]
- Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants. 2017;6:62-69.
- [Google Scholar]
- Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern Med. 2012;12(3):1472-6882.
- [Google Scholar]
- Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med Microbiol Immunol. 1975;162(1):23-27.
- [Google Scholar]
- Antimicrobial activity of South African medicinal plants. J Ethnopharmacol. 2008;119(3):462-472.
- [Google Scholar]
- Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52(12):4026-4037.
- [Google Scholar]
- Wide-scale evaluation of Origanum munzurense Kit Tan & Sorger using different extraction techniques: Antioxidant capacity, chemical compounds, trace element content, total phenolic content, antibacterial activity and genotoxic effect. Flavour and Fragrance Journal. 2020;35(4):394-410.
- [CrossRef] [Google Scholar]
- Evaluation of chemical composition, trace element content, antioxidant and antimicrobial activities of Verbascum pseudoholotrichum. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology. 2020;1–10
- [CrossRef] [Google Scholar]







