Translate this page into:
Evaluation of antiulcer potential of tambulin and ombuin isolated from Zanthoxylum armatum
⁎Corresponding authors. nasiratksu@gmail.com (Nasir A. Siddiqui), nsiddiqui@ksu.edu.sa (Nasir A. Siddiqui), khan.zulfanooreen7860@gmail.com (Zulfa Nooreen),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Abstract
Objectives
Zanthoxulym armatum fruit is traditionally used as carminative, anthelmintic, stomachic, and to relieve gastritis. It is commonly found in Nepal, Uttarakhand, and the Himalayan region of India. Tribal peoples used this fruit for various purposes and also as a spice ingredient. The present study aims to investigate the antiulcer potential of the fruit extract, and its isolated compounds.
Materials and methods
Fruits were extracted with successive extraction methods with hexane, ethyl acetate, and butanol. All the fractions were evaluated for phytochemical investigations. Further, the ethyl acetate fraction, tambulin, and ombuin were tested for antiulcer activity induced by ethanol and pylorus ligation-induced methods. The IL-1β, TNF-α, and IL-6 cytokines were also studied followed by histopathology.
Results
It has been demonstrated that the ethyl acetate fraction possesses a major class of secondary metabolites. No acute toxicity was observed, as all the organs (heart, kidney, lung, stomach, and liver) were found in normal range and no significant weight variation was found. The antiulcer activity was performed at two different doses of all samples. Tambulin possesses the most significant activity at the dose of 50 mg/kg. Ulcer index and percent ulcer inhibition of tambulin were found to be 2.8 ± 0.6 mm2 and 69.5 ± 0.18 % in pylorus –ligated model whereas 5.2 ± 0.7 mm2 and 70.2 ± 0.15 in the ethanol-induced model. Histopathology results also support the above-mentioned data.
Conclusion
Tambulin, a flavonoidal compound isolated from fruits of Z. armatum was found to be useful in the management of ulcers. Ethylacetate extract and ombuin also possess dose-dependent activity.
Keywords
Zanthoxulym armatum
Tambulin
Ombuin
Flavonoids
Antiulcer activity
1 Introduction
A frequent gastrointestinal illness that affects numerous individuals is peptic ulcer. Generally, this involves a damage in the outer layer of digestive tract’s membrane lining. Disturbance in normal equilibrium leads to enhancement in aggressiveness or reduction in mucosal resistance causing ulceration. It might be brought on by consistent drug use, strange eating patterns, stress, and other factors (Vimala et al., 2014). It is a non-fatal condition that mostly manifests as periodic indications of epigastric pain, which are frequently eased by meals or alkali, in addition to allowing patients to experience significant discomfort, disruption of their regular lives, and emotional distress (Hamedi et al., 2015). Although the prevalence of peptic ulcer has decreased recently, the condition still has a significant economic burden and causes morbidity and death. Proton pump inhibitors, histamine −2 receptor blockers, and prostaglandin analogs are effective medication families for treating peptic ulcers (Bhajoni et al., 2016). Plants and their components have long been utilized in folklore medicines across the globe to heal a variety of illnesses and disorders. Today, herbal therapy is supplanting synthetic medications sold commercially as an effective alternative for managing and curing peptic ulcers (Andrade et al., 2007). Modern healthcare seeks traditional systems to discover treatments and cures for many disorders. In many countries, specific plant components have been used for decades to treat illnesses. The quest for novel medications has switched due to the numerous adverse effects associated with utilizing mainstream antiulcer medications. Currently, several plants and their components have been investigated. The utilization of herbal medicine has increased dramatically during the past several years. Due to their natural origins and lack of negative side effects, this sector has become more popular in developing and developed nations.(See Fig. 1).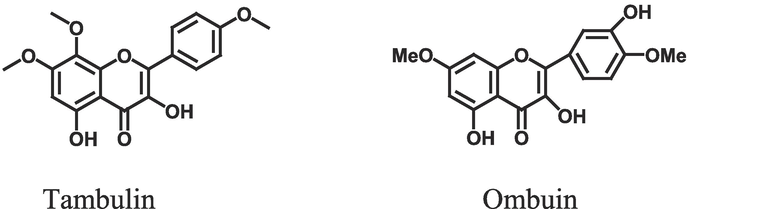
Chemical structures of compounds.
Zanthoxylum armatum DC. is a pungent, aromatic, entirely glabrous, and small tree situated in hot valleys of subtropical Himalayas in India while in Pakistan it spread in Swat, Hazara, and Poonch hills. The plant is also popularly known as ‘Nepali Dhania’, ‘Tomar’, ‘Timoor’, and ‘Timbar’. The seeds and bark are employed as an aromatic tonic to treat fever, cold, indigestion, and cholera.
The fruit along with its stems and thorns, is used as a fish poison, as well as a stomachic and carminative (Phuyal et al., 2019). In last few years, many chemical compounds have been investigated and reported. The twig is traditionally used for teeth cleansing, also toothpowder was prepared. Apart from this, various other pharmacological activities are also reported in the literature (Singh et al., 2011), Many indigenous medical systems have employed various Z. armatum components, including the fruits, roots, leaves, and bark, to treat gastric problems, fever, and as appetizers. It works well to reduce swelling, dental pain, and stomach discomfort. Due to its pharmacological benefits, it is in high demand in domestic and international markets (Rafique et al., 2023). Timur has long been utilized for treating many illnesses, including irritation, chills, headache, and stomach discomfort (Nooreen et al., 2019). Alkaloids, flavonoids, tannin, resins, glycosides, and other groups of compounds are found in the Z. armatum fruit, and pharmacological activities like antiplasmodial, cytotoxic, anthelminthic, antifungal, antibacterial, hepatoprotective, antioxidant, and larvicidal, have been reported (Negi et al., 2011). Z. nitidum root water extract inhibits H. pylori urease in dose-dependent manner which is necessary for the survival of bacteria at IC50 1.29 mg/mL (Lu et al., 2020), while ethanolic extract of root bark of Z. zanthoxyloides reduces gastric ulceration induced by indomethacin by 71 % and 85 % at a dose of 250 and 500 mg/kg, respectively (Boye et al., 2012). Traditionally the plant is reported to be used for stomachic, carminative, and anti-inflammatory profiles. The Ayurvedic Pharmacopoeia of India (API) reported that the plant is useful for treating stomach-related problems, dental care, balance vata, and Kapha roga (Anonymous, 2016). The present work is proposed to investigate the ulcer protective potential of the plant extract and the isolated compounds (flavonoids). Here, we selected the two compounds tambulin and ombuin,as they are present in a higher amount than other components.
2 Material and method
2.1 Chemical and reagent
All the solvents were purchased from Merck. The biochemical assessments were done in triplicate, and the results were expressed as mean ± standard deviations.
2.2 Plant material collection and preparation
The Zanthoxylum armatum fruits were purchased from Lucknow, Uttar Pradesh, and authenticated by the Botany and Pharmacognosy department in CSIR-CIMAP, Lucknow, with the specimen number ZA/F/14.
2.3 Extraction and isolation
Dried powder was extracted by methanol and further processed with hexane, ethyl-acetate, and n-butanol. Compound tambulin and ombuin were previously isolated from the hexane fraction. The isolation procedure comprises successive column chromatography and is further elucidated with the help of NMR, IR, Mass Spectroscopy, and HPLC methods. The detailed isolation procedure of both compounds is mentioned in Nooreen et al., 2017.
2.4 Phytochemical analysis
To know the major groups of chemicals such as flavonoids, saponins, phenols, steroids, tannins, glycosides, and alkaloids present in the extracts, confirming qualitative phytochemical examinations of plant materials was carried out using established techniques (Dubale et al., 2023).
2.4.1 Test for alkaloids
Hydrochloric acid (5 mL) was individually mixed with each sample before filtering and then used to examine further.
-
Mayer’s Test
A few drops of Mayer's reagent were added with a 2 mL of the supernatant (sample) in a test tube and the colour change of the precipitate was observed indicating the presence of alkaloids.
-
Wagner’s Test
2 ml of the filtrate was combined using a couple of droplets of wagner's reagent in the test tube,a mixture of iodine in potassium iodide. The reddish-brown precipitate confirms the presence of alkaloids
-
Hager's test
2 ml of filtrate and 2 to 3 drops of Hager's solution were added to the test tube and it was noted that the precipitate seemed yellow
2.4.2 Test for tannin and phenolic compounds
-
Ferric Chloride Test
The existence of phenols and tannins is shown by the production of a bluish-black colour when the sample was treated with a few drops of ferric chloride solution.
-
Gelatin Test
Gelatin (1 %) solution with sodium chloride (10 %) was treated with the sample. The emergence of a precipitate shows the existence of phenols and tannins.
-
Iodine Test
When the sample was mixed with dilute iodine solution, a transient red colour appeared, this confirms the presence of phenolic and tannin molecules.
2.4.3 Test for flavonoids
-
NaOH and Acid Test
A small amount of sodium hydroxide was added in the sample, and an intense yellow colour appears and becomes colourless by adding a few drops of dilute HCl confirming the existence of flavonoids.
-
Lead Acetate Test
The sample is added with a solution of lead acetate. After a few minutes yellow precipitate was observed that indicates the existence of flavonoids.
2.4.4 Test for glycosides
-
Borntrager's test
1 gm of sample with 5–10 mL of HCL was heated for 10 minutes in the water bath. A similar amount of CCl4 and ammonia mixture was incorporated after getting the filtrate with continuous stirring. The assessment was made to turn the pink solution into red
Modified Borntrager's test.
1 gm sample was heated with dilute 5 mL HCL and 5 mL ferric chloride. The solution was then cooled and filtered. Take filtrate and add 1 mL of each ammonia and benzene solution. The pink colour changes into red, indicating the presence of glycosides
2.4.5 Test for carbohydrate
-
Molischʹs test
Some droplets of Molisch reagent have been mixed with 2 mL of the sample's water-based extract. Slow addition of 1 mL of H2SO4 (Conc.) was done by the side wall of the test tube to create a layer. The comment was made depending on the purple colour intensity.
-
Barfoed’s test
The test solution was heated for 2 min in the water bath after adding Barfoed’s reagent. The occurrence of red precipitate served as the basis for the finding.
2.4.6 Test for saponin
-
Foam test
In a graduated cylinder, 20 mL of water and 1 gm of sample were combined. It was agitated for fifteen to twenty minutes. Based on the development of a stable form, an assessment was concluded.
2.4.7 Test for steroids
-
Salkowski test
Carefully mixed 3 mL conc. Sulphuric acid to a 5 mL sample and 2 mL chloroform, to demonstrate that terpenoids were present, and a reddish-brown colour at the interface appeared.
2.5 Animals
Male Wistar albino rats weighing between 170–220 gm were obtained from the animal house of PSIT, Kanpur by the Institutional Animal Ethics Committee (IAEC Before the studies animals were housed for one week at 26 ± 2 ◦C in the departmental animal house with light and dark cycles of 10 and 14 h, respectively. The animals were fed a normal rat pellet diet (Amrut, India) and the food was removed 18–24 h before the experiment; however, water was available ad libitum (Sini et al., 2011; Somagari et al., 2014). The experiment has been performed with the permission and approval of CPCSEA approved (Reg. no. 1273/PO/RE/09/CPCSEA), Government of India.
2.6 Acute toxicity
Ethyl acetate fraction of methanol extract (here onwards called as ethyl acetate extract) and isolated pure compounds (Tambulin and Ombuin) were assessed at the highest doses, orally. The dose of ethyl acetate extract (2000 mg/kg) and pure compounds (150 mg/kg) were selected for acute toxicity study. Health problems i.e. weight variation, eye redness, and behavioural changes were recorded up to 48 hrs of dose administration. All the vital organs (heart, liver, stomach, lung, and kidney) were closely evaluated and weighed during the autopsy. The water as the vehicle is administered to the normal control group, and the above-mentioned doses to the treated groups (Beaufay et al., 2017).
2.7 In-vivo antiulcer activity
This study aimed to evaluate the protective potential of ethyl acetate extract and isolated pure compounds against stomach ulcers caused by pylorus-ligation and ethanol-induced ulcers. The researchers induced gastric ulceration in animals by administering ethanol (1 mL/200 g b.w.) orally and by pylorus ligation using pentobarbitone (35 mg/kg i.p.).
2.7.1 Ethanol-induced ulcer
Disease control (ethanol), positive control (ranitidine) and, test samples {ZA/Ea, ethylacetate extract (250 mg/kg); ZA/Eb, ethylacetate extract (500 mg/kg); ZA/1a, tambulin (25 mg/kg); ZA/1b, tambulin (50 mg/kg); ZA/2a, ombuin (25 mg/kg); ZA/2b, ombuin (50 mg/kg)} were given to the rats twice a day orally for 5 days prior to ulcer induction. According to the method described by Hollander et al., 1985, rats were administered with ethanol orally on 6th day (1 mL/200 g, 1 h) to induce uniform gastric ulcers and ranitidine was given as positive control at the dose of 50 mg/kg, orally. After the rats were euthanized, their stomachs were examined along the greater curve to assess the presence of ulcers. To determine the ulcer index, the length and width of ulcers in the glandular part of the stomach were multiplied (mm2/rat). This method provides a reliable and efficient way of analysing the effects of ethanol on the development of gastric ulcers in rats. Ulcer index protection was determined by the following formula:
2.7.2 Pylorus ligated- ulcers
Disease control (induced due to accumulation of hydrochloric acid), positive control (ranitidine) and test samples, {ZA/Ea, ethylacetate extract (250 mg/kg); ZA/Eb, ethylacetate extract (500 mg/kg); ZA/1a, tambulin, (25 mg/kg); ZA/1b, tambulin (50 mg/kg); ZA/2a, ombuin (25 mg/kg); ZA/2b, ombuin (50 mg/kg)} were given to the rats for 5 days, and then they were kept in a cage for 18 h without food. Pentobarbitone (35 mg/kg, i.p.) was used for sedation of animals. The belly was then opened and the pylorus was tied off without hurting the animals' blood supply. It was carefully brought back in place, and the wall of the belly was closed in two layers with stitches that were broken so often. After the surgery, the animals were not given any water (Shay et al., 1945). After 4 h, the animals were sacrificed, and the stomach was carefully taken out and cut along the larger curve. It was then carefully washed with 5.0 mL of 0.9 % NaCl, and ulcers were scored in the glandular part of the stomach by someone who wasn't part of the experiment. The ulcer index was estimated by adding the number of ulcers on each stomach and how bad each ulcer was. The method given by Sanyal et al. (1982) was used to figure out the ulcer score for the whole group.
2.8 Measurement of inflammatory cytokines
2.8.1 IL-1β, TNF-α, and IL-6
Were measured using an ELISA kit within 2 hrs, and the concentration was calculated by following the standard procedure. Leukocyte infiltration was observed in the deep mucosal layer. A homogenate was prepared from the detached scarred fraction. The supernatant collected from the homogenate was used for further studies of interleukins (Almasaudi et al., 2016).
2.9 Histopathology
Histopathology was examined by rats' stomach which were fixed in 10 % neutral formalin, then embedded in paraffin blocks. The eosin and hematoxylin stains were used for the section visibility. The degree of stomach damage was determined using the standard procedure mentioned in MICRON desquamative changes, mucosal defects of varying depths, and the degree of infiltrative changes.
2.10 Gastric wall mucus measurement
Corne et al. (1974) developed a method for measuring gastric wall mucus in ethanol-induced ulcerated mice. Glandular segments from stomachs were isolated, weighed, and then incubated for 2 h in a 1 % Alcian blue solution (0.16 M sucrose in 0.05 M sodium acetate, pH 5.8) in test tubes. After centrifuging (100 g) the Alcian blue binding extract for 10 min, the absorbency of the supernatant was determined at 498 nm. The amount of Alcian blue taken from the glandular tissue was then determined (µg/g of glandular tissue).
2.11 Statistical analysis
The obtained data were processed to determine the significant difference between the groups. Standard mean and error were optimized. To summarise the results, a one-way analysis of variance (ANOVA) was employed. Version 5.01 of GraphPad PRISM® (GraphPad Software, Inc., USA) was used. The value p < 0.05 was regarded as a significant indication.
3 Results
3.1 Phytochemical investigation
The freshly prepared hexane, ethyl acetate, and butanol extracts of Z. armatum fruits were subjected to chemical tests for alkaloids, tannins, flavonoids, glycosides, carbohydrates, saponins, and steroids. It is found that the extracts possess a wide range of phytochemicals in all the above mentioned three extracts as mentioned in Table 1. -Negative; + Positive.
S. No
Phytochemical Tests
Observations
Hexane extract (ZA/H)
Ethyl acetate extract (ZA/E)
Butanol extract (ZA/B)
1.
Test for alkaloids
Mayerʹs test
−
+
+
Wagnerʹs test
−
+
−
Hagerʹs test
−
+
−
2.
Test for tannins
Ferric chloride test
+
−
+
Gelatin test
+
+
−
Iodine test
−
+
+
3.
Test for flavonoids
NaOH and Acid test
+
+
+
Lead acetate test
+
+
+
4.
Test for glycosides
Borntrager's test
−
+
+
Modified Borntrager's test
−
+
+
5.
Test for carbohydrates
Molisch’s test
+
+
+
Barfoed’s test
−
+
+
6.
Test for saponins
Foam test
−
−
−
7.
Test for steroids
Salkowski test
−
−
+
3.2 Extraction, isolation and characterization of compounds
Tambulin and ombuin mentioned in Fig. 1 were isolated from Z. armatum fruits as mentioned by Nooreen et al. (2017).
3.3 Estimation of acute toxicity
The results of the acute toxicity study witness no behavioural changes and no significant weight variation of vital organs at the highest doses (2000 mg/kg of extract and 150 mg/kg dose of pure compounds) tested for the purpose. This confirms that the extract (2000 mg/kg) and the compounds (150 mg/kg) are safe to use up to the above-mentioned tested doses. The results details are given in Table 2 and Fig. 2.
Sample
Heart
Liver
Stomach
Lung
Kidney
Normal
1.03 ± 0.1
7.25 ± 0.2
3.30 ± 0.1
1.28 ± 0.1
1.12 ± 0.2
ZAE
1.11 ± 0.1
7.12 ± 1.1
3.25 ± 0.1
1.29 ± 0.2
1.26 ± 0.1
ZA/1
1.15 ± 0.1
6.95 ± 1.3
3.23 ± 0.2
1.13 ± 0.1
1.25 ± 0.2
ZA/2
1.01 ± 0.1
7.43 ± 1.2
3.33 ± 0.2
1.39 ± 0.2
1.13 ± 0.2
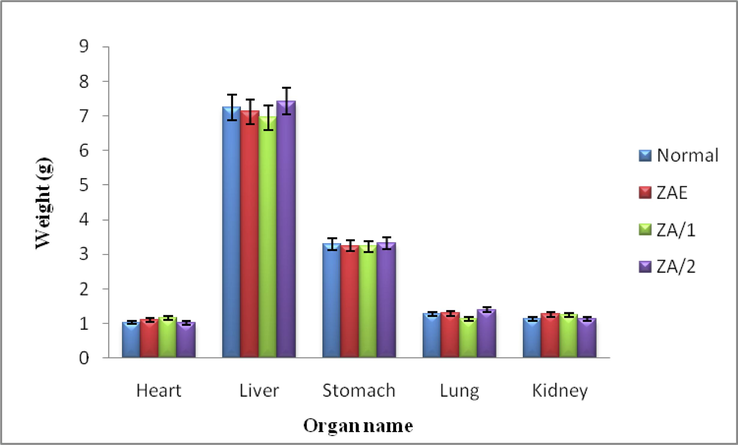
Weight of normal vital organs and treated rat’s organs showing the effect of, Zanthoxylum armatum extracts (ZAE), tambulin (ZA/1), and ombuin (ZA/2). Data indicated mean ± SD, n = 6.
3.4 In-vivo anti-ulcer activity
3.4.1 Ethanol (EtOH) −induced ulcer and pylorus ligated (PL)- caused ulcers
It has already been reported that the ethanol induced ulcers located in the glandular region of the stomach enhance the production of leukotrienes, secretory products, mast cells (Oates and Hakkinenet al., 1988), and reactive oxygen species (Peskaret al., 1986). All the above factors are mainly responsible for the gastric mucosal damage in the rats. Tambulin considerably reduced the loss of gastrointestinal mucus caused by ethanol. Gastric mucosal autodigestion and barrier disintegration are blamed for pylorus ligation-induced ulcers (Sairamet al., 2002). Both ethanol and pylorus ligation-induced ulcers were prevented by the tambulin (ZA/1b) at 50 mg/kg by 70.20 % and 69.50 % respectively as mentioned in Table 3 and Table 4.
Group
Treatment
Ulcer index mm2
% Ulcer inhibition
I
Normal control
−
−
II
Negative control
26.5 ± 0.02
−
III
Positive control
2.4 ± 0.13
78.5 ± 0.06
IV
ZA/Ea (250 mg/kg)
17.4 ± 0.13
49.3 ± 0.13
V
ZA/Eb (500 mg/kg)
13.6 ± 0.14
56.4 ± 0.12
VI
ZA1a (25 mg/kg)
8.5 ± 0.2
64.1 ± 0.13
VII
ZA1b (50 mg/kg)
5.2 ± 0.7
70.2 ± 0.15
VIII
ZA2a (25 mg/kg)
9.4 ± 0.6
59.3 ± 0.18
IX
ZA2b (50 mg/kg)
7.3 ± 0.2
67.5 ± 0.13
Group
Treatment
Ulcer index mm2
% Ulcer inhibition
I
Normal control
−
−
II
Negative control
18.4 ± 0.16
−
III
Positive control
2.5 ± 0.2
75.5 ± 0.12
IV
ZA/Ea (250 mg/kg)
8.4 ± 0.15
47.4 ± 0.09
V
ZA/Eb (500 mg/kg)
5.2 ± 0.3
58.6 ± 0.13
VI
ZA1a (25 mg/kg)
5.2 ± 0.1
63.3 ± 0.11
VII
ZA1b (50 mg/kg)
2.8 ± 0.6
69.5 ± 0.18
VII
ZA2a (25 mg/kg)
6.3 ± 0.4
59.4 ± 0.15
IX
ZA2b (50 mg/kg)
4.2 ± 0.3
62.6 ± 0.12
3.5 Measurement of IL-1β, TNF-α, and IL-6 level
The experiments involved an analysis of inflammatory cytokines IL-1β, TNF-α, and IL-6 in both ethanol and pylorus-induced ulcer models. The cytokines were estimated from the supernatant within 2 hrs using ELISA kits following the given instructions. The results were then compared to negative control, positive control, and treated groups. A significant increase was observed in the toxin group. However, the extract and compound showed a dose-dependent reduction in inflammation, as shown in Figs. 3 and 4. The compound tambulin at a dose of 50 mg/kg (ZA/1b) exhibited marked protection of ulcers and maximum inhibitory activity of cytokines in ethanol-induced and pylorus ligation-induced ulcers. The levels of TNF-α, IL-6, and IL-1β were found to be 307.4 ± 5, 138.8 ± 2, and 46.4 ± 0.2 pg/mL, respectively in ethanol-induced ulcers whereas in pylorus-ligated ulcers it was estimated as 326.4 ± 16, 134.2 ± 9, and 39.2 ± 3 pg/mL for the tambulin (50 mg/kg, ZA/1b), as mentioned in Table 5 and Figs. 3 and 4. Data are mean ± SEM; N = 6, Normal verses toxin, # Toxin verses test samples (ANOVA; Tukey test), p < 0.05.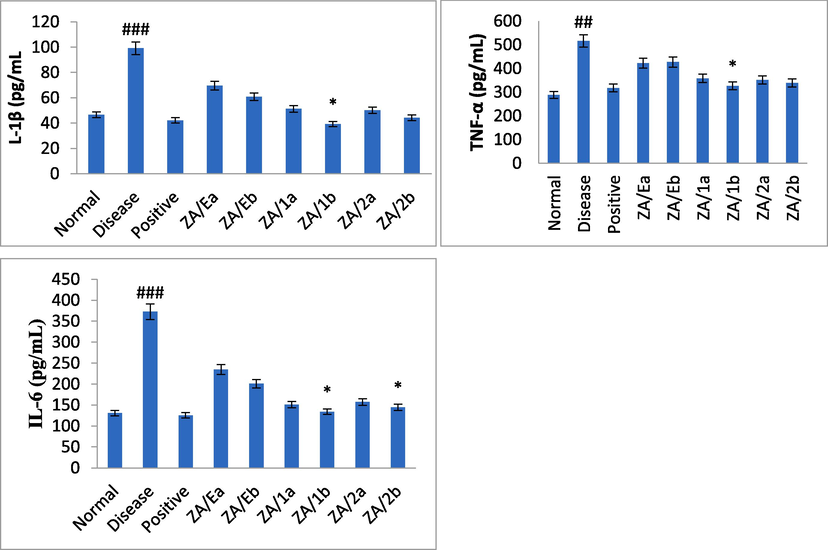
Effect of samples on inflammatory mediators in ethanol induced ulcer model. Data presented as mean ± SEM; n = 6 #Normal versus disease control, *Disease control versus treated, ANOVA, p < 0.05.
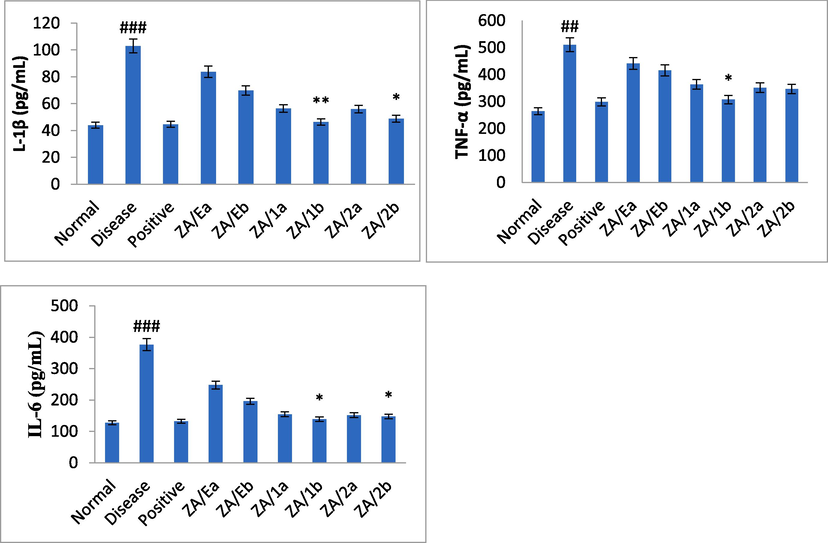
Effect of samples on inflammatory mediators in pylorus ligation induced ulcer model. Data presented as mean ± SEM; n = 6 #Normal versus disease control, *Disease control versus treated, ANOVA, p < 0.05.
Samples
Ethanol-induced ulcer
Pylorus-induced model
TNF-α(pg/mL)
IL-6(pg/mL)
IL-1β(pg/mL)
TNF-α(pg/mL)
IL-6(pg/mL)
IL-1β(pg/mL)
Normal
288.4 ± 11
130.6 ± 4
46.6 ± 1
264.2 ± 5
127.6 ± 2
44 ± 0.5
Disease
516.2 ± 25###
373 ± 7###
99.2 ± 3###
510.2 ± 16###
376.4 ± 1.5###
103 ± 2.5###
Positive
318 ± 15
125.4 ± 2
42.2 ± 2
298.2 ± 11
132.2 ± 5
44.6 ± 0.5
ZA/Ea
423 ± 17
234.8 ± 2
69.6 ± 5
440.8 ± 11
247.6 ± 4
83.8 ± 1.2
ZA/Eb
427.2 ± 15
201 ± 2
60.8 ± 3
415.6 ± 14
196 ± 4
69.8 ± 1.6
ZA/1a
363.8 ± 5
154.6 ± 4
56.4 ± 1.4
358.2 ± 7
151.2 ± 3
51.2 ± 3
ZA/1b
307.4 ± 5*
138.8 ± 2*
46.4 ± 0.2*
326.4 ± 16*
134.2 ± 9*
39.2 ± 3**
ZA/2a
351.2 ± 7
151.8 ± 2
56 ± 1.7
351.4 ± 16
157.2 ± 3
50.2 ± 4
ZA/2b
346.2 ± 5
147.6 ± 1.5*
48.8 ± 1.1
338.4 ± 21
144.6 ± 3*
44.2 ± 2*
3.6 Histopathology
Histopathological research was conducted on the stomachs’ slices of rats (Fig. 5A) to study the effects of pylorus-ligated ulcer as it showed maximum inhibition in the treatment groups of tambulin. The stomach slices from the control group showed normal anatomy with all layers intact. However, in negative and treated groups, it caused a decrease in the thickness of the mucous layer due to the erosion of the top epithelial cells. This led to the dysfunction of stomach glands in the mucosal layer, significant swelling, infiltration of inflammatory cells in the submucosa, and thinning of the muscular layer. On the other hand, tambulin was found to have a beneficial effect on the stomach of the animals. Histopathological findings revealed a normal glandular pattern with mild submucosal swelling in animals given tambulin (50 mg/kg). Moreover, when tambulin (50 mg/kg) was given to ulcerated mice, their stomachs were better protected than when they were given ombuin (50 mg/kg). The stomachs in this group showed almost normal structure with no epithelial loss, submucosal swelling, or leukocyte infiltration. The mucosal, submucosal, and muscle layers also returned to normal thickness as seen in Fig. 5B.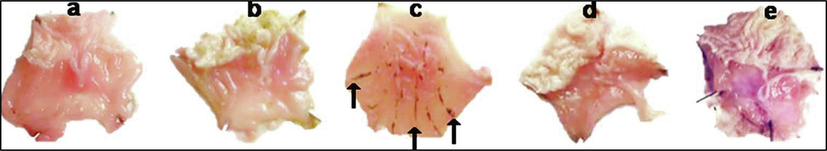
Pictures showing the effect of samples on stomach sections of pylorus ligated Wistar rat (a) Normal control, (b)positive control, (c) disease control (d) tambulin 25 mg/kg (ZA/1a), (e) tambulin 50 mg/kg (ZA/1b).
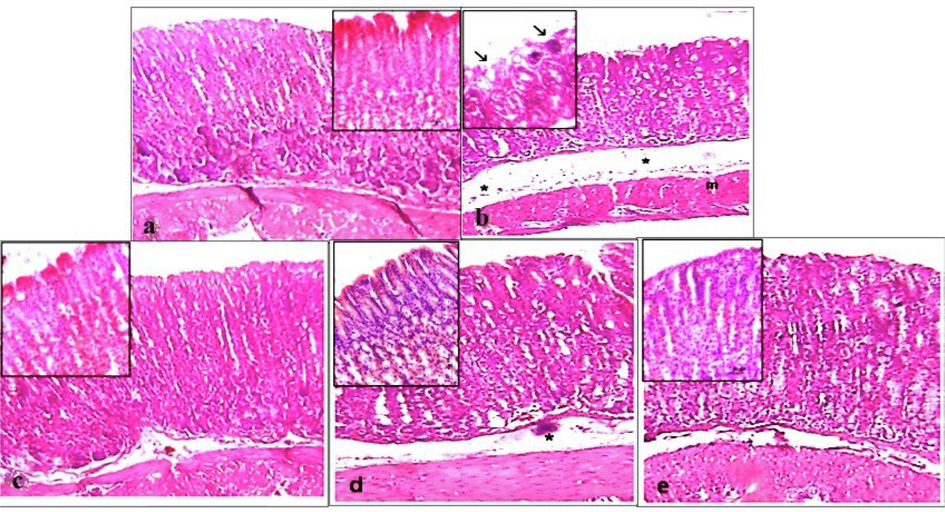
Histopathology of samples on stomach sections of pylorus ligated Wistar rat (a) Normal control, (b) disease control, (c) positive control (d) tambulin 25 mg/kg (ZA/1a), (e) tambulin 50 mg/kg (ZA/1b).
4 Discussion
The proposed study was based on the evaluation of the anti-ulcer potential of tambulin and ombuin, extracted from Z. armatum fruits. This fruit is commonly used as a toothache remedy and found in the subtropical Himalayan region of India, China, Bhutan, Malaysia, Japan, and Pakistan. According to various literature studies, the plant is safe and effective for pharmaceutical use (Ranjana et al., 2019). As described by Bandyopadhyay et al., 2001, peptic ulcers occur when there is an imbalance between endogenous aggressive factors (e.g.pepsin, leukotrienes, hydrochloric acid, and refluxed bile, etc.) and cytoprotective factors (e.g.mucus-bicarbonate barrier, enzymatic antioxidants, growth factors, cell renewal, and migration, etc.). It is now well known that nonsteroidal anti-inflammatory drugs such as aspirin and piroxicam have several detrimental effects on the gastrointestinal system. However, several flavonoids have been found to have curative properties for peptic ulcers without any adverse effects (Lira Mota et al., 2009).
Polyphenols, a class of flavonoids present in plants, have been found to have gastroprotective properties against peptic ulcers in both in-vitro and in-vivo studies. The probable mechanism is the stimulation of the defense mechanisms (such as the production of mucus and prostaglandins) and protection against potentially harmful substances through their antioxidative, anti-inflammatory, and antibacterial properties. Flavonoids have shown cytoprotective and rehabilitative effects. Although, there are currently few controlled clinical trials have reported that flavonoids demonstrated promising preventive and therapeutic potential for peptic ulcers (Zhang et al., 2020). Studies have also shown that flavonoids reduce stomach acidity in peptic ulcer patients. Citrus fruits contain a rich source of flavonoid called hesperidin, which was found to significantly reduce stomach ulcers caused by indomethacin in rats when added to gastric juice. In a model of hypothermic restraint stress-induced stomach ulcers, hesperidin at doses of 300 and 400 mg/kg considerably reduced the ulcer index, decreased the overall acidity of gastric juice, and raised pH (Bigoniya et al., 2014). Isorhamnetin, quercetin 3,7-dimethyl ether, and kaempferol 3,7-dimethyl ether, isolated from Cistus laurifolius, and irisolidone, tectorigenin, and genistein from the flowers and rhizomes of Pueraria thunbergiana, inhibited the growth of H. pylori in in-vitro studies (Bae et al., 2001). Musa sapientum, a plant, contains monomeric leucocyanidin, a naturally occurring flavonoid that appears to possess anti-ulcer properties. When administered prophylactically at doses of five milligrams and fifteen milligrams per day, it demonstrated protective properties towards aspirin-induced gastric damage, resulting in a significant reduction in the ulcer index (Lewis et al., 1999). In a restraint stress paradigm, it also lowers plasmatic corticosterone and lipid peroxidation. This flavonoid preserves the stomach mucosa in chronic models of ulcer formation and promotes the healing of gastric ulcers caused by acetic acid, a chronic model of ulcer induction (Motilva et al., 1992). The preliminary phytochemical analysis of hexane, ethyl-acetate, and butanol extract was assessed and found that the ethyl-acetate fraction possesses maximum secondary metabolites as compared to hexane and butanol extracts. Based on phytochemical and preliminary screening for biological activity ethyl acetate extract was selected for in-vivo study.
During the study, the ulcerated animals showed characteristic histopathological changes, such as a reduction in mucosal thickness, weakening of the muscle layer, erosion of the surface epithelial cells, loss of integrity in the stomach mucosa, submucosal edema, and infiltration of inflammatory cells. The tested compounds (Tambulin & Ombuin) showed dose-dependent protection of ulcerated mice but tambulin at 50 mg/kg showed significant protection as compared to ombuin. The overall effectiveness of tambulin over ombuin might be related to structural activity relationship and an additional methoxy group at carbon eight (C-8) of chromene nucleus may be responsible for better antiulcer effect of tambulin but it needs further investigations to confirm this hypothesis.
5 Conclusion
The study found that the ethyl acetate extract contains a high amount of bioactive phytomolecules such as glycosides, flavonoids, tannins, alkaloids, and carbohydrates that have medicinal properties. The fruit of Z. armatum can potentially prevent stomach ulcers caused by ethanol and pylorus ligation. In the pylorus-ligated model, the ulcer index and percent inhibition of tambulin are 2.8 ± 0.6 mm2 and 69.5 ± 0.18 %, respectively, while in the ethanol-induced model, the values are 5.2 ± 0.7 mm2 and 70.2 ± 0.15 %. The study also observed a reduction in the level of inflammatory cytokines (IL-1β, TNF-α, and IL-6), indicating that the plant has antiulcer activity. The compound tambulin, at a dose of 50 mg/kg, demonstrated significant activity which is additionally supported by histopathological findings.
CRediT authorship contribution statement
Nasir A. Siddiqui: Writing – review & editing, Funding acquisition, Conceptualization. Zulfa Nooreen: Methodology, Conceptualization. Pranay Wal: Software, Investigation, Conceptualization. Anil K. Yadav: Supervision, Investigation, Data curation. Omer I Fantoukh: Writing – review & editing, Supervision, Data curation. Saleh I. Alqasoumi: Writing – review & editing, Resources, Formal analysis. Ateeque Ahmad: Validation, Software, Project administration. Arhama Nasir: Writing – original draft, Methodology.
Acknowledgment
The authors of this study are highly thankful to the “Researchers Supporting Project” number (RSPD2024R981), King Saud University, Riyadh, Saudi Arabia, for supporting the current study and funding the research work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Almasaudi, S.B., El-Shitany, N.A., Abbas, A.T., Abdel-dayem, U.A., Ali, S.S., Al Jaouni, S.K., 2016. Antioxidant, Anti-inflammatory, and Antiulcer Potential of Manuka Honey against Gastric Ulcer in Rats. Oxid. Med. Cell. Longev. 1-10. Article ID 3643824, .
- De Andrade, S.F., Lemos, M., Comunello, E., Noldin, V.F., Cechinel Filho, V., Niero, R., 2007. Evaluation of the antiulcerogenic activity of Maytenus robusta (Celastraceae) in different experimental ulcer models. J. Ethnopharmacol. 113, 252–257. doi: 10.1016/j.jep.2007.06.002.
- Anonymous. The Ayurvedic Pharmacopoeia of India, Part-I, Vol IV, First Edition, Ministry of AYUSH, Government of India, New Delhi, 2016, Published by: Pharmacopoeia Commission for Indian Medicine & Homoeopathy, Ghaziabad, India.
- In vitro anti-helicobacter pylori activity of irisolidone isolated from the flowers and rhizomes of Pueraria thunbergiana. Planta Med.. 2001;67:161-163.
- [CrossRef] [Google Scholar]
- Gastric toxicity and mucosal ulceration induced by oxygen-derived reactive species, protection by melatonin. Curr. Mol. Med.. 2001;1:501-513.
- [CrossRef] [Google Scholar]
- In vivo anti-malarial activity and toxicity studies of triterpenic esters isolated form Keetialeucantha and crude extracts. Malar. J.. 2017;16:406.
- [CrossRef] [Google Scholar]
- Evaluation of the antiulcer activity of the leaves of Azadirachta indica: An experimental study. Integrative Med. Int.. 2016;3:10-16.
- [CrossRef] [Google Scholar]
- Ulcer protective potential of standardized hesperidin, a citrus flavonoid isolated from Citrus Sinensis. Rev. Bras. Farmacogn.. 2014;24:330-340.
- [CrossRef] [Google Scholar]
- Gastroprotective effect and safety assessment of Zanthoxylum Zanthoxyloides (Lam) Waterm root bark extract. Am. J. Pharmacol. Toxicol.. 2012;7:73-80.
- [CrossRef] [Google Scholar]
- A method for the quantitative estimation of gastric barrier mucus. J. Physiol.. 1974;242:116-117.
- [Google Scholar]
- Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J. Exp. Pharmacol.. 2023;15:51-62.
- [CrossRef] [Google Scholar]
- Gastroprotective effect of aqueous stem bark extract of Ziziphus jujube against HCL/ethanol-induced gastric mucosal injury in rats. J. Tradit. Chin. Med.. 2015;35:666-670.
- [CrossRef] [Google Scholar]
- Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat. Gastroenterology. 1985;88:366-374.
- [CrossRef] [Google Scholar]
- A natural flavonoid present in unripe plantain banana pulp (Musa sapientum L. var. paradisiaca) protects the gastric mucosa from aspirin-induced erosions. J. Ethnopharmacol.. 1999;65:283-288.
- [CrossRef] [Google Scholar]
- De Lira Mota, K.S., Dias, G.E.N., Pinto, M.E.F., Luiz-Ferreira, Â., Monteiro Souza-Brito, A.R., Hiruma-Lima, C.A., Barbosa-Filho, J.M., Batista., 2009. Flavonoids with gastroprotective activity. Molecules, 14, 979-1012. doi: 10.3390/molecules14030979.
- Insight into the inhibitory effects of Zanthoxylum nitidum against Helicobacter pylori urease and jack bean urease: Kinetics and mechanism. J. Ethnopharmacol.. 2020;249:112419
- [CrossRef] [Google Scholar]
- Effects of naringenin and quercetin on experimental chronic gastric ulcer in rat studies on the histological findings. Phytother. Res.. 1992;6:168-170.
- [CrossRef] [Google Scholar]
- Chemical constituents and biological activities of the genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem.. 2011;5:412-416.
- [Google Scholar]
- Characterization and evaluation of bioactive polyphenolic constituents from Zanthoxylum armatum DC., a traditionally used plant. Biomed. Pharmacother.. 2017;89:366-375.
- [CrossRef] [Google Scholar]
- New chemical constituents from the fruits of Zanthoxylum armatum and its in vitro anti-inflammatory profile. Nat. Prod. Res.. 2019;33:665-672.
- [CrossRef] [Google Scholar]
- Studies on the mechanism of ethanol induced gastric damage in rats. Gastroenterology. 1988;94:10-21.
- [CrossRef] [Google Scholar]
- Ethanol stimulates formation of leukotriene C4 in rat gastric mucosa. Prostaglandins. 1986;31:283-293.
- [CrossRef] [Google Scholar]
- Phuyal, N., Jha, P.K., Prasad, R.P., Rajbhandary, S., 2019. Zanthoxylum armatum DC.: Current knowledge, gaps and opportunities in Nepal, J. Ethnopharmacol. 30, 229, 326-341. doi: 10.1016/j.jep.2018.08.010.
- Investigation of the antimicrobial, antioxidant, hemolytic, and thrombolytic activities of Camellia sinensis, Thymus vulgaris, and Zanthoxylum armatum ethanolic and methanolic extracts. Food Sci. Nutr.. 2023;11:6303-6311.
- [CrossRef] [Google Scholar]
- Ranjana, Nooreen, Z., Bushra, U., Jyotshna, Bawankule, D.U., Shanker, K.,Ahmad, A., Tandon, S., 2019. Standardization and xanthine oxidase inhibitory potential of Zanthoxylum armatum fruits. , 1-8.doi: 10.1016/j.jep.2018.10.018.
- Antiulcerogenic activity of methanolic extract of Emblica officinalis. J. Ethnopharmacol.. 2002;82:1-9.
- [CrossRef] [Google Scholar]
- The effect of a traditional preparation of copper, tamrabhasma, on experimental ulcers and gastric secretion. J. Ethnopharmacol.. 1982;5:79-89.
- [CrossRef] [Google Scholar]
- A simple method for the uniform production of gastric ulceration. Gastroenterology. 1945;5:43-61.
- [CrossRef] [Google Scholar]
- Phytochemical and pharmacological profile of Zanthoxylum armatum DC. An overview. Indian J. Nat. Prod. Resour.. 2011;2:275-285.
- [Google Scholar]
- Protective effects of Capparis zeylanica Linn. Leaf extract on gastric lesions in experimental animals. Avicenna J. Med. Biotechnol.. 2011;3:31.
- [CrossRef] [Google Scholar]
- Phytochemical investigation of seeds of Achyranthes aspera Linn. J. Pharmacogn. Phytochem.. 2014;3:190-193.
- [Google Scholar]
- Vimala, G., Shoba, F.G., 2014. A review on antiulcer activity of few Indian medicinal plants, Int. J. Microbiol. 519590. doi: 10.1155/2014/519590.
- Preventative and therapeutic potential of flavonoids in peptic ulcers. Molecules. 2020;25:4626.
- [CrossRef] [Google Scholar]







