Translate this page into:
Antibacterial evaluation of 2-(6-Chloro-2-p-tolylquinazolin-4-ylthio) acetonitrile against pathogenic bacterial isolates with special reference to biofilm formation inhibition and anti-adherence properties
⁎Corresponding author at: College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia. irfancsmmu@gmail.com (Irfan Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Evaluating the effectiveness of a specific quinazoline molecule with antibacterial activity on microorganisms as a potential antibiotic substitution.
Methods
A variety of microorganisms were tested with the designated quinazoline molecule. Differences within two groups were analyzed using the two-tailed Student’s t-test,.
Results
Susceptibility tests revealed that the chemical has stronger antibacterial action against S. saprophyticus than other isolates, with just a slight effect on E. coli and M. smegmatis. The bacterial cells were subjected to varying concentrations of a certain molecule, and the results showed that the inhibition of bacterial adhesion was not consistent. This suggests that the effect of the molecule on bacterial adhesion is dependent on its concentration. After 24 h of treatment with varying chemical doses, all of the periodontal bacterial strains examined showed considerable inhibition of biofilm formation.
Conclusions
According to the findings, the chemical molecule quinazoline could be utilized as an alternative therapeutic approach for microorganism-caused infections.
Keywords
Quinazoline
Antibacterial
Microorganisms
Biofilm
EGFR
Data availability
Data will be made available on request.
1 Introduction
The use of antibiotics and the management of treatment control and hospital stays are of interest to healthcare providers. Antibiotics attack microorganisms through several mechanisms, including inhibition of bacterial cell wall synthesis, blocking deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and protein synthesis (Zaman et al). However, the emergence of bacterial resistance to the existing medications has caused numerous challenges for humans and healthcare systems (van Duin and Paterson, 2016). Misuse and overuse of antibiotics and a lack of alternative approaches established by the pharmaceutical industry for commercial reasons are among the factors contributing to the antibiotic resistance crisis (Ventola, 2015). Hence, it is imperative to undertake an estimation of the disease and fatalities linked to multidrug-resistant bacteria (Colomb-Cotinat et al., 2012). Although scientists are now battling pathogens' resistance by manipulating the existing antibiotics, several alternative therapy strategies were designed (León-Buitimea et al., 2020).
Lapatinib is a highly effective quinazoline derivative that functions as an inhibitor of epidermal growth factor receptors 1 (ErbB1) and 2 (ErbB2) within the human cells, demonstrated antimicrobial efficacy by inhibiting Staphylococcus aureus growth and biofilm formation (Patel et al., 2018; Rohini et al., 2009; El-Azab et al., 2010; Liu et al., 2022). Gefitinib, erlotinib, and afatinib are EGFR tyrosine kinase inhibitors (TKIs) that demonstrated to be an effective threapy for advanced non-small cell lung cancer with EGFR-mutant because of their advantages over palliative platinum-based chemotherapy regarding the rate of response to treatment, the pace of progression, and the quality of life (Dsouza and Kumar, 2017; Califano et al., 2015; Sogi et al., 2017). A team of scientific collaborators created unique quinazoline derivatives agents. These compounds were evaluated against human cervix cell lines (HELA), human liver cell lines (HEPG2).
2 Materials and methods
2.1 Chemistry
According to a documented process, 2-(6-Chloro-2-p-tolylquinazolin-4-ylthio)acetonitrile was obtained by refluxing 6-chloro-2-p-tolylquinazolin-4(3H)-thione with 2-chloroacetonitrile in pyridine (El-Azab et al., 2010).
2.2 Bacterial strains
A collection of eight microbial strains was used in this study, encompassing both Gram positive and Gram negative bacteria. Bacteria such as Enterococcus faecalis, Salmonella spp., Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, and Staphylococcus saprophyticus were among the strains examined. The different strains were cultivated in lysogenic broth (LB) medium using ideal conditions of 37 °C.
2.3 Agar well diffusion assay for determining antibacterial susceptibility
To perform an initial assessment of the susceptibility of the bacteria under study to the compound, the bacterial strains were cultivated until they reach the logarithmic growth phase, the optical density reading at a wavelength of 610 nm (O.D.610) is determined within the range of 0.4 to 0.6. This cultivation was carried out using LB (lysogenic broth) medium. As a result, the experimental bacterial strains were diluted in LB broth to achieve a theoretical optical density at 610 nm of 0.01. To determine the antibacterial efficacy of the compound, agar well diffusion was utilized (Magaldi et al., 2004). To summarize, LB agar wells measuring 6 mm in diameter were generated by employing a sterile syringe tip. The next step was to use the diluted culture to create a bacterial lawn culture on the agar using a sterilized cotton swab. In a Petri dish, 20 μl of a compound with a concentration of 2 mg/ml and dimethyl sulfoxide (DMSO) were put into three separate wells. Subsequently, the samples were subjected to aerobic incubation at a temperature of 37 °C for a duration of 24 h. Measurements were taken in millimeters to determine the diameter of the inhibition zone, which included the well diameter.
2.4 Analysis of MBC and MIC values
The determination of the Minimum Bactericidal Concentration (MBC) and Minimum Inhibitory Concentration (MIC) of the chemical was conducted following standard techniques, with certain changes (Wei et al., 2011). The chemical concentrations used to determine minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) on certain bacterial strains were diluted twice, going from 2 mg/ml to 0.015 mg/ml. To determine the MIC, the bacterial strains were cultivated until they reached the logarithmic phase, as indicated by an optical density (O.D.610) range of 0.4–––0.6. Subsequently, the bacterial cultures were diluted in LB broth in order to get a theoretical optical density (O.D.610) of 0.01. Consequently, a total of 180 μl of bacterial culture was introduced into the wells of polystyrene sterile flat-bottom 96-well plates. Wells were supplied in triplicate with 20 μl of the 2-fold dilution of the compound. As a control, triplicate wells were filled with 20 μl of DMSO (5 %). Additional aerobic incubation was conducted on the dishes for a period of 24 h at a temperature of 37 °C. Each well was tested hourly for the appearance of a pink color after adding 20 μl of alamar blue (Thermo Fisher, USA) following a 24-hour incubation period. The determination of the MIC involved identifying the smallest quantity of the compound that could be detected in a well without inducing a visible alteration in the color of the alamar blue solution to pink.
In order to determine the MBC of the compound against bacterial strains, a subculture consisting of 10 μl was incubated aerobically on LB agar for 24 h at 37 °C using wells that did not exhibit any change in the color of Alamar blue pigment. The MBC of the compound for the organisms under investigation was determined by ascertaining the minimum concentration at which there was no observed growth.
2.5 Adherence assay
The previously elucidated method was successfully executed (Fazly et al., 2013). In order to conduct this experiment, a total of 96 wells on a plate were filled with 100 μl of bacterial cells that were tested and had an optical density at 610 nm of 0.01. These bacterial cells were cultured in RPMI 1640 medium and were supplemented with 0.165 M morpholinepropanesulphonic acid buffer at a pH of 7.0. After being exposed to the chemical at a number of different doses (MIC x 0.5, MIC x 1, and MIC x 2), the bacteria were cultured in an aerobic environment at 37 °C for a period of six hours. Negative controls were untreated bacterial cells in each investigation. As a result, the medium used for incubation was removed, and the non-adherent bacterial cells that were present in each well were discarded by rinsing them twice with 200 μl of phosphate-buffered saline. After loading 100 μl of alamar blue at a concentration of 5 % absolute in RPMI 1640 medium, every well was subjected to aerobic incubation at 37 °C for a period of six hours. Consequently, the incubation media was disposed of and bacterial cells that did not adhere were removed by rinsing them twice with 200 μl PBS. Following the loading of 100 μl of alamar blue at a 5 % absolute concentration in RPMI 1640 media, each well was aerobically incubated at 37 °C for a duration of 6 h. The fluorescence indications were investigated using a Synergy HT microplate reader (BioTek Instruments, WA, USA) with excitation at 555 and emission at 585 nm.
2.6 Biofilm formation
By inoculating bacterial cell suspensions (O.D.610 = 0.01) that were cultivated in RPMI 1640 media was buffered with solution containing 0.165 M Mops at a pH of 7.0, biofilm formation was accomplished. The suspensions that had been inoculated were exposed to aerobic incubation at 37 °C for a period of 6 h in sterile 96-well plates with flat bottoms (Raut et al., 2013). After a 6-hour period of adhesion, the media was carefully extracted without inducing any disturbance to the development of the biofilm. Following that, various chemical concentrations (MIC x 0.5, MIC x 1, MIC x 2) were cultured in fresh RPMI 1640 media and subsequently transferred to the designated well. As a negative control, unprocessed bacterial cells were utilized in each experimental cohort. Additional aerobic incubation was performed at 37 °C for 24 h on the 96-well plate. The assessment of the tested compound's effects on biofilm formation was conducted following the methodology specified (Jin and Samaranayake, 2004).
2.7 Growth kinetic assay
To depict the kinetic growth plots, which gave a general idea of the survivability of bacteria, we used the logarithm transformation of the relative population size with respect to time. The compound’s antimicrobial activity of the was assessed using these plots. O.D.600 was utilized to analyze the growth curve throughout sixteen hours of cultivation..
To assess the influence of the compound on the bacterial cells being studied, a volume of 20 μl of the compound was introduced into 180 μl of the bacterial culture, which exhibited an optical density of 0.01at a wavelength of 600 nm at varying concentrations (MIC x 0.5, MIC x 1, MIC x 2). The control group was designated as the culture lacking any chemicals. The aerobic incubation of 96-well plates was performed at a temperature of 37 °C. The FLUOstar Omega plate reader, manufactured by BMG Labtech of Allmendgrun, Ortenberg, Germany, was used to detect absorbance at a 600 nm wavelength at regular 2-hour intervals. The measurements were replicated thrice in order to calculate the average value. A plot was generated with the mean absorbance values plotted against time.
2.8 Statistical analysis
The trials were repeated three times, and each time the mean and standard deviation were obtained. Graphpad Prism, version 6.0, developed by Graphpad Software Inc., was used to conduct the statistical analysis. An analysis of variance was carried out using a Student's t-test (two-tailed) to compare two separate groups. A p-value of less than 0.0001 (***) shows that there is a significant difference between the different groups, meaning that the results are statistically significant.
3 Results
3.1 Antibacterial activity of the compound
To evaluate the antibacterial effectiveness of the chemical, eight different microbial strains were treated (Table 1). The results of the susceptibility studies indicate that the compound exhibits greater antibacterial efficacy against S. saprophyticus in comparison to other isolates. Additionally, a modest impact was observed against E. coli and M. smegmatis. (Table 1). It was determined that a zone size greater than 8 mm was indicative of the sensitivity of the bacterial strains to the investigated compound. Bacterial isolates with a zone size greater than 8 mm were subjected to various compound concentrations to establish the MIC and MBC.
Organism
Zone of inhibition
Mean ± SD (mm)
MIC
(mg/ml)
MBC
(mg/ml)
P. aeruginosa
14 ± 1.5
0.125
0.5
E. coli
12 ± 1.2
0.25
0.5
K. pneumonae
15 ± 0.75
0.125
0.25
Salmonella
14 ± 1
0.125
0.25
S. aureus
20 ± 1.5
0.062
0.25
S. saprophyticus
21 ± 1.5
0.031
0.125
S. Pyogenes
19 ± 2
0.031
0.25
E. faecalis
18 ± 1
0.062
0.5
To ascertain the MBC and MIC, specific bacterial strains were subjected to a predetermined volume of the compound and subsequently incubated for a period of 24 h. By examining a low concentration of the drug at which the colour of the alamar blue dye remained unaltered, the MIC was established. The results presented in Table 1 demonstrate a significant susceptibility of all bacterial strains to the tested compound. The study found that the bacterial growth was effectively suppressed, with MBC values ranged from 0.125 to 0.5 mg/ml, and MIC values ranged from 0.031 to 0.25 mg/ml. The findings were corroborated by the subsequent increase in the zone of inhibition, which ranged from 12 to 21 mm.
3.2 Compound inhibits bacterial adhesion
The impact of the compound on bacterial adherence was evaluated through an adhesion assay utilising the alamar blue dye. The bacterial cells exhibited variable inhibition of bacterial adherence subsequent to exposure to different concentrations of the compound, indicating a concentration-dependent effect (Fig. 1). The compound under investigation exhibited inhibitory effects on bacterial adhesion across all bacterial strains. The degree of inhibition ranged from 25 % to 50 %, 47 % to 71 %, and 59 % to 86 % at concentrations of MIC x 0.5, MIC x 1, and MIC x 2, respectively. The group of bacterial known as the control consisted of strains that did not exhibit any concentration of the compound.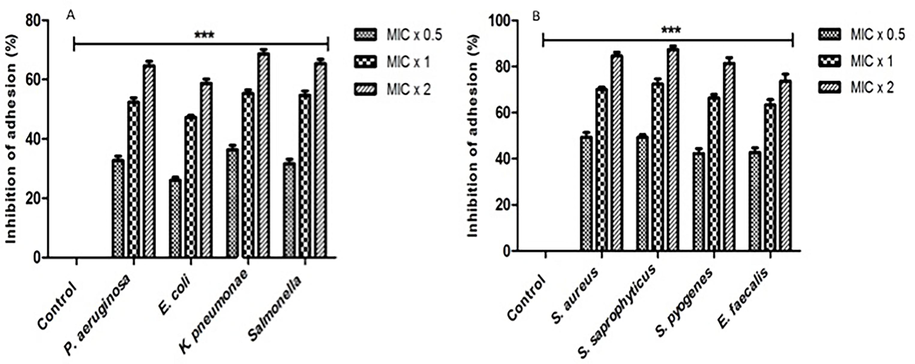
The inhibition of the microorganism adhesion by 2-((6-chloro-2-(p-tolyl)quinazolin-4-yl)thio)acetonitrile. The study employed the Alamar Blue-based polystyrene adhesion assay to assess the impact of 2-((6-chloro-2-(p-tolyl)quinazolin-4-yl)thio)acetonitrile on the adherence of different microorganisms. The bacterial strains that were subjected to experimentation were exposed to three different concentrations of the drug, namely MIC x 0.5, MIC x 1, and MIC x 2, for a duration of 6 h at a temperature of 37 °C. All untreated bacterial isolates are represented by control bars, which represent 0 % inhibition. The results of three distinct experiments are presented with means ± SD***p < 0.0001.
3.3 Compound inhibits bacterial biofilm formation
It is noteworthy that all the bacterial strains that were subjected to testing exhibited a substantial reduction in biofilm formation subsequent to exposure to diverse compound concentrations for a duration of 24 h. Following a 24-hour period, the test entities exhibited biofilm formation inhibition against all bacterial strains at concentrations of MIC x 0.5, MIC x 1, and MIC x 2. The observed inhibition percentages ranged from 22 % to 40 %, 47 % to 63 %, and 67 % to 85 %, respectively (Fig. 2). The control group consisted of bacterial strains that lacked any concentration of the compound.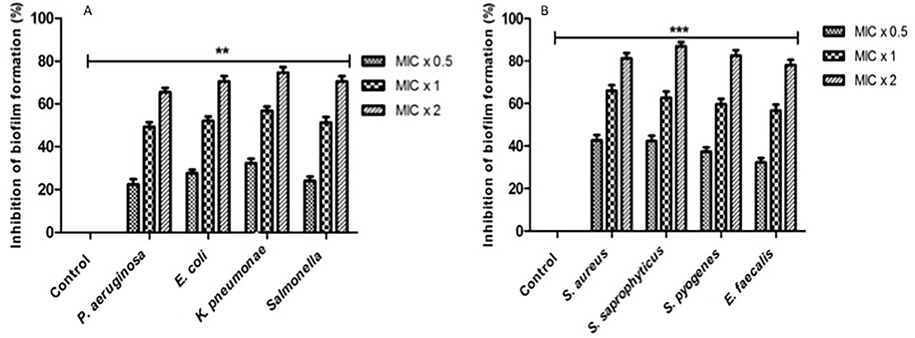
The reduction of biofilm formation by 2-((6-chloro-2-(p-tolyl)quinazolin-4-yl)thio)acetonitrile. Under biofilm growth conditions, microorganisms were incubated for 24 h with 2-((6-chloro-2-(p-tolyl)quinazolin-4-yl)thio)acetonitrile at MIC x 0.5, MIC x 1, and MIC x 2 values. All untreated bacterial isolates are represented by control bars, which represent 0 % inhibition. The results of three distinct experiments are presented with means ± SD***p < 0.0001.
3.4 Effect on bacterial growth
A real-time study was conducted to examine the impact of the compound on bacterial growth at various time intervals. In order to ascertain the growth kinetics, a bacterial culture measuring 180 μl with an OD 610 of 0.01 was subjected to treatment with a compound at concentrations of MIC x 0.5, MIC x 1, and MIC x 2, using 20 μl of the aforementioned compound. For every two hours. The bacterial growth rate was recorded, as depicted in Fig. 3.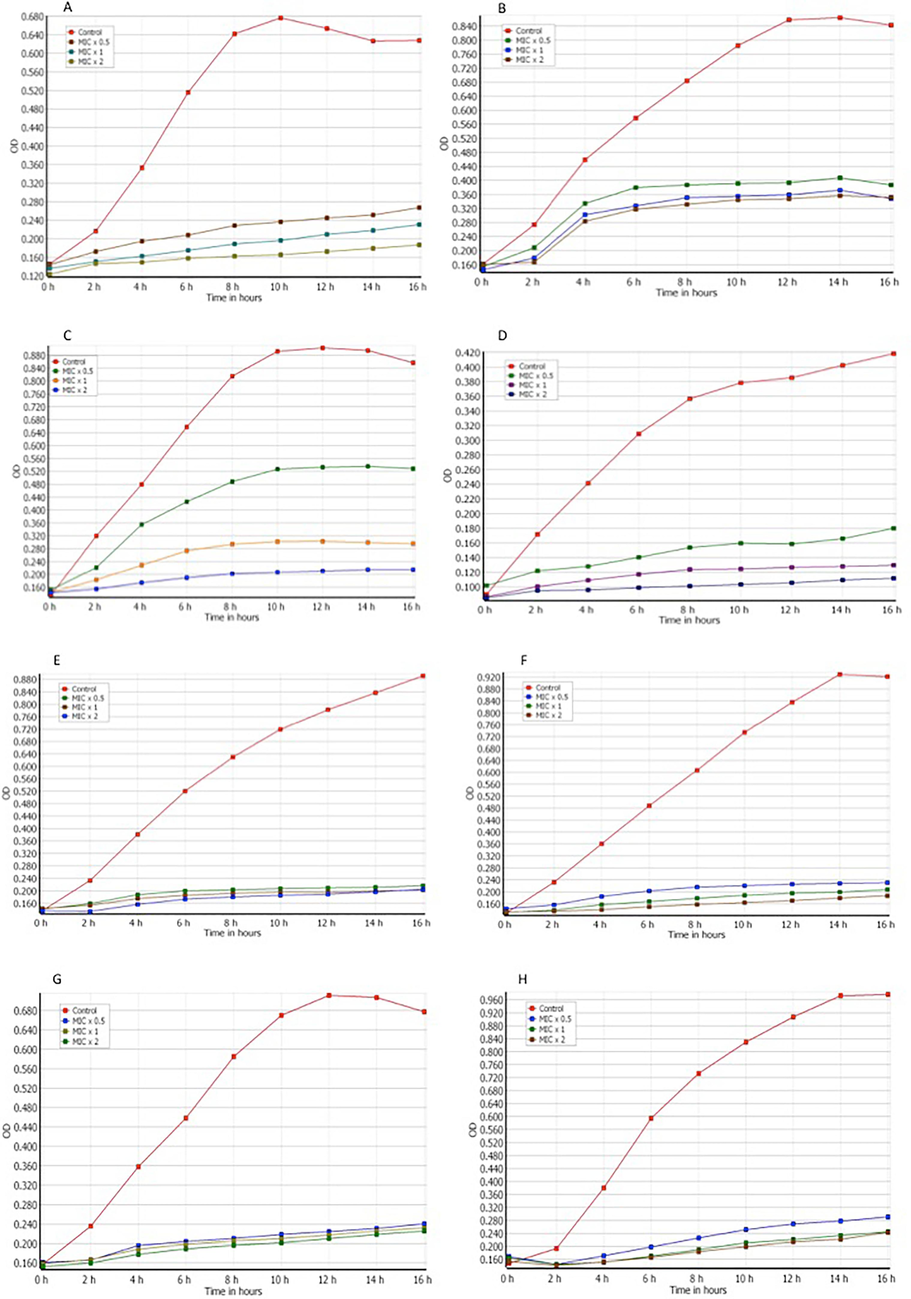
The impact of bacterial growth kinetics by 2-((6-chloro-2-(p-tolyl)quinazolin-4-yl)thio)acetonitrile. Different concentrations of the chemical (MIC x 0.5, MIC x 1, and MIC x 2) were applied to various strains. The strains are as follows: (A) Pseudomonas aeruginosa (B) Escherichia coli (C) Klebsiella pneumoniae (D) Salmonella (E) Staphylococcus aureus (F) Staphylococcus saprophyticus (G) Streptococcus pyogenes (H) Enterococcus faecalis. The growth cycle of organisms that were not subjected to any treatment was utilized as a control for growth. The absorbance at a wavelength of 610 nm was measured at consistent time intervals of 2 h. The results of three distinct experiments are presented with means ± SD***p < 0.0001.
4 Discussion
The use of alternative strategies for infection dissemination is critical due to the overuse and misuse of antimicrobial agents, which leads to drug resistance (Leekha et al., 2011). It has been demonstrated that several substances, including those based on indole and quinazolines, are efficient for treating microbial activity (Nandwana et al., 2018). Quinazoline, a compound derived from the benzene and pyrimidine ring fusion, exhibited a wide array of biological actions. These included effects against bacteria, cancer, hypertension, analgesia, convulsions, malaria, and tuberculosis (Gupta et al., 2018; Abdel-Aziz et al., 2016; Alanazi et al., 2014; Alanazi et al., 2013).
The newly developed quinazoline molecular compound was evaluated for antibacterial activity and shown to be highly effective against specific pathogens (Al-Obaid et al., 2009; Al-Suwaidan et al., 2016; Al-Suwaidan et al., 2013; El-Azab et al., 2013). Consequently, the chemical might be utilized as an alternate therapeutic protocol for microbial infection (El-Azab and Eltahir, 2012; El-Azab et al., 2012; El-Azab et al., 2019). The viability of using the compound as an antimicrobial agent was confirmed by a number of study methods, as detailed in the result section. The formation of biofilms and adhesion both increase bacterial virulence (El-Azab et al., 2011; Mohamed et al., 2016; Alanazi et al., 2016; El-Azab et al., 2017). Attachment (or adhesion) is the initial stage of bacterial infection, promotingolonization and host cell invasion (El-Azab et al., 2017; Al-Omary et al., 2010). Anti-adhesion treatment is thus required to prevent the adhesion step and host cell attachment. Bacteria engage in the formation of biofilms as a means to enhance their survival stratigies (El-Azab and ElTahir, 2012; Abdel-Aziz et al., 2016). Biofilms are clusters of bacteria embedded in a self-produced matrix composed of numerous elements such as proteins, DNA, and polysaccharides that are linked to a surface and/or each other (El-Azab et al., 2020). According to the findings, the Quinazoline derivative molecule prevented the adhesion and biofilm formation of the tested microorganisms, demonstrating its efficiency as an antimicrobial agent (Hamdi et al., 2022). The molecules developed, namely 4-substituted 6-chloro-2-p-tolylquinazolin, possess a pharmacophore of 4-substituted quinazoline that bears structural similarity to Erlotinib and Lapatinib (El-Azab et al., 2010). As a result, the compound may target microbial growth via EGFR.
It has been proven that the unique quinazoline molecule, as indicated above, is effective as an anticancer therapeutic. Our research showed that the compound is also effective as an antimicrobial therapeutic. The current study indicates using the chemical molecule quinazoline as an alternative therapeutic strategy for treating infectious diseases caused by microorganisms. Further research is still required to understand the molecule's function against infection better. However, the current work provides the basis for future research.
5 Conclusion
The study's findings revealed that 2-((6-chloro-2-(p-tolyl)quinazolin-4-yl)thio) acetonitrile exhibited a growth inhibitory effect on pathogenic bacterial strains in a dose-dependent manner, thereby decreasing their pathogenic potential. The results of this study suggest that the tested compound exhibits potential as a appropriate contender for the advancement and establishment of effective therapeutics against infections caused by these pathogenic microorganisms, thereby warranting further investigation into its potential as a drug candidate. It is imperative to assess the potential deleterious effects, pharmacokinetic characteristics, and associated adverse reactions. Moreover the tested compound exhibits promising antibacterial properties, making it a viable candidate for incorporation into pharmaceutical formulations.
CRediT authorship contribution statement
Sultan Z. Alasmari: Writing – original draft, Investigation, Data curation, Conceptualization. Mohammed H. Makkawi: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Irfan Ahmad: Writing – review & editing, Writing – original draft, Methodology, Investigation, Funding acquisition, Conceptualization. Abdulrahim R. Hakami: Writing – review & editing, Validation, Supervision, Resources. Abdulrahman A. Almehizia: Writing – review & editing, Validation, Resources, Data curation. Adel S. El-Azab: Writing – review & editing, Visualization, Validation, Investigation, Conceptualization. Alaa A.-M. Abdel-Aziz: Writing – review & editing, Supervision, Formal analysis, Data curation. Mohammed Ghazwani: Writing – review & editing, Supervision, Formal analysis, Data curation.
Acknowledgements
The authors express their gratitude to the Deanship of Research and Graduate Studies at King Khalid University for sponsoring this study under the large group project grant number RGP.2/507/45.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4(3H)-quinazolinones. Eur. J. Med. Chem.. 2016;121:410-421.
- [Google Scholar]
- Design, synthesis of 2,3-disubstitued 4(3H)-quinazolinone derivatives as anti-inflammatory and analgesic agents: COX-1/2 inhibitory activities and molecular docking studies. Bioorg. Med. Chem.. 2016;24(16):3818-3828.
- [Google Scholar]
- Design, synthesis and biological evaluation of some novel substituted 2-mercapto-3-phenethylquinazolines as antitumor agents. Med. Chem. Res.. 2013;22(11):5566-5577.
- [Google Scholar]
- Design, synthesis and biological evaluation of some novel substituted quinazolines as antitumor agents. Europ. J. Med. Chem.. 2014;79:446-454.
- [Google Scholar]
- Synthesis, antitumor and antimicrobial activity of some new 6-methyl-3-phenyl-4(3H)-quinazolinone analogues: in silico studies. J. Enzyme Inhib. Med. Chem.. 2016;31(5):721-735.
- [Google Scholar]
- Substituted quinazolines, part 3. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur. J. Med. Chem.. 2009;44(6):2379-2391.
- [Google Scholar]
- Al-Omary, F. A.; Abou-Zeid, L. A.; Nagi, M. N.; Habib, E.-S. E.; Alaa, A.-M.; El-Azab, A. S.; Abdel-Hamide, S. G.; Al-Omar, M. A.; Al-Obaid, A. M.; El-Subbagh, H. I., Non-classical antifolates. Part 2: synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg. Med. Chem. 2010, 18, (8), 2849–2863.
- Design, synthesis and biological evaluation of 2-mercapto-3-phenethylquinazoline bearing anilide fragments as potential antitumor agents: molecular docking study. Bioorg. Med. Chem. Lett.. 2013;23(13):3935-3941.
- [Google Scholar]
- Synthesis, antitumor activity and molecular docking study of some novel 3-benzyl-4(3H)quinazolinone analogues. J. Enzyme Inhib. Med. Chem.. 2016;31(1):78-89.
- [Google Scholar]
- Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs. 2015;75:1335-1348.
- [Google Scholar]
- Colomb-Cotinat M, J. Lacoste, C. Brun-Buisson, V. Jarlier, B. Coignard and S. Vaux. Estimating the morbidity and mortality associated with infections due to multidrug-resistant bacteria (MDRB), france, 2012. Antimicrob. Resist Infect. Control 2016; 5.
- Role of systemic antibiotics in preventing epidermal growth factor receptor: Tyrosine kinase inhibitors-induced skin toxicities. Asia Pac J. Oncol. Nurs.. 2017;4:323-329.
- [Google Scholar]
- Design and synthesis of novel 7-aminoquinazoline derivatives: antitumor and anticonvulsant activities. Bioorgan. Med. Chem. Lett.. 2012;22(5):1879-1885.
- [Google Scholar]
- Synthesis and anticonvulsant evaluation of some novel 4 (3H)-quinazolinones. Monatshefte Für Chemie-Chemical Monthly. 2011;142(8):837-848.
- [Google Scholar]
- 6-Methyl-3-phenyl-2-sulfanylidene-1, 2, 3, 4-tetrahydroquinazolin-4-one. Acta Crystallographica Section e: Structure Reports Online. 2012;68(3):o862-o.
- [Google Scholar]
- Novel 4 (3H)-quinazolinone analogs: synthesis and anticonvulsant activity. Med. Chem. Res.. 2013;22(6):2815-2827.
- [Google Scholar]
- Synthesis, in vitro antitumour activity, and molecular docking study of novel 2-substituted mercapto-3-(3,4,5-trimethoxybenzyl)-4(3H)-quinazolinone analogues. J. Enzyme Inhib. Med. Chem.. 2017;32(1):1229-1239.
- [Google Scholar]
- Synthesis of benzensulfonamides linked to quinazoline scaffolds as novel carbonic anhydrase inhibitors. Bioorg. Chem.. 2019;87:78-90.
- [Google Scholar]
- Antitumor activity, multitarget mechanisms, and molecular docking studies of quinazoline derivatives based on a benzenesulfonamide scaffold: Cell cycle analysis. Bioorg. Chem.. 2020;104:104345
- [Google Scholar]
- Synthesis and anticonvulsant evaluation of some new 2,3,8-trisubstituted-4(3H)-quinazoline derivatives. Bioorg. Med. Chem. Lett.. 2012;22(1):327-333.
- [Google Scholar]
- Synthesis, anticancer and apoptosis-inducing activities of quinazoline-isatin conjugates: epidermal growth factor receptor-tyrosine kinase assay and molecular docking studies. J. Enzyme Inhib. Med. Chem.. 2017;32(1):935-944.
- [Google Scholar]
- El-Azab AS, Mohamed A. Al-Omar, Alaa A. -M Abdel-Aziz, Naglaa I. Abdel-Aziz, Magda A. -A el-Sayed, Abdulaziz M. Aleisa, et al. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. Eur. J. Med. Chem. 2010; 45: 4188–4198.
- Chemical screening identifies filastatin, a small molecule inhibitor of candida albicans adhesion, morphogenesis, and pathogenesis. Proc. Natl. Acad. Sci. U S A. 2013;110:13594-13599.
- [Google Scholar]
- Md Jawaid Akhtar, Md Rafi Haider and M. Shahar Yar. Current perspectives on quinazolines with potent biological activities: A review. Synth. Commun.. 2018;48:1099-1127.
- [Google Scholar]
- Design, synthesis, antitumor, and VEGFR-2 inhibition activities of novel 4-anilino-2-vinyl-quinazolines: Molecular modeling studies. Bioorg. Chem.. 2022;122:105710
- [Google Scholar]
- Yuthika Samaranayake and Hak Kong Yip. Biofilm formation of candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789-798.
- [Google Scholar]
- The demand for new antibiotics: Antimicrobial peptides, nanoparticles, and combinatorial therapies as future strategies in antibacterial agent design. Front. Microbiol.. 2020;11
- [Google Scholar]
- Liu Y, Yiyi Shi, Hang Cheng, Junwen Chen, Zhanwen Wang, Qingyin Meng, et al. Lapatinib acts against biofilm formation and the hemolytic activity of staphylococcus aureus. ACS Omega 2022; 7: 9004-9014.
- Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis.. 2004;8:39-45.
- [Google Scholar]
- Synthesis and antitum r evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur. J. Med. Chem.. 2016;112:106-113.
- [Google Scholar]
- Design and synthesis of imidazo/benzimidazo[1,2-c]quinazoline derivatives and evaluation of their antimicrobial activity. ACS Omega. 2018;3:16338-16346.
- [Google Scholar]
- Patel H, Atul Shirkhedkar, Sanjay Bari, Kamalkishor Patil, Amit Arambhi, Chandrakantsing Pardeshi, et al. Quinazolino-thiadiazoles as antimicrobial agents. Bull. Faculty Pharm. Cairo University 2018; 56: 83-90.
- Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by candida albicans. Biofouling. 2013;29:87-96.
- [Google Scholar]
- Yen-Peng Ho and Vadde Ravinder. Mono and bis-6-arylbenzimidazo[1,2-c]quinazolines: a new class of antimicrobial agents. Eur. J. Med. Chem.. 2009;44:3330-3339.
- [Google Scholar]
- The tyrosine kinase inhibitor gefitinib restricts mycobacterium tuberculosis growth through increased lysosomal biogenesis and modulation of cytokine signaling. ACS Infect. Dis.. 2017;3:564-574.
- [Google Scholar]
- Multidrug resistant bacteria in the community: Trends and lessons learned. Infect. Dis Clin North Am. 2016;30:377-390.
- [Google Scholar]
- Depletion of antibiotic targets has widely varying effects on growth. Proc. Natl. Acad. Sci. U S A. 2011;108:4176-4181.
- [Google Scholar]
- Zaman SB, Muhammed Awlad Hussain, Rachel Nye, Varshil Mehta, Kazi Taib Mamun and Naznin Hossain. A review on antibiotic resistance: Alarm bells are ringing. Cureus 9: .
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103316.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







