Translate this page into:
pH-switchable hydrophobic deep eutectic solvent-based liquid phase microextraction for detecting morphine and codeine in whole blood samples followed by HPLC-UV
⁎Corresponding author. DNBanjawhar@pnu.edu.sa (Dalal N. Binjawhar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Prescription opioids are used in clinics for reducing pain, but overdoses and addiction can lead to poisoning. Herein, we report a rapid, straightforward, and cost-effective hydrophobic deep eutectic solvent-based liquid phase microextraction process with HPLC-UV detection for extracting and analyzing morphine and codeine from whole blood samples. The procedure involved synthesizing seven deep eutectic solvents and investigating their pH switchability. Deep eutectic solvents with pH-switchable properties were employed as extractants. Under optimal conditions, the relative standard deviation of 50 μg/L of morphine and codeine in blood samples was 5.4–6.2 % for inter-day measurements and 3.7–4.3 % for intra-day measurements. For both analytes, the calibration graphs showed a linear range of 1.5–300 μg/L and a limit of detection of 0.5 μg/L. The enrichment factor and the extraction recovery of morphine and codeine were 152–––166 and 76 − 83 %, respectively. The results revealed that the addicted person’s blood sample contained both morphine and codeine. The real blood samples spiked with varying doses of codeine and morphine had relative recoveries ranging from 91.8 to 107.0 %, suggesting the method is suitable for real sample analysis.
Keywords
Opioids
Addiction
Blood testing
Liquid phase microextraction
Recovery
pH-switchable deep eutectic solvent
Morphine
Codeine
Blood analysis
- DES
-
deep eutectic solvent
- LPME
-
liquid phase microextraction
- DLLME
-
dispersive liquid–liquid microextraction
- EG
-
ethylene glycol
- HBA
-
hydrogen bound acceptor
- HBD
-
hydrogen bound donor
- CSA
-
(1S)-(+)-camphor-10-sulfonic acid
- SA
-
salicylic acid
- MTOAC
-
methyltrioctylammoniumchloride
- BA
-
n-butanoic acid
- SDS
-
sodium dodecyl sulfate
Abbreviations
1 Introduction
Morphine and codeine are natural alkaloids extracted from the poppy plant's seeds and have been used as medicine since ancient times (Vadhel et al., 2023). Morphine is a strong analgesic that relieves severe pain, while codeine is a mild analgesic and cough suppressant (Plueschke et al., 2022). Opioids are the cornerstone of pharmacotherapy for cancer treatment, and in more than 70 % of patients, cancer pain is relieved with opioids (Paice et al., 2023).On the other hand, these drugs make use of these compounds, causing poisoning (Sobczak and Goryński, 2020). Morphine overdoses typically produce symptoms such as sweating, nausea, vomiting, diarrhea, itching, and so on (Bonifonte et al., 2021). As a result, examination of opioid levels in biological fluids is important for clinical medicine, drug abuse control, and forensic cases to prevent poisoning from opioid overdose.
To quantify and separate pharmaceuticals and opioids in complex matrices, high-performance liquid chromatography (HPLC) (Yang et al., 2016; Sharma et al., 2022), electrophoresis (Zhang et al., 2007), gas chromatography (GC) (Chericoni et al., 2014; Papoutsis et al., 2011), and electrochemical methods (Wong et al., 2021) are commonly used. Chromatographic methods have more sensitivity than other approaches. The use of GC is limited by the requirement for derivatization of certain medications due to their heat stability, particularly if the GC is equipped with a mass spectroscopy (Rodríguez-Ramos et al., 2020). Alternatively, HPLC has been used to separate a wide range of pharmaceuticals without being sensitive to temperature or requiring derivatization. It can also be coupled with a wide range of detectors, including the more contemporary mass spectrometry (Zhang et al., 2022; Yang et al., 2016; Shamsipur and Fattahi, 2011), UV (Ahmadi-Jouibari et al., 2013), fluorescence (Toker et al., 2021), diode array detection (Moreno et al., 2014); chemiluminescence (Terry et al., 2013), and others.
Despite developing various exceedingly sensitive instrumental analysis techniques, sample preparation is still required before analysis. Rezaee et al. (2006) developed dispersive microextraction (DLLME), which is mostly used to concentrate analytes because real sample matrices are too complicated and incompatible with analytical instrumentation (Rezaee et al., 2006). DLLME approach resolves all of the concerns with earlier methods. However, issues remain, such as excessive disperser solvents in conventional DLLME and hazardous solvent extraction. Researchers and scientists have modified the DLLME approach to address these issues, resulting in significant achievements. The toxic extractants in DLLME have been replaced with deep eutectic solvents (DESs) (Lin et al., 2021), supramolecular (AltunayN et al., 2023), ionic liquids (ILs) (SehrawatH et al., 2020), and organic solvents lighter than water (Farajzadeh et al., 2018). DESs have a higher activity level than individual components (Golpayegani et al., 2022). DESs can be produced by combining chemicals that serve as hydrogen bond donors (HBD) and acceptors (HBAs) (Ahmadi Jouybari et al., 2022). There are instances where these solvents have three or more constituents. Various organic and inorganic species have been tested for safety, and review articles have been published on this subject (Osamede Airouyuwa et al., 2024; Morgan et al., 2021). In recent years, vortexing and ultrasonication have been used to disperse the extraction solvent rather than the dispersant solvent in an aqueous solution. Pirsaheb et al. demonstrated the extraction of amoxicillin and ceftriaxone from hospital waste water using DES through vortexing and detection by HPLC-UV (Pirsaheb et al., 2019). Ahmadi-Jouibari employed ultrasonic to disperse strobilurin fungicide residues from apple samples, which were then extracted and measured using HPLC-UV (Ahmadi-Jouibari et al., 2022). Vortex and ultrasonic processes are also time-consuming, taking more than 30 min to achieve acceptable efficiency. The problems with existing methods can be addressed by developing a strategy based on pH-switchable deep eutectic solvents.
This research aims to demonstrate a simple, affordable, and broadly accessible method for the precise and selective extraction of codeine and morphine from whole blood samples. Seven DESs were developed specifically for this application, and their pH switchability was studied. By using pH-switchable DESs, morphine and codeine were isolated from blood samples and preconcentrated. The influence of experimental parameters on extracting the target analyte and recovery was studied one variable at a time.
2 Material and methods
2.1 Materials
Sigma-Aldrich Chemical Company (St. Louis, MO, USA) supplied the l-menthol, ethylene glycol (EG), salicylic acid (SA), (1S)-(+)-camphor-10-sulfonic acid (CSA), (BA), methyltrioctylammonium chloride (MTOAC), n-butanol, 1-undecanol, n-butanoic acid, codeine phosphate and morphine sulfate. 1-decyl-3-methylimidazolium chloride ([DMIM]Cl) and sodium dodecyl sulfate (SDS) were supplied by Merck (Darmstadt, Germany). A 1000 mg/L stock solution of morphine and codeine was prepared with a water: methanol (1:1 v/v) ratio. The necessary test solution was prepared from stock daily with adequate dilution.
2.2 Instrumentation
Morphine and codeine were examined by a Knauer high-performance liquid chromatography system (Germany) attached with an H5-ODS C18 column, binary pumps, a variable wavelength UV detector and Chromgate software (version 3.1). A Rheodyne model 7725i manual sample injector with a 20 μL loop was used for injection. The isocratic elution technique used a mobile phase of 10.0 mM Na2HPO4 and 0.70 mM SDS (pH = 6.5). The flow rate used was 1.0 mL per minute. The analytes has absorbance at 285 nm, hence used for the detection.
2.3 Sample collection and preparation
With informed consent, blood samples for a blank study were taken from a healthy 28-year-old male volunteer who had not used medication or opium in at least six months. The clinical microbiology lab at local Hospitals, supplied a real plasma sample from a 43-year-old man who had surgery and was administered morphine and codeine. The samples were preserved at −18 °C before analysis.
The sample was prepared by incubating 1.0 mL of plasma as received from the hospital without dilution was mixed with 800 μL of 15 % w/v ZnSO4 in a test tube at 4 °C for 10 min. The acquired samples were centrifuged at 4000 rpm to prevent any matrix impact. The obtained supernatant was carefully transferred to a new, clean container and diluted with 10 mL of distilled water. The analyte in these solutions was investigated in this study.
2.4 Preparation of deep eutectic solvents
Table 1 shows the seven DESs synthesized based on previous research, their switchability, and references. DES-1 − l-menthol: ethylene glycol (1:1 M ratio); DES-2 − l-menthol: salicylic acid (4:1 M ratio); DES-3 − l-menthol: camphor sulphonic acid (5:1 M ratio); DES-4 − l-menthol: phenol (1:1 M ratio); DES-5 − MTOAC:n-butanol (1:1 M ratio); DES-6 − [DMIM]Cl:1-undecanol (1:2 M ratio) and DES-7 − [DMIM]Cl:n-butanoic acid (1:2 M ratio) (Abri et al., 2019; Ahmadi Jouybari et al., 2023; Raj, 2020; Pirsaheb and Fattahi, 2018).
Reference
Component-1
Component-2
Molar ratio
Abbreviation
pH-Switchability
(Ahmadi Jouybari et al., 2022)
l-menthol
EG
1:1
DES-1
Switchable
(Osamede Airouyuwa et al., 2024)
l-menthol
SA
4:1
DES-2
Switchable
(Morgan et al., 2021)
l-menthol
CSA
5:1
DES-3
Switchable
(Pirsaheb et al., 2019)
l-menthol
Phenol
1:1
DES-4
Switchable
(Golpayegani et al., 2022)
MTOAC
n-butanol
1:3
DES-5
Non-switchable
(Ahmadi-Jouibari et al., 2022)
[DMIM]Cl
1-Undecanol
1:2
DES-6
Non-switchable
(Abri et al., 2019)
[DMIM]Cl
n-Butanoic acid
1:2
DES-7
Non-switchable
2.5 Extraction procedure
For extraction, about 10.0 mL of the dilute plasma sample (which may or may not spike with morphine and codeine) was mixed with 50 μL of selected DES-3 in a 20 mL test tube. Addition of 100 μL of KOH solution (5 mol/L) and mixed for 3 min by shaking produced a single-phase DES and sample system. Following the drop-by-drop addition of 95 μL of HCl (5 mol/L), the target analytes and DES-3 were extracted as a single phase on the sample solution surface without centrifugation. The extracted phase was solidified by immersing the sample tube in an ice-cold bath for 5 min. The solidified phase was collected in a clean container, allowed to liquefy at room temperature, and injected into the HPLC system for analysis. The pH-switchable DES-LPME approach for quantitative analysis was assessed with the following parameters: accuracy, precision, repeatability along with intra- and inter-day repeatability, LR (linear range), LOD (limit of detection), LOQ (limit of quantification), ER (extraction recovery), and EF (enrichment factor).
3 Results and discussion
3.1 Deep eutectic solvent selection
DESs interact differently with different analytes because of variations in polarity, structure, and other physical and chemical properties. Herein, seven different DESs were developed and tested for extracting morphine and codeine from diluted plasma samples using pH-switchability (Fig. 1). Table 1 illustrates that three DESs (DES-5, DES-6, and DES-7) were eliminated from the study due to their inability to turn on or off pH. The other DESs are effective at the molar ratio of l-menthol: CSA is 5:1. It is noted from Table 1 that DES-3 was found to be a suitable extractant based on its efficacy.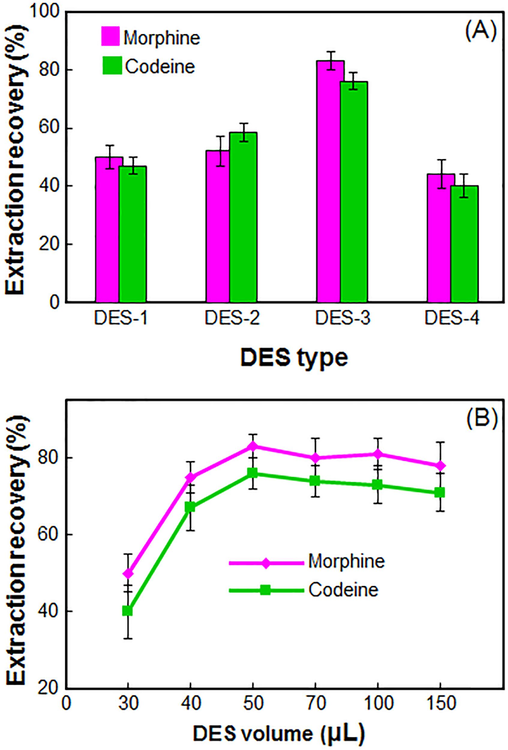
The effect of the DES type (A) and volume of DES (B) on the extraction recovery of morphine and codeine in blood samples obtained from DES–LPME/HPLC–UV.
3.2 Deep eutectic solvent volume selection
One important factor in liquid-phase microextraction that needs to be carefully studied is the extraction solvent volume. However, it should not be set so high that it reduces the enrichment factor. The influence of DES volume on morphine and codeine extraction recovery was tested by varying the volume from 30 to 150 μL. Fig. 2(B) shows that raising the volume of DES from 30 to 50 μL enhanced the ER of the required analytes. Additional DES volume increases will keep the extraction recovery steady or slightly lower. The decrease in extraction efficiency is likely related to variations in the alkali utilized (100 μL) and problematic extractant phase collection with increasing DES volume. Thus, 50 μL DES was selected as the optimal volume.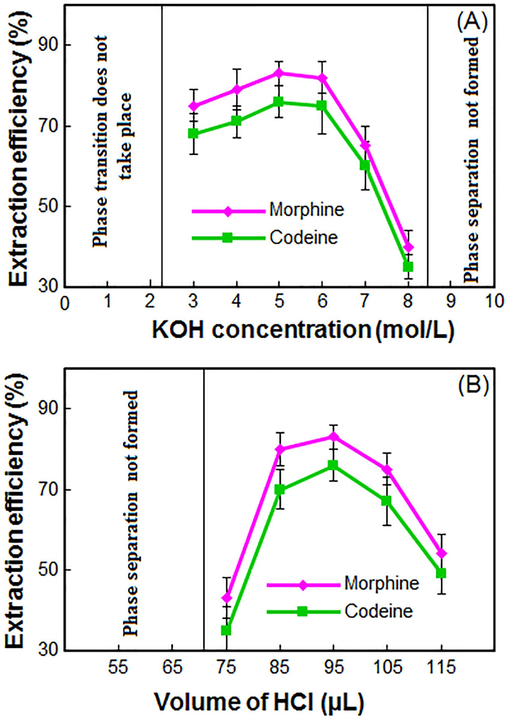
The effect of the KOH concentration (A) and volume of HCl (B) on the extraction recovery of morphine and codeine in blood samples obtained from DES–LPME/HPLC–UV.
3.3 KOH concentration selection
DESs used in this study are hydrophobic and immiscible with water. The present process transfers the DES into the sample system without needing a vortex, ultrasonic, or disperser solvent. As a result, the analytes' surface area in contact with the extraction solvent increases significantly, increasing extraction recovery. High concentrations of KOH are inefficient for achieving phase separation, while low concentrations do not show phase transition. Hence, KOH concentrations were optimized. Experiments were conducted with 100 μL of KOH at 1 to 10 mol/L concentrations. Fig. 2(A) shows that KOH concentrations below 3 mol/L do not result in phase transition or solvent transfer into the aqueous phase. The system is converted into a single phase during extraction by raising the KOH concentration from 3 to 5 mol/L. When the concentration is between 5 and 7 mol/L, the recommended volume of HCl is insufficient for phase separation, resulting in lower ER. Thus, the optimal KOH concentration was chosen as 5 mol/L.
3.4 HCl volume selection
From section 3.3, KOH concentration (7 mol/L) was preferred for the phase transition and single-phase system. Different HCl solutions (7 mol/L) with different volumes were used to neutralize the environment's alkalinity and separate phases. The phase separation was achieved by gradually increasing the HCl volume from 75 to 95 µL. Fig. 2(B) shows that the volume less than 75 µL does not result in phase separation. Strikingly, the phase separation gradually occurs at 95 µL, but the extraction recovery reduces with increasing HCl volume. Thus, 95 µL of the HCl was selected for testing.
3.5 Influence of salt
Analytes become less soluble in water and more inclined when salt is added to an aqueous solution. These two mechanisms partially neutralize the effects of salt on liquid-phase microextraction efficiency. The proposed procedure involves mixing acid-base and adjusting pH to obtain a high-concentration KOH solution. It is reasonable to assume that increasing the salt concentration does not influence extraction efficiency. However, a series of studies were conducted using NaCl solution at 0 to 5 % (w/v) concentrations. The findings of the studies that were carried out without the addition of NaCl showed that the addition of varied amounts of NaCl did not affect the analyte extraction recovery. Therefore, the subsequent studies were carried out in the absence of NaCl.
3.6 Influence of extraction time
According to the method outlined, the ER is considered as the interval between adding the KOH solution and starting to add the HCl solution. To examine the impact of extraction time (ET) on the recovery of morphine and codeine, we utilized an ET of 0 for the sample solution, resulting in a single-phase system. The extractant takes over 30 s for complete miscibility with the aqueous solution, and hampers ER due to the unavailability of the high contact surface. Intervals longer than 30 s have minimal influence on ER. Thus, the ideal extraction time was set at 30 s.
3.7 Quantitative analysis
An overview of the parameters used to construct the calibration curve is given in Table 2. The linear range of morphine and codeine was achieved by spiking blank blood with varying dosages of target analytes, and the samples were examined in triplicate. While considering days as a coefficient, the inter-day studies revealed a linear range of 1.5–300 μg L-1. It was accomplished by adding predetermined doses of codeine and morphine to the defined concentration of blood samples. Following analyte extraction and HPLC analysis with seven replications, the analytes were determined to be 3.7–4.3 % and 5.4–6.2 %, respectively. An accuracy of 93.0 − 107.0 and 91.8 − 106.0 % was found for the inter-day and intra-day, respectively. The signal-to-noise ratio of 3 (S/N = 3) resulted in LODs of 0.5 µg/L, whereas S/N = 10 gave LOQs of 1.5 µg/L. At 100 μg L-1, the EF and ER of morphine and codeine were found to be 152–166 % and 76–83 %, respectively.
Compounds
RSDa (intra-day, n = 7)
RSD (inter-day, n = 7)
r2
LODb (μg L−1)
LOQc (μg L−1)
LRd (μg L−1)
EFe
ERf (%)
Morphine
3.7
5.4
0.9988
0.5
1.5
1.5 − 300
166
83
Codeine
4.3
6.2
0.9951
0.5
1.5
1.5 − 300
152
76
3.8 Real samples analysis
After optimizing all parameters and creating a calibration plot, the method was tested on real blood samples. Initially, a blank blood sample free from drugs was collected from a 28-year-old healthy male volunteer and evaluated using the pH-switchable DES-LPME method. According to the results, the analytes were absent in the blank sample collected from the healthy male volunteer. Next, a blood sample from a 61-year-old opium user was collected and evaluated using the current approach. The samples tested positive for morphine and codeine at concentrations of 158.5 and 78.2 μg.L-1, respectively. Furthermore, blood samples from two patients − a 25-year-old male patient and a 43-year-old female patient − who were undergoing morphine and codeine treatment showed the presence of both analytes. The results are presented in Table 3.
Human blood samples
Analyte
Added (µg/L)
Found (SDa, n = 3) (µg/L)
Relative recovery (%)
Blank (taken from 28-year-old healthy male volunteer)
Morphine
0
−
−
10
9.7 (0.6)
97
50
53.4 (2.1)
106.8
100
99.4 (5.2)
99.4
Codeine
0
−
−
10
10.4 (0.3)
104
50
47.3 (2.2)
94.6
100
91.8 (4.5)
91.8
Taken from addicted male to opium (61-year-old)
Morphine
0
158.5 (8.4)
−
10
169.1 (7.0)
106
50
206.3 (12.5)
95.6
100
257.9 (16.1)
99.4
Codeine
0
78.2 (4.3)
−
10
88.7 (3.6)
105
50
129.0 (5.9)
101.6
100
175.6 (8.1)
97.4
Taken from a patient under morphine and codeine treatment (25-year-old male)
Morphine
0
118.6 (5.5)
−
10
129.3 (6.4)
107
50
166.9 (7.8)
96.6
100
223.2 (10.5)
104.6
Codeine
0
14.8 (1.3)
−
10
24.3 (2.0)
95
50
63.6 (3.3)
97.6
100
116.2 (5.1)
101.4
Taken from a patient under morphine and codeine treatment (43-year-old female)
Morphine
0
98.1 (4.7)
−
10
107.4 (5.0)
93
50
146.2 (6.8)
96.2
100
203.4 (9.7)
105.3
Codeine
0
26.3 (1.8)
−
10
36.9 (2.4)
106
50
73.8 (3.2)
95
100
119.7 (5.9)
93.4
Real blood samples were tested for the matrix effect by adding morphine (10, 50, and 100 μg L-1). The findings of the triplicate experiment showed that the relative recoveries of morphine and codeine in blood samples had a standard deviation of less than six and ranged from 91.8 to 107.0 %. The relative recoveries that were obtained indicated that the matrix unaffected the performance of the suggested method for extracting analytes from blood samples.
3.9 Comparison to other methods
Table 4 compares the analytical figures obtained from the pH-switchable DES-LPME technique with HPLC-UV for the preconcentration and identification of morphine and codeine in biosamples. Compared to other analytical procedures, the present method has a low LOD (0.5 μg/L) and a broader linear range (1.5–300 μg/L) with RSD < 5 %. The approach had a lower extraction time (<2 min) and a greater preconcentration factor (152 − 166) compared to other methods. The reported analytical methodology proved to be an effective, sensitive, and reliable method for analyzing pharmaceuticals in biological samples, as evidenced by the satisfactory results.
Extraction technique
Sample
Linear range (µg/L)
LOD
(µg/L)
RSD%
Extraction time (min)
Reference
MSPE − HPLC − UV
Blood, urine
0.01 − 10
0.0018–0.0021
1.02
20
(Bonifonte et al., 2021)
0.01 − 10
0.0018–0.0021
5.10
SPE − HPLC − UV
Blood, urine
3 − 1000
0.8
4.8
25
(Plueschke et al., 2022)
3 − 1000
0.9
6.8
Rapid Extraction − GC − MS
Urine
250 − 1000
250
7.1 − -8.7
−
(Raj, 2020)
100 − 1000
50
8.4 − 11.7
SPE − MEKC
Urine
0.30 − 10
90
2.70
15
(Paice et al., 2023)
0.22 − 10
70
3.39
DLLME − SFO − HPLC − UV
Plasma
15 − 1000
5
7.4
10
(Zhang et al., 2022)
15 − 1000
5
6.5
DLLME − HPLC − UV
Urine
20 − 500
7
6.1
4
(Rodríguez-Ramos et al., 2020)
30 − 500
10
5.7
DES − LPME − HPLC − UV
Whole blood
1.5 − 300
0.5
3.7
<2
This work
1.5 − 300
0.5
4.3
4 Concluding remarks
The present study employed an improved analytical methodology for the preconcentration and measurement of morphine and codeine in human plasma samples. This methodology was based on pH-switchable deep eutectic solvents for liquid phase microextraction (DES − LPME) followed by HPLC − UV. The method used here made it simple to identify morphine and codeine in actual blood samples, even at extremely low quantities (0.5 μL). The proposed method's key advantage was using pH to switch for the extraction process. An accuracy of the method was 93.0 − 107.0 for the inter-day and 91.8 − 106.0 % for the intra-day. The sensitivity of the method was judged by LODs of 0.5 µg/L and LOQs of 1.5 µg/L, which was useful in detecting the opioids in the real samples. The high sensitivity, accuracy, LOD, and LR and the method validity demonstrated by recovery tests with spiked samples indicate the method has huge potential to analyze biological samples with various drugs.
CRediT authorship contribution statement
Dalal N. Binjawhar: Writing – review & editing, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Walaa Mohammedsaeed: Writing – review & editing, Writing – original draft, Methodology.
Acknowledgements
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R155), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Spectral and thermophysical properties of some novel deep eutectic solvent based on l-menthol and their mixtures with ethanol. J. Mol. Liq.. 2019;285:477-487.
- [Google Scholar]
- Trace determination of triazine herbicides in fruit and vegetables using novel hydrophobic deep eutectic solvent-based dispersive liquid-liquid microextraction followed by high-performance liquid chromatography-ultraviolet. J. Sep. Sci.. 2022;45:4448-4459.
- [Google Scholar]
- Evaluation of blood lead levels in opium addicts and healthy control group using novel deep eutectic solvent based dispersive liquid–liquid microextraction followed by GFAAS. Environ. Sci. Pollut. Res.. 2023;30:24553-24561.
- [Google Scholar]
- Dispersive liquid–liquid microextraction followed by high-performance liquid chromatography–ultraviolet detection to determination of opium alkaloids in human plasma. J. Pharm. Biomed. Anal.. 2013;85:14-20.
- [Google Scholar]
- Extraction and determination of strobilurinfungicides residues in apple samples using ultrasound-assisted dispersive liquid-liquid microextraction based on a novel hydrophobic deep eutectic solvent followed by HPLC-UV. FoodAdditContam. Part A.. 2022;39:105-115.
- [Google Scholar]
- Determination and extraction of acrylamide in processed food samples using alkanol-based supramolecular solvent-assisted dispersive liquid-liquid microextraction coupled with spectrophotometer: Optimization using factorial design. J. Food Compost. Aal.. 2023;11:105023
- [Google Scholar]
- Morphine equivalent total dosage as predictor of adverse outcomes in opioid prescribing. Pain Med.. 2021;22(12):3062-3071.
- [Google Scholar]
- Simultaneous determination of morphine, codeine and 6-acetylmorphine in human urine and blood samples using direct aqueous derivatisation: Validation and application to real cases. J. Chromatogr. B.. 2014;949–950:127-132.
- [Google Scholar]
- Simultaneous derivatization and lighter-than-water air-assisted liquid–liquid microextraction using a homemade device for the extraction and preconcentration of some parabens in different samples. J. Sep. Sci.. 2018;41:3105-3112.
- [Google Scholar]
- Sensitive determination of vincristine in plasma ofchildren with leukaemia using vortex-assisteddispersive liquid–liquid microextraction based on hydrophobic deep eutectic solvent. RSC Adv.. 2022;12:3611-3617.
- [Google Scholar]
- Ultrasound-assisted dispersive liquid-phase microextraction by solidifying L-menthol-decanoic acid hydrophobic deep eutectic solvents for detection of five fungicides in fruit juices and tea drinks. J. Sep. Sci.. 2021;44:3870-3882.
- [Google Scholar]
- HPLC-DAD determination of CNS-acting drugs in human blood, plasma, and serum. Crit. Rev. Anal. Chem.. 2014;44(1):68-106.
- [Google Scholar]
- A comparative analysis of conventional and deep eutectic solvent (DES)-mediated strategies for the extraction of chitin from marine crustacean shells. Molecules. 2021;26(24):7603.
- [Google Scholar]
- Sustainable green extraction of anthocyanins and carotenoids using deep eutectic solvents (DES): A review of recent developments. Food Chem.. 2024;448:139061
- [Google Scholar]
- Use of Opioids for Adults With Pain From Cancer or Cancer Treatment: ASCO Guideline. J Clin Oncol.. 2023;41(4):914-930.
- [Google Scholar]
- Development and validation of a gas chromatography-mass spectrometric method for the determination of sildenafil and desmethyl-sildenafil in whole blood. J. Sep. Sci.. 2011;34:3037-3042.
- [Google Scholar]
- Development of a liquid-phase microextraction based on the freezing of a deep eutectic solvent followed by HPLC-UV for sensitive determination of common pesticides in environmental water samples. RSC Adv.. 2018;8:11412-11418.
- [Google Scholar]
- preconcentration and determination of amoxicillin andceftriaxone in hospital sewage using vortex-assisted liquid phase microextraction based on the solidification of the deep eutectic solvent followed by HPLC–UV. Int. J. Envron. Anal. Chem.. 2019;99:112-123.
- [Google Scholar]
- Prescribing Patterns of Codeine and Alternative Medicines in Children in Europe. Drug Saf.. 2022;45(10):1069-1081.
- [CrossRef] [Google Scholar]
- Thin-layer chromatography with eutectic mobile phases—preliminary results. J. Chromatogr. A.. 2020;1621:461044
- [Google Scholar]
- Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A.. 2006;1116:1-9.
- [Google Scholar]
- Critical review and re-assessment of analyte protectants in gas chromatography. J. Chromatogr. A.. 2020;1632:461596
- [Google Scholar]
- Synthesis and characterization of novel 1,3-benzodioxole tagged noscapine based ionic liquids with in silico and in vitro cytotoxicity analysis on HeLa cells. J. Mol. Liq.. 2020;302:112525
- [Google Scholar]
- Extraction and determination of opium alkaloids in urine samples using dispersive liquid–liquid microextraction followed by high-performance liquid chromatography. J. Chromatogr. B.. 2011;879:2978-2983.
- [Google Scholar]
- Synthetic pharmaceutical peptides characterization by chromatography principles and method development. J. Sep. Sci.. 2022;45(13):2200-2216.
- [Google Scholar]
- Pharmacological aspects of over-the-counter opioid drugs misuse. Molecules. 2020;25(17):3905.
- [Google Scholar]
- Chemiluminescence detection of heroin in illicit drug samples. Talanta.. 2013;116:619-625.
- [Google Scholar]
- Determination of levofloxacin by HPLC with fluorescence detection in human breast milk. Bioanalysis. 2021;13(13):1063-1070.
- [Google Scholar]
- Opium alkaloids, biosynthesis, pharmacology and association with cancer occurrence. Open Biol.. 2023;13(5):220355
- [Google Scholar]
- A new electrochemical platform based on carbon black paste electrode modified with α-cyclodextrin and hierarchical porous carbon used for the simultaneous determination of dipyrone and codeine. Microchem. J.. 2021;164:106032
- [Google Scholar]
- Yang Z, Wang L, Xu M, Gu J, Yu L, Zeng S. Simultaneous analysis of gemfibrozil, morphine, and its two active metabolites in different mouse brain structures using solid-phase extraction with ultra-high performance liquid chromatography and tandem mass spectrometry with a deuterated internal standard. J. Sep. Sci. 2016; 39: 2087−2096.
- Simultaneous analysis of gemfibrozil, morphine, and its two active metabolites in different mouse brain structures using solid-phase extraction with ultra-high performance liquid chromatography and tandem mass spectrometry with a deuterated internal standard. J. Sep. Sci.. 2016;39:2087-2096.
- [Google Scholar]
- Utilization of magnetic pomelo peel-derived biochar for extraction and liquid chromatography-mass spectrometry determination of opioid drugs in wastewaters. J. Sep. Sci.. 2022;45:4099-4106.
- [Google Scholar]
- Determination of morphine and codeine in urine using poly(dimethylsiloxane) microchip electrophoresis with electrochemical detection. J. Pharm. Biomed. Anal.. 2007;43:237-242.
- [Google Scholar]







