Translate this page into:
Narirutin ameliorates polystyrene microplastics induced nephrotoxicity by modulating oxidative stress, inflammation and Nrf2/Keap1 pathway
⁎Corresponding author. umar.ijaz@uaf.edu.pk (Muhammad Umar Ijaz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polystyrene microplastics (PSMPs) have emerged as potentially hazardous materials, which significantly affect various body organs including kidneys. Narirutin (NRT) is a flavanone that exhibits a wide range of pharmacological properties. Therefore, this study was planned to appraise the nephro-protective effects of NRT on PSMPs-instigated kidney damages in male albino rats. In this study, 24 male albino rats were randomly distributed in 4 groups (n = 6/group); control group, PSMPs (0.01 mgkg−1) treated group, PSMPs + NRT (0.01 mgkg−1 + 50 mgkg−1) co-treated group, and NRT (50 mgkg−1) only treated group. PSMPs exposure reduced the expressions of Nrf-2 and anti-oxidant enzymes coupled with increased expressions of Keap-1. PSMPs treatment reduced the activities of heme oxygenase-1 (HO-1), catalase (CAT), glutathione reductase (GSR), glutathione peroxidase (GPx), glutathione (GSH), glutathione S-transferase (GST), and superoxide dismutase (SOD), whereas escalated the levels of malondialdehyde (MDA) and reactive oxygen species (ROS). Moreover, PSMPs administration substantially elevated the levels of kidney function markers such as creatinine, urea, kidney injury molecules-1 (KIM-1) and neutrophil gelatinase associated lipocalin (NGAL). Conversely, it reduced the level of creatinine clearance. Besides, PSMPs significantly escalated the levels of inflammatory markers such as nuclear factor kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and cyclooxygenase-2 (COX-2) activity. In contrast, NRT restored all these damages and abnormalities to their normal level. According to these findings, NRT may act as a potential flavanone with the ability to mitigate the kidney toxicity induced by PSMPs in male albino rats.
Keywords
Narirutin
Polystyrene microplastics
Oxidative stress
Nephrotoxicity
1 Introduction
Plastic pollution is ubiquitous in both terrestrial and aquatic ecosystems. In 2019, worldwide plastic production was 368 million metric tons (Mt), but it is expected to double over the next 20 years (Lebreton and Andrady, 2019). Microplastics (MPs) are widely recognized as environmental contaminants with a diameter less than 5 mm and may persist in the environment for a longer duration. MPs can enter living organisms and humans via inhalation, cutaneous contact, or ingestion (Deng et al., 2017). Food, drinking water, soil, marine food, and hot beverages have all been found to contain MPs. Overall, the buildup of microplastics in tissues may result in a number of adverse effects, including oxidative stress, immunological responses, physical damage, decreased feeding activity, stunted development and growth, energy deficiencies, genotoxicity, metabolic diseases and neurological damage (Rochman et al., 2014).
Polystyrene (PS) is one of the most commonly used plastics due to its unique physical characteristics. It is widely employed as an essential element in plastics, textiles, electronics, building materials made of plastic, and other flammable items, to increase their fire resistance. Previous research has shown that PSMPs can surpass several biological barriers, ultimately resulting in hepatotoxicity, neurotoxicity and reproductive toxicity (Huang et al., 2021). According to previous reports, exposure to PSMPs raises the amount of ROS, which causes oxidative stress and promotes apoptosis and inflammation in the kidneys. High ROS generation damages lipids, proteins, DNA, and disrupts several normal cell signaling pathways, ultimately leading to cellular death (Herb and Schramm, 2021).
Phytochemicals derived from plants are used as an alternative treatments for various diseases. Despite significant advancements in modern medicine, there is still a persistent lack of effective and safe therapies (Hasan et al., 2018). Narirutin (NRT) is a naturally occurring flavanone, which is mainly present in citrus peel, orange, and grapefruit juice. According to Manach et al. (2003), it shows potent anti-allergic, anti-oxidant, anti-inflammatory, neuroprotective, and anti-tumor activities. Additionally, oral administration of narirutin was observed to reduce the symptoms of inflammation in animals suffering from colitis and peritonitis (Napimoga et al., 2013). Therefore, this study was designed to ascertain the protective efficacy of NRT against PSMPs-instigated renal impairment in rats.
2 Materials and methods
2.1 Chemicals
NRT (CAS NO. 14259-46-2) & PSMPs (CAS No. 9003-53-6) were procured from the Sigma-Aldrich (Germany).
2.2 Animals
Twenty-four albino male rats weighing 180–220 g were used in this research. Animals were housed in steel cages at animal care facility of university of Agriculture, faisalabad (UAF). Rats were allocated into four different groups and confined in separate enclosures. Standard laboratory conditions such as 26±2 °C and 12-hour day/night cycles were maintained. Experimental animals were handled in accordance with the European Union guidelines for animal care and experimentation (CEE council 86/609), which were also approved by UAF ethical committee.
2.3 Experimental layout
24 rats were divided into four groups of equal size (n = 6/group) after a week of acclimatization to the lab environment. Group I was termed as control group, while group II (PSMPs-treated group) was supplied with 0.01 mg/kg of PSMPs orally. In group III (PSMPs + NRT co-treated group), 0.01 mg/kg PSMPs, and 50 mg/kg were administered orally, whereas group IV (only NRT treated group) received 50 mg/kg NRT daily by oral gavage. Doses of PSMPs (0.01 mg/kg) and NRT (50 mg/kg dose) were given according to the previous investigations of Wang et al. (2022) and Fang et al. (2023), respectively. After the completion of experiment, rats were euthanized with ketamine (60 mg/kg) and xylazine (6 mg/kg) prior to decapitation. Kidneys were removed, rinsed with saline, and preserved in zip-lock bags at −80 °C for biochemical analysis. Tissue samples were homogenized and spun at 3000 rpm in centrifuge for fifteen minutes
2.4 Biochemical profile
CAT activity was appraised in renal tissues using Chance and Maehly (1955) approach. Activity of SOD was quantified based on Kakkar et al. (1984) colour intensity technique. For quantifying GSR activity, the Carlberg and Mannervik (1975) protocol was followed. The evaluations of GSH content and GST activity were carried out using the techniques described by Jollow et al. (1974) and Habig et al. (1974), respectively. MDA level was evaluated based on a method developed by Ohkawa et al. (1979). Hayashi et al. (2007) methodology was employed for the determination of ROS concentration.
2.5 Quantitative real-time PCR (qRT-PCR)
qRT-PCR was used to analyze the variations in the expressions of antioxidant genes and Nrf2-Keap1 pathway. Total ribonucleic acid was extracted via TRIzol reagent, which was then reverse transcribed into cDNA employing Thermo Scientific RevertAid Reverse Transcriptase Kit. β-actin was considered as internal control and 2−ΔΔCT was used to assess the variations in the expressions of these parameters (Livak and Schmittgen, 2001). Table 1 demonstrates the primers sequences of targeted genes, as previously reported by Ijaz et al. (2022) and Hamza et al. (2023).
Gene
Primers 5′—3′
Accession number
Nrf2
Forward: ACCTTGAACACAGATTTCGGTG
NM_031789.1
Reverse: TGTGTTCAGTGAAATGCCGGA
Keap1
Forward: ACCGAACCTTCAGTTACACACT
NM_057152.1
Reverse: ACCACTTTGTGGGCCATGAA
CAT
Forward: TGCAGATGTGAAGCGCTTCAA
NM_012520.2
Reverse: TGGGAGTTGTACTGGTCCAGAA
SOD
Forward: AGGAGAAACTGACAGCTGTGTCT
NM_017051.2
Reverse: AAGATAGTAAGCGTGCTCCCAC
GPx
Forward: TGCTCATTGAGAATGTCGCGTC
NM_030826.4
Reverse: ACCATTCACCTCGCACTTCTCA
GSR
Forward: ACCAAGTCCCACATCGAAGTC
NM_053906.2
Reverse: ATCACTGGTTATCCCCAGGCT
GST
Forward: TCGACATGTATGCAGAAGGAGT
NM_031509.2
Reverse: CTAGGTAAACATCAGCCCTGCT
HO-1
Forward: AGGCTTTAAGCTGGTGATGGC
NM_012580.2
Reverse: ACGCTTTACGTAGTGCTGTGT
β-actin
Forward: AGGAGATTACTGCCCTGGCT
NM_031144
Reverse: CATTTGCGGTGCACGATGGA
2.6 Renal function markers assessment
The renal function markers (creatinine clearance, creatinine & urea) were estimated by using AMP standardized diagnostic kit (AMEDA Labor Diagnostics Gmbh, Austria). The assay was performed in accordance with the instructions of the manufacturer.
2.7 Inflammatory biomarkers assessment
The levels of inflammatory markers in renal tissues (IL-1β, TNF-α, IL-6, NF-κB, and COX-2 activity) were estimated by using ELISA kits procured from Shanghai YL Biotech Company Ltd., located in China.
2.8 Statistical analysis
The acquired data were displayed as Mean ± SE. The Shapiro-Wilk test was used to check the normal distribution of the data, and the Levene test was used to confirm the homogeneity of variances. For the statistical analysis of data one-way ANOVA followed by Tukey's test was applied. Graphs were made with GraphPad Prism 5. p < 0.05 was chosen as the significance level for analysis.
3 Results
3.1 Effects of PSMPs + NRT on Nrf2-Keap1 signaling pathway
PSMPs intoxication led to a considerable (p < 0.05) reduction in the expression of Nrf2 and its cytoprotective genes, while significantly (p < 0.05) increasing the Keap1 expressions as compared to the control group. However, NRT co-treatment substantially (p < 0.05) elevated the expressions of Nrf2 and its cytoprotective genes, while substantially (p < 0.05) reducing the expression of Keap1 in comparison to PSMPs-treated group. Besides, in NRT only treated rats these values were comparable to the control group (Fig. 1 and Fig. 2).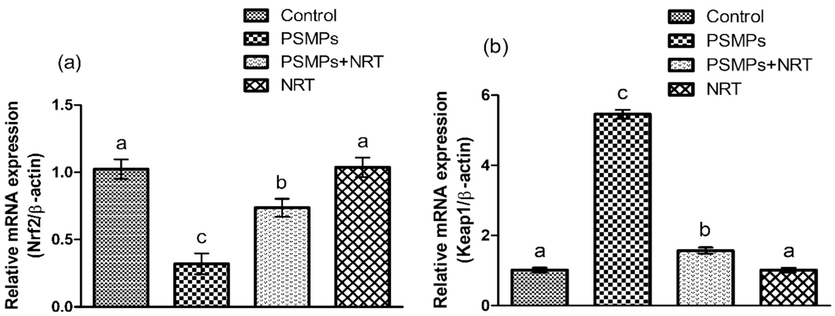
Displays the impact of NRT & PSMPs a) Nrf-2, b) Keap-1 expression in 4 different groups. Dissimilar superscripts on bars exhibit substantial difference.
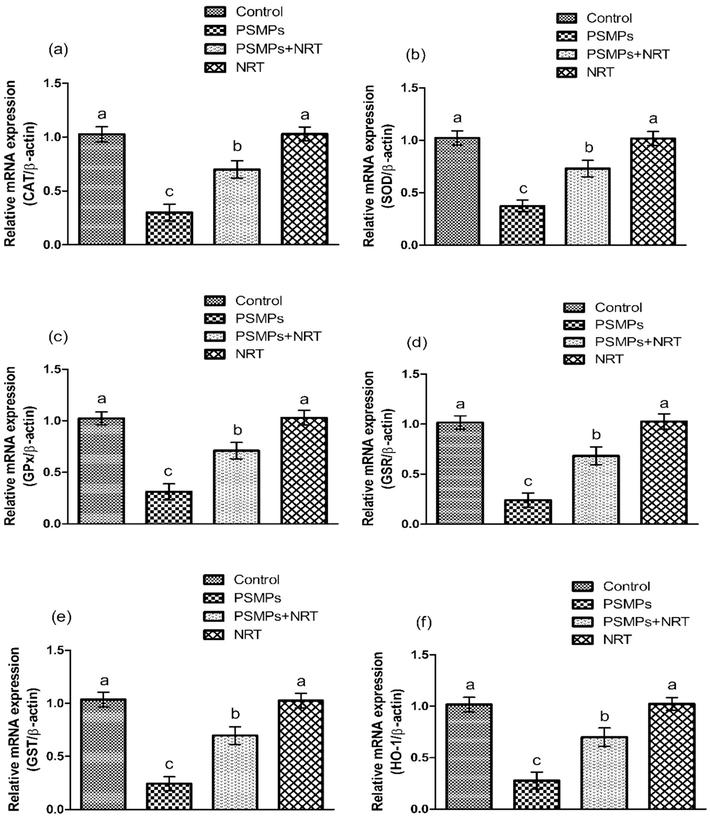
Represents the impact of NRT & PSMPs on a) CAT, b) SOD, c) GPx, d) GSR, e) GST & f) HO-1 expressions in 4 different groups. Different superscripts on bars demonstrates substantial alterations.
3.2 Effects of PSMPs + NRT on antioxidant enzymes activity
The activities of GPx, CAT, GSR, SOD, GST, GSH as well as HO-1 were noticeably (p < 0.05) decreased in PSMPs-treated group in comparison to the control group. On the other hand, NRT co-treated group showed substantial (p < 0.05) escalation in the activities of above-mentioned antioxidant enzymes as compared to PSMPs treated group. The activities of antioxidant enzymes NRT (alone) treated group were comparable to the control group (Table 2). Values having dissimilar letters are considerably different from other groups.
Parameters
Groups
Control
PSMPs
PSMPs + NRT
NRT
CAT (Umg−1 protein)
12.32 ± 0.48a
6 ± 0.14c
9.52 ± 0.63b
12.84 ± 0.59a
SOD (Umg−1 protein)
10.53 ± 0.71a
4.87 ± 0.18c
8.34 ± 0.27b
11.07 ± 0.87ab
GPx (Umg−1 protein)
27.52 ± 0.87a
14.42 ± 0.73c
22.42 ± 0.49b
27.71 ± 0.88a
GSR (nM NADPH oxidized/min/mg tissue)
10.15 ± 0.33a
3.77 ± 0.21c
7.76 ± 0.39b
10.34 ± 0.42a
GST (nM/min/mg protein)
34.99 ± 0.95a
14.70 ± 0.87c
26.22 ± 0.84b
35.96 ± 0.72a
GSH (μM/g tissue)
20.16 ± 0.75a
7.21 ± 0.31c
15.40 ± 0.43b
20.33 ± 0.82a
HO-1(pmoles bilirubin/mg protein/h)
303.93 ± 5.97a
57.52 ± 3.65c
235.33 ± 8.03b
323.79 ± 7.92a
ROS (Umg−1 tissue)
1.52 ± 0.15a
8.41 ± 0.29c
2.48 ± 0.22b
1.49 ± 0.134a
MDA (nmol/mg protein)
0.39 ± 0.09a
3.08 ± 0.26c
1.29 ± 0.14b
0.38 ± 0.12a
3.3 Effects of PSMPs + NRT on oxidative stress markers
The administration of PSMPs prompted a notable (p < 0.05) increase in ROS and MDA levels as compared to the control group. However, co-treatment with PSMPs + NRT resulted in a significant (p < 0.05) decrease in MDA and ROS levels as compared to PSMPs administered group. Moreover, the levels of ROS and MDA in NRT (alone) treated rats were comparable to the control group (Table 2).
3.4 Effects of PSMPs + NRT on kidney function markers
PSMPs administration resulted in a significant (p < 0.05) increase in the levels of kidney function markers i.e., creatinine, urea, KIM-1 and NGAL, while reducing the concentration of creatinine clearance as compared with control group. Conversely, concomitant treatment of PSMPS + NRT lowered the levels of urea, creatinine KIM-1 and NGAL, while improving creatinine-clearance concentration that demonstrates the therapeutic efficacy of NRT against PSMPs induced nephrotoxicity. In NRT treated group, serum levels of aforementioned markers were similar to the control group (Table 3). Values having dissimilar letters are considerably different from other groups.
PARAMETERS
GROUPS
Control
PSMPs
PSMPs + NRT
NRT
Urea (mg/dl)
14.48 ± 0.96a
47.86 ± 2.25c
25.88 ± 1.40b
14.29 ± 1.03a
Creatinine (mg/dl)
1.39 ± 0.15a
4.66 ± 0.23c
2.10 ± 0.09b
1.35 ± 0.17a
Creatinine clearance (mL/min)
2.34 ± 0.13a
0.53 ± 0.18c
1.58 ± 0.15b
2.26 ± 0.15a
Urinary KIM-1 (ng/ml)
0.26 ± 0.09a
5.1 ± 0.17c
1.61 ± 0.22b
0.23 ± 0.10a
NGAL (ng/ml)
0.89 ± 0.13a
7.22 ± 0.19c
2.01 ± 0.06b
0.82 ± 0.17a
3.5 Effects of PSMPs + NRT on inflammatory markers
The levels of inflammatory markers i.e., TNF-α, IL-6, NF-kB, IL-1β and COX-2 activity were substantially (p < 0.05) increased in PSMPs administered group as compared to the control group. Conversely, NRT supplementation combined with PSMPs indicated significant (p < 0.05) reduction in the levels of inflammatory marker as compared to PSMPs group. Moreover, these inflammatory makers in NRT alone treated rats were similar to the control group (Table 4). Values having dissimilar letters are considerably different from other groups.
Parameters
Groups
Control
PSMPs
PSMPs + NRT
NRT
NF-κB (ng/g tissue)
23.51 ± 0.88a
86.61 ± 0.95c
38.10 ± 1.92b
22.99 ± 1.01a
TNF-α (ng/g tissue)
9.98 ± 0.80a
64.93 ± 1.78c
18.46 ± 1.67b
9.85 ± 0.82a
IL-1β (ng/g tissue)
17.59 ± 1.05a
68.61 ± 1.97c
31.69 ± 1.62b
17.42 ± 1.15a
IL-6 (ng/g tissue)
14.87 ± 0.73a
48.97 ± 1.24c
26.20 ± 1.62b
14.69 ± 0.65a
COX-2 (ng/g tissue)
17.63 ± 1.04a
78.47 ± 1.61c
28.15 ± 2.12b
17.38 ± 0.92a
4 Discussion
The current study was designed to determine the protective efficacy of narirutin against nephrotoxicity prompted by PSMPs in rats. Our finding showed that PSMPs administration decreased the activities of CAT, SOD, GSR, GST, GSH, GPx, and HO-1, while elevating the levels of ROS and MDA. ROS, contains OH−, O2− and H2O2, which are metabolic byproducts, when produced excessively can damage body tissues (Seddiki et al., 2017). MDA, a toxic byproduct produced in lipid peroxidation, indicates the damage caused by lipid peroxidation and ROS (Yu et al., 2018). Antioxidants function as a first line of defense against oxidative stress in the body by decreasing ROS formation and protecting lipids, DNA, and proteins (Ighodaro and Akinloye, 2018). CAT and SOD are the two important antioxidants that protect the body from the adverse effects of lipid peroxidation (LP) induced by oxidative stress (OS). SOD protects the cells from ROS by catalyzing the highly reactive superoxide into H2O2 (Yang et al., 2017). CAT and GPx mediates the degeneration of hydrogen peroxide into less toxic forms H2O and O2, to counter the effects of oxidative stress (Wang et al., 2021). GSH plays a significant role in the reduction of H2O2, while GSR facilitates in the transformation of GSSG into GSH. HO-1, a cytoprotective enzyme, helps to maintain cellular homeostasis by breaking down heme (Bai et al., 2017). The antioxidant defense mechanism of the body is disrupted by the overproduction of ROS, which leads to OS. Nevertheless, NRT supplementation lowered ROS and MDA levels while improving the activities of antioxidant enzyme due to its antioxidant properties.
The Nrf2/Keap1 signaling pathway is an important regulator of the cytoprotective response, involved in both endogenous and external stress generated by ROS (Yamamoto et al., 2018). Nrf2 increases the expressions of antioxidant enzymes that scavenge ROS by improving the cellular antioxidant defense system. Keap1, which is an inhibitory regulator of Nrf2, is responsible for inducing Nrf2 disintegration (Bellezza et al., 2018). In our study, PSMPs treatment upregulated Keap1 expression, while lowering the expressions of Nrf2, which in turn reduced the cytoprotective genes (GST, SOD, GSR, CAT, GPx & HO-1) expressions. However, concurrent supplementation of NRT + PSMPs significantly regulated the expression of the above-mentioned cytoprotective genes via Nrf2-Keap1 signaling pathway modulation.
According to our findings, PSMPs exposure increased the levels of urea and creatinine, while a notable reduction was observed in creatinine clearance level. These changes in renal serum markers, such as urea and creatinine, and reduction in creatinine clearance, which shows kidney damage (Şener et al., 2007). The rise in urea and creatinine levels in the blood is regarded as a significant indicator of renal impairment caused by PSMPs-induced nephrotoxicity (Farooqui et al., 2017). Urea is produced from the breakdown of proteins, whereas creatinine is a nitrogen-containing molecule that is eliminated from the body through urine during glomerular filtration, and their increased level in the blood indicates impaired renal function (Sepulveda, 2019). Both urea and creatinine are commonly used to assess normal function of kidney (Ramsey et al., 2018). However, these markers were restored to normal followed by NRT supplementation, which indicates reno-protective role of NRT.
Our result showed significant increase in the levels of KIM-1 and NGAL after PSMPs exposure. NGAL, a member of the lipocalin family, and KIM-1, a type 1 transmembrane glycoprotein, were used to detect the development of acute kidney damage (AKI) (Lei et al., 2018). KIM-1 is a trans-membrane protein that is used as an initial diagnostic indicator for AKI. It is not expressed in normal or healthy kidney, but it can be detected during the early stages of nephrotoxicity (Song et al., 2019). NGAL is a cytosolic protein released by neutrophils and renal tubular cells in case of acute injury associated with inflammation and oxidative stress. Subsequently, it is eliminated from the body via urine, as it is in line with the study conducted by Khawaja et al. (2019). Conversely, these markers were restored to normal followed by NRT treatment, which indicates nephroprotective potential of NRT.
PSMPs treatment increased the levels of inflammatory markers such as NF-κB, TNF-α, IL-6, IL-1β, and COX-2 activity. Activation of NF-κB significantly affects the upregulation of cytokines such as TNF-α, IL-6, COX-2, and IL-1β, which are associated with inflammation, oxidative stress (Khan et al., 2020), and kidney dysfunction (Kandemir et al., 2018). TNF-α is an important cytokine that promotes inflammation, stimulates the secretion of IL-1β and cyclooxygenase (COX). The cytokine IL-1β is released during early inflammation and triggers the generation of other inflammatory mediators, exacerbating the inflammatory response (Zhang et al., 2022). COX-2, an inducible type of COX, is another important factor that contributes in the process of inflammation (Kim et al., 2019). However, the concurrent administration of NRT with PSMPs effectively decreased the levels of above-mentioned inflammatory markers, indicating the anti-inflammatory function of NRT in renal tissues.
5 Conclusion
In conclusion, the findings of this study revealed that NRT has the ability to alleviate PSMP-induced kidney damage in albino rats. NRT supplementation effectively ameliorated nephrotoxicity by inhibiting lipid peroxidation, reducing inflammation, and restoring the antioxidant state. This protective effect may be due to NRT’s ability to increase the expressions of Nrf2, consequently increasing the activity of antioxidant enzymes via its intrinsic antioxidant potential. These findings suggest that NRT has the potential to be a promising therapeutic candidate for renal protection against PSMP-induced toxicity.
CRediT authorship contribution statement
Muhammad Umar Ijaz: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. Maria Ghaffar: Writing – original draft, Methodology, Investigation, Conceptualization. Rabia Azmat: Writing – review & editing, Visualization, Validation, Conceptualization. Moazama Batool: Visualization, Validation, Formal analysis, Data curation. Hammad Ahmed Khan: Validation, Software, Formal analysis, Data curation. Shaik Althaf Hussain: Writing – review & editing, Software, Resources, Funding acquisition. Mian Nadeem Riaz: Visualization, Validation, Formal analysis, Data curation.
Acknowledgement
The authors would like to acknowledge the funding support by the Researchers Supporting Project number (RSP2024R371), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci.. 2017;96:74-82.
- [CrossRef] [Google Scholar]
- Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta. Mol. Cell Res.. 2018;1865:721-733.
- [CrossRef] [Google Scholar]
- Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep.. 2017;7:46687.
- [CrossRef] [Google Scholar]
- Narirutin activates TFEB (transcription factor EB) to protect against Acetaminophen-induced liver injury by targeting PPP3/calcineurin. Autophagy.. 2023;19:2240-2256.
- [CrossRef] [Google Scholar]
- Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed. Pharmacother.. 2017;85:7-15.
- [CrossRef] [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [CrossRef] [Google Scholar]
- Hepatoprotective effects of astragalin against polystyrene microplastics induced hepatic damage in male albino rats by modulating Nrf-2/Keap-1 pathway. J. Funct. Foods.. 2023;108:105771
- [CrossRef] [Google Scholar]
- Hepatoprotective, antihyperglycemic and antidiabetic effects of Dendrophthoe pentandra leaf extract in rats. Clin. Phytosci.. 2018;4:1-7.
- [CrossRef] [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [CrossRef] [Google Scholar]
- Functions of ROS in macrophages and antimicrobial immunity. Antioxidants. 2021;10:313.
- [CrossRef] [Google Scholar]
- Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater.. 2021;405:124187
- [CrossRef] [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;2018(54):287-293.
- [CrossRef] [Google Scholar]
- Chemoprotective effect of vitexin against cisplatin-induced biochemical, spermatological, steroidogenic, hormonal, apoptotic and histopathological damages in the testes of Sprague-Dawley rats. Saudi Pharm. J.. 2022;30:519-526.
- [CrossRef] [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [CrossRef] [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed. Pharmacother.. 2018;105:981-991.
- [CrossRef] [Google Scholar]
- Naringenin prevents doxorubicin-induced toxicity in kidney tissues by regulating the oxidative and inflammatory insult in Wistar rats. Arch. Physiol. Biochem.. 2020;126:300-337.
- [CrossRef] [Google Scholar]
- The utility of neutrophil gelatinase-associated Lipocalin (NGAL) as a marker of acute kidney injury (AKI) in critically ill patients. Biomark. Res.. 2019;7:1-6.
- [CrossRef] [Google Scholar]
- Anti-inflammatory actions of folate-functionalized bioactive ion-releasing nanoparticles imply drugfree nanotherapy of inflamed tissues. Biomaterials. 2019;207:23-38.
- [CrossRef] [Google Scholar]
- Future scenarios of global plastic waste generation and disposal. Palgrave. Commun.. 2019;5:1-11.
- [CrossRef] [Google Scholar]
- Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep.. 2018;8:1-9.
- [CrossRef] [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402-408.
- [CrossRef] [Google Scholar]
- Manach, C.1., Morand, C., Gil-Izquierdo, A., Bouteloup-Demange, C., Remesy, C., 2003. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 57, 235–242. doi: 10.1038/sj.ejcn.1601547.
- Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J. Nat. Prod.. 2013;76:2316-2321.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [CrossRef] [Google Scholar]
- Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist. 2018;23:52-61.
- [CrossRef] [Google Scholar]
- Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ.. 2014;493:656-661.
- [CrossRef] [Google Scholar]
- Seddiki, Y., Silva, F.M., da Silva, F.M., 2017. Antioxidant properties of polyphenols and their potential use in improvement of male fertility: a review. Biomed. J. Sci. Tech. Res. 1, 612–617. 10.26717/BJSTR.2017.01.000259.
- Chronic renal failure-induced multiple-organ injury in rats is alleviated by the selective CysLT1 receptor antagonist montelukast. Prostaglandins Other Lipid Mediat.. 2007;8:257-267.
- [CrossRef] [Google Scholar]
- Sepulveda, J.L., 2019. Challenges in routine clinical chemistry testing analysis of small molecules. In: Accurate results in the clinical laboratory. Elsevier 2019, 101–140. doi: 10.1016/B978-0-12-813776-5.00009-1.
- Understanding kidney injury molecule 1: a novel immune factor in kidney pathophysiology. Am. J. Transl. Res.. 2019;11:1219.
- [Google Scholar]
- Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine. Food Chem. Toxicol.. 2022;162:112904
- [CrossRef] [Google Scholar]
- The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ. Health Perspect.. 2021;129:057003
- [Google Scholar]
- The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev.. 2018;98:1169-1203.
- [CrossRef] [Google Scholar]
- Assessment of doxorubicin-induced mouse testicular damage by the novel second-harmonic generation microscopy. Am. J. Transl. Res.. 2017;9:5275-5288.
- [Google Scholar]
- Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol.. 2018;200:28-36.
- [CrossRef] [Google Scholar]
- Ameliorative effect of scutellarin on acute alcohol brain injury in mice. J. Zhejiang Univ. Sci. B.. 2022;23:258-264.
- [CrossRef] [Google Scholar]







