Translate this page into:
Exploring the development of a promising mucosal adjuvant vaccine for human respiratory syncytial virus (RSV) infection
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Human respiratory syncytial virus (RSV) is one of the common causes of respiratory illnesses and hospitalizations among infants and young children worldwide, yet an effective vaccine remains unavailable. Cytokines that can activate B-cells and stimulate antibody production, such as thymic stromal lymphopoietin (TSLP), could be used to produce robust and long-lasting mucosal responses to respiratory virus infection. TSLP cytokine is expressed by many immune and non-immune cells, for example airway epithelial cells and mediates a crucial role in activating and maturing dendritic cells and T helper 2 cells. TSLP also promotes cytokine production from activated Th2 cells, such as IL-5, that enhance antibody class switching recombination to IgA, which can protect against viral respiratory infections at the mucosal surfaces. This review is focused on the effect of TSLP on local B-cell responses and IgA antibody production during respiratory viral infections. We suggest that mucosal vaccines could provide better protection against RSV. A novel RSV mucosal vaccine that uses TSLP as an immunostimulant with the RSV Fusion protein (RSV-F) could enhance the local adaptive immune response, generating extended lasting tissue resident memory cells and block early RSV replication, and increase viral clearance from airways. However, additional investigations are required to determine the challenges and limitations of utilizing TSLP cytokine as an immunostimulant combined with RSV-F as it may lead to excessive Th2 immune responses.
Keywords
Mucosal vaccine
B cells
RSV
Adjuvant
IgA
TSLP
RSV-F
Th2 cell
1 Introduction
Respiratory syncytial virus (RSV) infection is a bronchial illness that commonly affects young children as well as infants (Harcourt et al., 2016). However, it can also infect older adults and immunocompromised individuals (Brandao-Rangel et al., 2021). Severe RSV infection may result in life-threatening conditions such as pneumonia or bronchiolitis (Prill, Langley, Winn, & Gerber, 2021). RSV infections are estimated to cause around 33 million significant cases of acute pneumonia in the lower respiratory tract and result in 3.2 million hospitalizations globally each year, and most of these cases occur in countries with low and middle income (Li et al., 2020). Thus, infection with this virus may lead to significant impact on the public health, emphasizing the importance of ongoing efforts to create and improve strategies for prevention and treatment.
RSV is a member of the Pneumoviridiae family and has two circulating strains, including A and B, and both are known to cause similar symptoms (Muñoz-Escalante et al., 2019). The virus genome is negative sense, single-stranded, non-segmented, and encloses ten genes encoding eleven proteins, including three non-structural and eight structural proteins (Ramilo, Rodriguez-Fernandez, & Mejias, 2023). The viral surface membrane comprises three structural proteins, including small hydrophobic (SH), glycoprotein (G), and fusion (F) glycoproteins, while internal proteins are five and exists inside the virus (Ramilo et al., 2023). The (F) and (G) proteins have essential for virus infection as well as pathogenesis, with the G protein sticking to host cells and targeting ciliated cells, and the F protein initiating viral penetration and causing cell fusion. Although SH protein not essential for infection, it can sometimes cause syncytia to form late in the infection (Ramilo et al., 2023).
The primary neutralizing antibody against the F protein, which can allow viral fusion with respiratory cells, but RSV, can alter it to evade neutralization. The G protein targets ciliated cells and subtype classification varies accordingly (Capella et al., 2017). Therefore, both these viral proteins can be targeted for development of novel vaccines as well as monoclonal antibodies. Although RSV has been studied for many years, there are still no licensed vaccines available yet. Conventional vaccine approaches, such as live attenuated, subunit, or inactivated vaccines, have failed to provide significant protection against RSV, because the virus has the capacity to avoid being detected by the host immunity (Soto et al., 2020). Therefore, one potential strategy for developing a vaccine against RSV is the use of mucosal adjuvants (Lavelle & Ward, 2022). Mucosal adjuvants have been found to increase the immune response to RSV antigens when delivered via mucosal routes such as intranasal or oral delivery (Lavelle & Ward, 2022). Mucosal adjuvants can stimulate the immune system at mucosal surfaces, which are the primary sites of pathogen entry and replication (Stevceva & Ferrari, 2005). Mucosal adjuvants have the potential to induce systemic and mucosal immunity, providing better protection against RSV infection (Stevceva & Ferrari, 2005). One of the main obstacles in creating an effective RSV vaccine is identifying an appropriate adjuvant that can improve the immune response to the vaccine antigen. Cytokines that stimulate B cell responses and antibody production are potential vaccine adjuvants that could improve vaccine efficacy. TSLP cytokine is expressed by several of cells and tissues cells, including airways in the lungs and mediates its biological functions after binding to its receptor, TSLP-R (Ebina-Shibuya & Leonard, 2023).
This review summarizes current insights into mucosal adjuvants for RSV vaccines, with a focus on the role of TSLP as an adjuvant in RSV-F-based vaccine development. Recent studies conducted in this field will be highlighted and considered to assess the potential of TSLP as an effective adjuvant in RSV vaccine development.
2 RSV infection and host immune response
RSV primarily infects epithelial cells of the airways, and in response, the host immunity activates pattern recognition receptors (PRRs), for instance, Toll-like receptors (TLRs), to identify and respond to viral pathogens (Ouyang et al., 2022). Recent study has shown TLRs are essential for establishing protective host immune responses against RSV (Alshaghdali, Saeed, Kamal, & Saeed, 2021). Airway epithelial cells express both TLR-4 and TLR-3, which recognize RSV infection and activate the innate immune response. TLR-4 is presented normally on the surface of the epithelial cells in the airways and has been shown to recognize fusion protein of RSV (Yuan et al., 2018).
In addition, TLR-3 recognizes certain viral mediators such as double-stranded RNA (dsRNA) during RSV replication. Activation of TLR-3 and TLR-4 triggers signalling pathways, leading to the release of inflammatory mediators and type-I interferers (IFN), particularly IFN-β and IFN-α, with infected host cells, and the release of IFN-β can act as an antiviral drug and prevent RSV replication and facilitate clear virus. In contrast, IFN-β deficiency leads to an inflammatory response, resulting in lung damage in human and mouse models of infection (Alshaghdali et al., 2021; Hijano et al., 2018). However, RSV has the ability to modulate the host immune system, and evade immune response (Ouyang et al., 2022).
Understanding the interaction between RSV, airway epithelial cells, TLRs, and IFN-β is therefore essential for devising effective treatments and vaccines against RSV infection (Ouyang et al., 2022). Furthermore, current studies have established that TLR2/TLR6 and TLR7 are implicated in RSV identification and consequent innate immune activation. (Alshaghdali et al., 2021; Klouwenberg, Tan, Werkman, van Bleek, & Coenjaerts, 2009).Therefore, TLRs have an essential function in initiation the early host innate immune response to RSV infection, and additional investigations are necessary to define the precise mechanism of TLRs in RSV recognition and subsequent initiation of the natural immune response. In addition, administering IFN-α to high-risk infants who have not been exposed to RSV can reduce lung injury and enhance virus elimination. However, RSV has mechanisms that avoid the immune system, such as NS1 and G proteins that impede the type- I IFN response (Tognarelli, Bueno, & González, 2019). Therefore, an effective RSV vaccine candidate should elicit a strong type I IFN response. Overall, early detection and recognition of RSV by the host innate immune response is an essential for determining the expansion of adaptive immune responses (Sun & López, 2017).
The cellular adaptive immunity, which includes T cells (CD4 andCD8) and specific antibodies (IgG and secretory IgA), is necessary to control RSV infection and protect against re-infection (Lambert, Sagfors, Openshaw, & Culley, 2014; Russell, Unger, Walton, & Schwarze, 2017). However, most adults fail to achieve sufficient antibody titres for protection against RSV, which permits the virus to infect again and succeed (Lambert et al., 2014). The cause of this incomplete immunity is not yet clear, but it is likely a result of inadequate and persistence of humoral and cellular immune responses (Openshaw & Chiu, 2013). Therefore, improved understanding of RSV immunology is essential for effective vaccine design. In addition, CD8 T cells are crucial in clearing RSV infection, and studies in mice show that CD8 memory T cells can protect against reinfection (Graham, Bunton, Wright, & Karzon, 1991; Schmidt et al., 2018). However, natural RSV infection prompts low CD8 T cells levels, so a vaccine that stimulate a Th-1mediated immune response and generates memory CD8 T cells would be beneficial (Bueno et al., 2008; Cautivo et al., 2010; Céspedes et al., 2017). In addition, the precise role of CD4 T cells during RSV infection remains debatable since they can either trigger immunopathology or provide protection in mouse models. Thus, to create an effective vaccine, it is important to promote both(CD8 and CD4) T cells by inducing a Th-1 immune response to foster protective immunity against RSV(Soto et al., 2020). Overall, the development of potent RSV is challenging. Thus, a deeper comprehension of how the host response react to RSV infection can help us to identify the crucial elements of the immune response that need to be targeted by mucosal adjuvants.
3 Current vaccines against RSV
Although there have been attempts since the 1960 s to create a safe and reliable RSV vaccine, it has not been successfully achieved yet. One such attempt was the formalin-inactivated RSV (FI-RSV) vaccine, which has been examined on infants around fifty years ago, which caused some complication and deaths upon following exposure to natural RSV infection (Kapikian, Mitchell, Chanock, Shvedoff, & Stewart, 1969). Those who received the FI-RSV vaccine showed reduced levels of neutralizing antibodies after spontaneous infection. This could be because formalin inactivation causes alterations in the protein processing of the RSV surface glycoproteins, including (F and G). Consequently, the resulting antibody response was mostly nonfunctional and did not offer neutralization(Murphy et al., 1986). Nowadays, there are no active therapies to cure an RSV infection. Nevertheless, administration of monoclonal antibody (Palivizumab) that particularly interacts with the F protein of the RSV can possibly prevent severe disease and lower respiratory tract infections during the RSV season. However, its use is not advised for healthy infants and does not prevent upper respiratory system infections (Anderson et al., 2013; Blanken et al., 2013).
Several vaccines have been generated to produce neutralizing antibodies in at-risk groups, including pregnant women, to prevent early RSV infections by transferring maternal antibodies. However, these vaccines can provide only short immunity. In contrast, intranasal vaccines can generate significant quantities of IgA neutralizing antibodies, which has an important role in providing protecting against RSV infections. Intranasal vaccines are therefore a potential alternative approach to the existing vaccines (Ascough et al., 2019; Hijano et al., 2018; J.-Y. Lee & Chang, 2017; Salisch et al., 2019; Yang & Varga, 2014).
Furthermore, mucosal immunity plays a crucial role in protecting mucosal surfaces that are susceptible to inhaled pathogens and since numerous microbes, for example RSV, penetrate via mucosal membranes, it is imperative to develop vaccines that can protect these vulnerable entry points (Alturaiki, 2022a; Lycke, 2012). Thus, for effective mucosal immune responses, it is necessary to employ suitable methods of administration, along with specific adjuvants and delivery systems. Mucosal vaccination can trigger a better immune protection at mucosal sites than parenteral immunization. Among the various methods of administering vaccines to mucosal surfaces, the most efficient delivery method is intranasal, as it can elicit pro powerful and comprehensive immune responses at various mucosal sites, surpassing alternative routes of administration (Holmgren & Czerkinsky, 2005; Rose, Zielen, & Baumann, 2012).
Recurring RSV infections can be caused by insufficient levels of nasal mucosa-specific IgA antibodies(Walsh & Falsey, 2004). The vaccine must induce IgA antibodies in the respiratory mucosa to avert RSV infection (Yang & Varga, 2014). Administering the vaccine through the nasal route is more easily accepted and safer, particularly for children (Lavelle & Ward, 2022). A recent study testing a potential vaccine for RSV, which is not yet approved and is under phase three clinical trial (registration number: NCT04908683), demonstrated that the Ad26.RSV.preF vaccine, which encodes the RSV F, reduced RSV infections by approximately 40 % compared to the control after four weeks of injection(Sadoff et al., 2022).
Moreover, the mRNA vaccine platform has demonstrated effective protection against SARS-CoV-2 (Toutoudaki, Dimakakou, & Androutsakos, 2023). Currently, a phase III clinical trial (NCT05127434) is underway for an mRNA vaccine that encodes for RSV-F glycoprotein. Both vaccine candidates primarily stimulate systemic immunity and are administered intramuscularly, with repeated immunizations resulting in low to modest levels of adaptive immunity in the mucosal membranes (Salisch et al., 2019). However, the mucosal vaccination using the Adenovirus and Modified Vaccinia Ankara RSV vector has shown promising results in inducing strong cellular and mucosal immune responses against RSV, particularly against the F protein, and this can protect mice from lethal infection caused by RSV (Pierantoni et al., 2015). These findings propose that such a vaccine could potentially be developed for use in humans to prevent RSV infection.
Furthermore, a recent study has revealed that the Ad-RSVF vaccine, which encodes the fusion protein and uses a molecular adjuvant adenovirus serotype 5, is safe, immunogenic, and induces robust specific antibodies capable of neutralizing the virus. This provides effective immunity protection against RSV when administered through various methods, including orally, intranasally, or intramuscularly in cotton rats, as compared to the FI-RSV vaccine. The study suggests that the Ad-RSVF vaccine has the potential to become a more effective and safer vaccine against RSV (Joyce et al., 2018).
Furthermore, studies have revealed that delivering mucosal vaccines is crucial for the development of respiratory tissue-resident memory T cells (Lapuente et al., 2018; Zens, Chen, & Farber, 2016).Therefore, mucosal vaccines that elicit both local and systemic immunity can provide protective mucosal immunity against respiratory viral infections. Additionally, a recent study, utilized adenoviral vector that codes for the RSV fusion protein along with genetic adjuvant encoding for Interleukin (IL)-1β, showed that, the vaccine has led to elevated high specific IgA mucosal against F protein of RSV as well as generation of extended lasting tissue-resident memory T cells (TRM), which effectively controlled viral load and reduced lung damage (Maier et al., 2022). This suggests that the vaccine can stimulate both mucosal immune responses and systemic immunity.
4 Expression of the TSLP cytokine and its receptor TSLP-R
TSLP is one member cytokine of the IL-2 family and has a structure that is composed of four different −helical bundles. It was discovered in 1994 in the culture supernatant of a mouse thymic stromal cell line, and was found to enhance the expansion and maturation of immature B cells (Levin et al., 1999). TSLP is secreted by various cells, including airways epithelial, smooth muscle, fibroblast, mast, dendritic, T cells, B cells, granulocyte, skin, gastrointestinal tract, and macrophage/monocyte cells (Alturaiki, 2022b; Kurihara, Kabata, Irie, & Fukunaga, 2023; Mitchell & O'Byrne, 2017; Pandey et al., 2000; Verstraete et al., 2017).
Furthermore, TSLP expression has been reported in other diseases such as autoimmune disorders, allergic diseases as well as cancer (Matera, Rogliani, Calzetta, & Cazzola, 2020; Varricchi et al., 2018) TSLP can induce naïve CD4 + T lymphocytes to differentiate into type 2 cells, that produce cytokines, including IL-4, IL-5, IL-13, and decrease IFN-γ expression related to type 1 cells (Mitchell & O'Byrne, 2017; Verstraete et al., 2017). Moreover, TSLP expression is regulated by cytokines, including TNF-α and IL-1β (H.-C. Lee & Ziegler, 2007), as well as proteases such as tryptase and papain (Kouzaki, O'Grady, Lawrence, & Kita, 2009). TSLP expression can also be stimulated by microbial infection in the lungs, including viral, bacterial and fungal infections (Han et al., 2017; Vu et al., 2010), as well as environment factors such cigarette smoke (Nakamura et al., 2008), allergens (Zhou et al., 2005), and mechanical injury (Oyoshi, Larson, Ziegler, & Geha, 2010). Furthermore, TSLP exert its biological activity after binding to a double-chain receptor, IL-7Rα and TSLPR. TSLPR on its own has weak binding affinity for TSLP, but when TSLPR binds with IL-7Rα, it generates a high attraction site and initiates signalling (He & Geha, 2010; Ziegler et al., 2013).
5 Role of the TSLP cytokine in B cell response and antibody production
The initial studies have suggested that TSLP cytokine has an impact on B cells lymphopoiesis, and abnormal expression of TSLP can have an important consequence on B cells (Levin et al., 1999; Ziegler et al., 2013). Moreover, TSLP has been shown to activate B cells in the spleens of mice (Iseki et al., 2012). TSLP can directly induce activation and proliferation of T cells, enhance production of Th2 cytokines, and support B-cell activation, expansion and antibody production (Domeier, Rahman, & Ziegler, 2023; Kim, Jin, Nam, & Park, 2011). Furthermore, TSLP can enhance and promote the differentiation of pro-B cells and pre-B cells (Scheeren et al., 2010). Therefore, TSLP is vital for B cell response and antibody production as it stimulates B-cell expansion and differentiation. Moreover, recent study has also established that TSLP can induce the activation and differentiation of T follicular cells (TFH) as well as germinal centre (GC) response, suggestion that TSLP could be a useful adjuvant for activation and differentiation of TFH cells as well as humoral response in antibody production (Marschall et al., 2021). In addition, TSLP is expressed by tonsil follicular dendritic cells and plays an important role in IgA class switching, leading to increased levels of IgA in the serum, which may be related to the development of IgA nephropathy, a kidney disease characterized by the deposition of IgA in the glomeruli of the kidney (Meng et al., 2016). Furthermore, the active and well-controlled GC response is essential for generating efficient and long-lived antibodies, and TSLP has a significant role in regulating this activity, and deletion of TSLP-r has resulted in distinct antibody production and memory cells (Domeier et al., 2023). Finally, TSLP plays an essential role in antibody class-switching recombination in humans (Xu et al., 2007). Overall, TSLP has been shown to have a major impact on the development as well as on the production and regulation of antibodies.
6 TSLP cytokine as potential mucosal vaccine adjuvant
To prevent pulmonary microbial infections, there is a serious requirement for the development of novel mucosal vaccines and adjuvants. Therefore, mucosal vaccines are considered ideal for preventing pathogens invading through mucosal sites. Secretory IgA (SIgA) antibodies are responsible for to provide immune protection at the mucosal surfaces (Cerutti, 2008). Thus, designing effective mucosal vaccines, it is crucial to develop strategy to that stimulates SIgA responses specific to the antigen. However, the mechanisms that trigger and sustain immune responses in mucosal tissues, especially in the respiratory tracts after mucosal vaccination, are not yet completely known.
One possible alternative method for inducing strong and lasting mucosal responses to respiratory viral infection involves using certain cytokines that are associated with B-cells, such as TSLP (Cerutti, Chen, & Chorny, 2011). Earlier study has shown that TSLP could be a beneficial adjuvant for enhanced the efficiency of Bacillus Calmette-Guerin vaccine ( BCG), as it can lead to activation of DCs and expansion of cytotoxic T cell (CD8) (Sugimoto et al., 2010). In addition, a study conducted in mice using TSLP as vaccine adjuvant against HIV, has demonstrated that can stimulate robust local cellular and specific antibody responses in both the serum and mucosa, which can last for long time (Van Roey, Arias, Tregoning, Rowe, & Shattock, 2012). Besides, a recent study indicated that the levels of TSLP and its receptor (TSLP-R) were increased in mucosal dendritic cells (DCs) of mice that received nasal immunization with CT (cholera toxin) and pneumococcal surface protein A, and mice lack of TSLP-R gene the specific IgA response was significantly decreased (Joo et al., 2017), suggesting that TSLP and its receptor play a major role in the adjuvant effect of CT for mucosal immunity and highlights the importance of TSLP-TSLP-R signaling in the production of IgA.
Additionally, a recent study has shown that TSLP can act as a strong adjuvant of IFN-λ in mice vaccinated against influenza; TSLP has the ability to induce activation and migration of the DCs from the lungs to the nearest lymph nodes and prompt activation of the germinal center, which leads to the production of specific neutralizing antibodies, including IgG and IgA against influenza. However, this vaccine-enhancing activity was not observed in TSLPr-/- mice, indicating that the existence of endogenous TSLP signaling is critical in determining the immune-enhancing effect of IFN-λ(Ye et al., 2019). Although the TSLP cytokine has been shown to support the airway immune response, it has also been shown to enhance the excessive expression of TH-2 cytokines, such as IgE, which can facilitate to the progression of allergy disorders, such as asthma (de Lima et al., 2023).
From the above mentioned, it is evident that TSLP has a significant role in providing and enhancing local immune response following pulmonary infection. Therefore, we can suggest that TSLP can be used as a mucosal adjuvant vaccine combined with RSV-F protein, which may induce robust local immune cells and enhance the formation of tissue-resident cells (Fig. 1). However, the importance of TSLP/TSLP-r and its potential role in the worsening allergy disorders following respiratory viral infection require more research to be defined.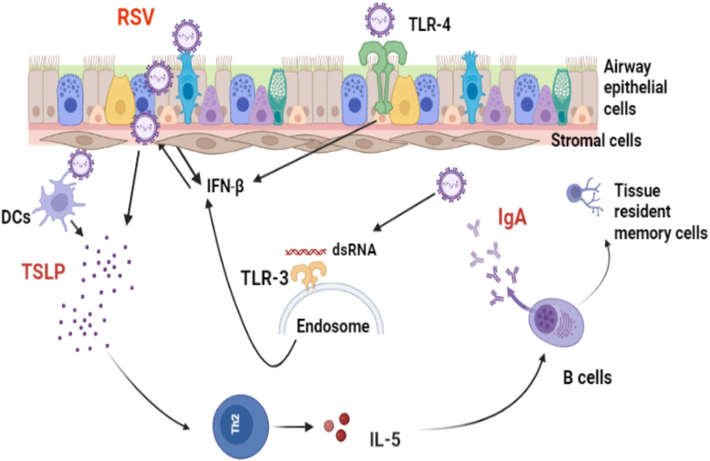
Role of TSLP during RSV- infected epithelial cells and enhanced production of IgA antibody. Upon RSV infection, innate immune sensors expressed on the airway epithelial cells and endoplasmic reticulum, such as TLR-3 and TLR-4, can identify RSV through viral nucleic acids and the RSV-F surface protein, respectively. This recognition leads to the releasing of Interferon beta (IFN-β), which clears the virus and stimulates the antiviral process in nearby cells. Furthermore, RSV-infected airway epithelial cells trigger the expression of TSLP, which can activate dendritic cells and enhance antigenic viral peptide presentation to Th2 cells and enhanced production of IL-5 cytokine, which plays an essential role in activating antibody class switching to IgA in the lungs and increasing protective immunity against RSV. Therefore, TSLP cytokine may serve as an effective vaccine adjuvant when combined with RSV-F protein, as it can promote IgA class switching, stimulate antibody production in mucosal tissues, and generate long-lasting tissue resident memory B cells.
7 Conclusion and future perspectives
As RSV primarily affects the respiratory mucosa, mucosal vaccine adjuvants are crucial in preventing RSV infection. At mucosal surfaces, a secretory IgA antibody has an essential role in providing protection against pulmonary infectious agents. An alternative approach to inducing long-lasting mucosal responses and combating respiratory viral infections is using B-cell-associated cytokines, such as TSLP. Combining TSLP cytokines with RSV-F could enhance vaccine efficacy against RSV and serve as novel mucosal adjuvants. However, there are several limitations and challenges to address while using TSLP as a vaccine adjuvant, such as the risk of excessive Th2 responses and allergic reactions. The optimal formulation, dose, and administration route of TSLP as an adjuvant remain undetermined. Therefore, further research is necessary to define TSLP's exact role and receptors, including its safe utilization as a vaccine. Future studies should investigate TSLP's synergistic effects with other adjuvants to improve vaccine efficacy and explore cellular and molecular pathways involved in immune responses to RSV to promote rational vaccine design. Ongoing research in this field is critical to developing efficient and safe vaccines, thereby preventing RSV spread among high-risk populations.
Funding
This work was supported by the Deanship of Graduate Studies and Scientific Research at Majmaah University [grant number R-2024-1155].
Acknowledgments
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Interaction of ectodomain of Respiratory Syncytial Virus G protein with TLR2/TLR6 heterodimer: An in vitro and in silico approach to decipher the role of RSV G protein in pro-inflammatory response against the virus. Curr. Pharm. Des.. 2021;27(44):4464-4476.
- [Google Scholar]
- Considerations for novel COVID-19 mucosal vaccine development. Vaccines. 2022;10(8):1173.
- [Google Scholar]
- High plasma levels of the TSLP cytokine in Saudi patients with chronic stable asthma. J. King Saud Univ. – Sci.. 2022;34(7):102271
- [CrossRef] [Google Scholar]
- Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31:B209-B215.
- [Google Scholar]
- Local and systemic immunity against respiratory syncytial virus induced by a novel intranasal vaccine. A randomized, double-blind, placebo-controlled clinical trial. Am. J. Respir. Crit. Care Med.. 2019;200(4):481-492.
- [Google Scholar]
- Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med.. 2013;368(19):1791-1799.
- [Google Scholar]
- Pulmonary function changes in older adults with and without metabolic syndrome. Sci. Rep.. 2021;11(1):17337.
- [Google Scholar]
- Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc. Natl. Acad. Sci.. 2008;105(52):20822-20827.
- [Google Scholar]
- Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis. 2017;216(11):1398-1406.
- [Google Scholar]
- Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guerin promotes virus clearance and protects from infection. J. Immunol.. 2010;185(12):7633-7645.
- [Google Scholar]
- Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol.. 2011;29:273-293.
- [Google Scholar]
- A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine. 2017;35(5):757-766.
- [Google Scholar]
- de Lima, L. C., Cruz, Á. A., Costa, R. d. S., Silva, H. d. S., Coelho, R. S., Teixeira, H. M. P., et al., 2023. TSLP and IL25 variants are related to asthma and atopy. Gene Reports, 30, 101727. doi: 10.1016/j.genrep.2022.101727.
- B cell–and T cell–intrinsic regulation of germinal centers by thymic stromal lymphopoietin signaling. Sci. Immunol.. 2023;8(79)
- [Google Scholar]
- Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol.. 2023;23(1):24-37.
- [CrossRef] [Google Scholar]
- Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest.. 1991;88(3):1026-1033.
- [Google Scholar]
- The innate cytokines IL-25, IL-33, and TSLP cooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus-infected immature mice. J. Immunol.. 2017;199(4):1308-1318.
- [Google Scholar]
- Human cathelicidin, LL-37, inhibits respiratory syncytial virus infection in polarized airway epithelial cells. BMC. Res. Notes. 2016;9:1-6.
- [Google Scholar]
- Type I interferon potentiates IgA immunity to respiratory syncytial virus infection during infancy. Sci. Rep.. 2018;8(1):1-12.
- [Google Scholar]
- Thymic stromal lymphopoietin (TSLP)-induced polyclonal B-cell activation and autoimmunity are mediated by CD4+ T cells and IL-4. Int. Immunol.. 2012;24(3):183-195.
- [Google Scholar]
- Critical role of TSLP-responsive mucosal dendritic cells in the induction of nasal antigen-specific IgA response. Mucosal Immunol.. 2017;10(4):901-911.
- [Google Scholar]
- Orally administered adenoviral-based vaccine induces respiratory mucosal memory and protection against RSV infection in cotton rats. Vaccine. 2018;36(29):4265-4277.
- [CrossRef] [Google Scholar]
- An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol.. 1969;89(4):405-421.
- [CrossRef] [Google Scholar]
- Role of TSLP in Nasal Polyp Inflammation. Allergy Asthma Immunol. Res.. 2011;3(3):146-147.
- [Google Scholar]
- The role of Toll-like receptors in regulating the immune response against respiratory syncytial virus. Critical Reviews™ in Immunology. 2009;29(6)
- [Google Scholar]
- Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J. Immunol.. 2009;183(2):1427-1434.
- [Google Scholar]
- Current summary of clinical studies on anti-TSLP antibody, Tezepelumab, in asthma. Allergol. Int.. 2023;72(1):24-30.
- [CrossRef] [Google Scholar]
- Lapuente, D., genannt Bonsmann, M. S., Maaske, A., Stab, V., Heinecke, V., Watzstedt, K., et al., 2018. IL-1β as mucosal vaccine adjuvant: the specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucosal immunology, 11(4), 1265-1278.
- Mucosal Vaccines - Fortifying the Frontiers.. 2022;22(4):236-250.
- [CrossRef]
- Universal vaccine against respiratory syncytial virus A and B subtypes. PLoS One. 2017;12(4):e0175384.
- [Google Scholar]
- Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc. Natl. Acad. Sci.. 2007;104(3):914-919.
- [Google Scholar]
- Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J. Immunol.. 1999;162(2):677-683.
- [Google Scholar]
- Health and economic burden of respiratory syncytial virus (RSV) disease and the cost-effectiveness of potential interventions against RSV among children under 5 years in 72 Gavi-eligible countries. BMC Med.. 2020;18:1-16.
- [Google Scholar]
- Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol.. 2012;12(8):592-605.
- [Google Scholar]
- Mucosal immunization with an adenoviral vector vaccine confers superior protection against RSV compared to natural immunity. Front Immunol. 2022;13(920256)
- [Google Scholar]
- Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol.. 2021;147(5):1778-1794.
- [Google Scholar]
- TSLP inhibitors for asthma: current status and future prospects. Drugs. 2020;80:449-458.
- [Google Scholar]
- Thymic stromal lymphopoietin in tonsillar follicular dendritic cells correlates with elevated serum immunoglobulin A titer by promoting tonsillar immunoglobulin A class switching in immunoglobulin A nephropathy. Transl. Res.. 2016;176:1-17.
- [Google Scholar]
- Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol. Ther.. 2017;169:104-112.
- [Google Scholar]
- Respiratory syncytial virus A genotype classification based on systematic intergenotypic and intragenotypic sequence analysis. Sci. Rep.. 2019;9(1):1-12.
- [Google Scholar]
- Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J. Clin. Microbiol.. 1986;24(5):894-898.
- [Google Scholar]
- Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to TH2-type immune responses and airway inflammation. J. Allergy Clin. Immunol.. 2008;122(6):1208-1214.
- [Google Scholar]
- Protective and dysregulated T cell immunity in RSV infection. Curr. Opin. Virol.. 2013;3(4):468-474.
- [Google Scholar]
- Innate immune evasion by human respiratory syncytial virus. Front. Microbiol.. 2022;13:865592
- [Google Scholar]
- Mechanical injury polarizes skin dendritic cells to elicit a TH2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol.. 2010;126(5):976-984. e975
- [Google Scholar]
- Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol.. 2000;1(1):59-64.
- [Google Scholar]
- Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol. Therapy-Methods Clin. Develop.. 2015;2:15018.
- [Google Scholar]
- Respiratory syncytial virus-associated deaths in the United States according to death certificate data, 2005 to 2016. Health Sci. Rep.. 2021;4(4)
- [Google Scholar]
- Respiratory syncytial virus infection: old challenges and new approaches. J. Infect. Dis. 2023
- [CrossRef] [Google Scholar]
- Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines. 2012;11(5):595-607.
- [Google Scholar]
- The human immune response to respiratory syncytial virus infection. Clin. Microbiol. Rev.. 2017;30(2):481-502.
- [Google Scholar]
- Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26. RSV. preF in a human challenge study. J. Infect Dis.. 2022;226(3):396-406.
- [Google Scholar]
- Adenovectors encoding RSV-F protein induce durable and mucosal immunity in macaques after two intramuscular administrations. npj Vaccines. 2019;4(1):54.
- [Google Scholar]
- Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. Eur. J. Immunol.. 2010;40(4):955-965.
- [Google Scholar]
- Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog.. 2018;14(1):e1006810.
- [Google Scholar]
- Current insights in the development of efficacious vaccines against RSV. Front. Immunol.. 2020;11:1507.
- [Google Scholar]
- Thymic stromal lymphopoietin plays an adjuvant role in BCG-mediated CD8+ cytotoxic T cell responses through dendritic cell activation. Clin. Immunol.. 2010;136(2):205-216.
- [Google Scholar]
- The innate immune response to RSV: Advances in our understanding of critical viral and host factors. Vaccine. 2017;35(3):481-488.
- [CrossRef] [Google Scholar]
- Immune-modulation by the human respiratory syncytial virus: focus on dendritic cells. Front. Immunol.. 2019;10:810.
- [Google Scholar]
- Efficacy, Safety and Immunogenicity of Anti-SARS-CoV-2 Vaccines in Patients with Cirrhosis: A Narrative Review. Vaccines. 2023;11(2):452.
- [Google Scholar]
- Thymic stromal lymphopoietin (TSLP) acts as a potent mucosal adjuvant for HIV-1 gp140 vaccination in mice. Eur. J. Immunol.. 2012;42(2):353-363.
- [Google Scholar]
- Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front. Immunol.. 2018;9:1595.
- [Google Scholar]
- Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun.. 2017;8(1):14937.
- [CrossRef] [Google Scholar]
- Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2–Toll-like receptor 6 pathway. J. Allergy Clin. Immunol.. 2010;126(5):985-993. e983
- [Google Scholar]
- Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190(2):373-378.
- [Google Scholar]
- Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol.. 2007;8(3):294-303.
- [Google Scholar]
- Mucosal vaccines against respiratory syncytial virus. Curr. Opin. Virol.. 2014;6:78-84.
- [Google Scholar]
- Interferon-λ enhances adaptive mucosal immunity by boosting release of thymic stromal lymphopoietin. Nat. Immunol.. 2019;20(5):593-601.
- [Google Scholar]
- Respiratory syncytial virus prolifically infects N2a neuronal cells, leading to TLR4 and nucleolin protein modulations and RSV F protein co-localization with TLR4 and nucleolin. J. Biomed. Sci.. 2018;25(1):13.
- [CrossRef] [Google Scholar]
- Vaccine-generated lung tissue–resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1(10)
- [Google Scholar]
- Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol.. 2005;6(10):1047-1053.
- [Google Scholar]
- The biology of thymic stromal lymphopoietin (TSLP) Adv. Pharmacol.. 2013;66:129-155.
- [CrossRef] [Google Scholar]







