Translate this page into:
Melatonin triggers salinity tolerance in pansy (Viola tricolor) by regulation of defense system
⁎Corresponding authors at: School of Tropical Agriculture and Forestry (School of Agricultural and Rural Affairs, School of Rural Revitalization), Hainan University, Haikou, PR China. anwar_uaar@yahoo.com (Muhammad Anwar), ahsanaltaf8812@gmail.com (Muhammad Ahsan Altaf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Salinity poses a significant threat to the floral plants. Melatonin has emerged as a potential phytohormone to cope with the adverse effects of salinity on plants due to its multifaceted roles in regulating abiotic stresses. The current study aimed to investigate the impact of melatonin on pansy by modifications in physiological indices under salinity stress. Pansy plants were subjected to salinity stress (0, 50, and 100 mM NaCl) and melatonin (control, 50, and 100 µM). Salinity-induced oxidative stress was apparent through enhanced generation of toxic substances. However, melatonin supplementation improved chlorophyll ‘a’, chlorophyll ‘b’, total chlorophyll, glycine betaine (GB), superoxidase dismutase (SOD), peroxidase (POD), catalases (CAT), and glutathione-reductase (GR), thus promoting improved growth under salinity. The activation of plant defense system and regulation of signaling molecules is indication of lessening of toxic substances within the plant cells. Current findings revealed that supplementation of melatonin enhanced pansy tolerance against salinity stress.
Keywords
Antioxidants
Cellular damage
Signaling molecules
Toxic substances
1 Introduction
Pansy is a common choice in ornamental gardens and landscapes because of its bright and colorful petals (Hamlin and Mills, 2001). The pansy has distinctive traits that render it vulnerable to stress brought on by salinity. For their sustainable development and economic viability, it is crucial to comprehend negative effects of abiotic stresses (Hakim et al., 2017; Mudassir et al., 2021; Lal et al., 2023). Salinity stress has a negative impact on pansy production, beginning at the cellular level (Qin et al., 2022). Reduced water intake and plant wilting are caused by the excess accumulation of Na+ and Cl- in the root zone, which alters the water potential (Li et al., 2022; Islam et al., 2021; Manzoor et al., 2023). Salinity stress causes an excessive buildup of reactive oxygen species (ROS), which can further oxidize biological components (Kohli et al., 2019; Nasir et al., 2021; Mansoor et al., 2022). Melatonin is a hormone well known for its numerous and amazing effects on plants (Siddiqui et al., 2020; Kaya et al., 2020; Altaf et al., 2021). Ornamental plants are sensitive to elevated salinity that can seriously hinder their growth and yield (Ahmad et al., 2023). When exceeded soluble salts build up in the soil, it causes salinity stress, which in turn causes osmotic imbalance, ion toxicity, and oxidative damage in plants (Rehman et al., 2019). About one-third of irrigated land facing salinity-related problems, this situation has become a major global concern for agricultural productivity (Li et al., 2019a; Zhang et al., 2021).
Melatonin was once primarily thought to be a hormone found in plants, leading to substantial research into its role and effects on plant physiology (Fan et al., 2018; Li et al., 2019a). Although its fundamental function in plants has not yet been fully understood, it has been linked to a wide range of physiological functions, including the control of growth, the timing of blooming, the response to biotic and abiotic stressors, and antioxidant defense mechanisms (Akram et al., 2021; Eisa et al., 2023). Exogenous melatonin treatment improves numerous plant species' ability to tolerate salt, including improvements in seed germination, root growth, and photosynthetic efficiency in salty environments (Roopin and Levy, 2012). Melatonin had capability to neutralize ROS and regulated antioxidant defense mechanisms in order to reduce stress adversities. Moreover, melatonin controls the expression of genes and signaling pathways that are responsive to stress and are involved in the perception and adaption to stress (Posmyk and Janas, 2009). These signaling pathways are essential for cellular homeostasis regulation and stress tolerance enhancement. The current study aims to explore melatonin potential against salinity in pansy by focusing on changes in photosynthetic pigments, physiological indices, signaling molecules, and defense system.
2 Materials and methods
2.1 Planting materials
Pansy seeds were purchased from a seed company More Green Company, Pakistan. Seeds were sown in pots filled with well-drained soil on 30 October 2022 at Department of Horticulture, Bahauddin Zakariya University, Multan. Soil physical and biochemical characteristics were measured before and at the research trial termination stage on 27 February 2023 (Table 1). The urea of 5 g was applied to every pansy plant. However, insecticides were not applied to pansy plants.
Characteristics
Before research trial
After research trial
50 mM NaCl
100 mM NaCl
Soil texture
Silt loam
Silt loam
Silt loam
Saturation (%)
30
28
28
Organic matter (%)
0.41
0.36
0.35
EC (mScm−1)
1.79
1.81
1.82
pH
8.0
8.1
8.1
P (mg kg−1)
4.10
3.50
3.50
K (mg kg−1)
123
120
108
Na (mg kg−1)
135
223
245
Cl (mg kg−1)
131
187
193
2.2 Experimental details
The uniform-size seedlings were tagged for treatment applications as salinity (0, 50, and 100 mM NaCl) and melatonin (control, 50, and 100 µM). Melatonin was purchased from the well-known company of Sigma–Aldrich Chemicals Pvt. Ltd. Stock solution of the melatonin was prepared in the ethanol and preserved at −20 °C for regular spraying. Desired concentration of melatonin was mixed in the ethanol (100 %). A volume of 40 mL of melatonin was sprayed on each plant after covering the soil. All irrigations were made with salinity levels, while melatonin concentrations were applied after fifteen days intervals till crop harvesting.
2.3 Observation recorded
2.3.1 Growth and yield measurements of plants
Plant height and canopy were measured using a vernier caliper. The fresh weight of the plant and its flowers was measured using a digital weighing balance (Wang et al., 2023). The number of leaves, flowering buds, and flowers were also counted on the tagged pansy plants.
2.3.2 Evaluation of photosynthetic pigments
Photosynthetic pigments (chlorophyll ‘a’, chlorophyll ‘b’, and total chlorophyll) were measured using a common method of Arnon (1949) by use of 80 % acetone. After extraction, samples were centrifuged and the supernatant was used to record absorbance via spectrophotometer at 665 and 652 nm. Acetone was used as a blank to subtract their absorbance value from the pansy leaf samples.
Chlorophyll ‘a’ (mg/g fresh weight basis) = (A663 × 0.0127 − A645 × 0.00269) × 100.
Chlorophyll ‘b’ (mg/g fresh weight basis) = (A645 × 0.0229 − A663 × 0.00468) × 100.
Total chlorophyll (mg/g fresh weight basis) = (A645 × 0.0202 + A663 × 0.00802) × 100.
2.3.3 Determination of malondialdehyde (MDA) and hydrogen peroxide (H2O2)
To measure MDA and H2O2 in pansy plants, a simple and commonly used method called the thiobarbituric acid reactive substances (TBARS) assay was adopted for MDA and the titanium sulfate (Ti-SO4) assay for H2O2. About, 200 μL supernatant was used homogenized in 0.5 % thiobarbituric acid for MDA, and 0.5 Ti-SO4 for H2O2 (Nakano and Asada, 1981).
2.3.4 Assessment of antioxidant activities
Grind the plant material in liquid nitrogen to obtain a fine powder and stop metabolic activities. the powder was transferred to a pre-chilled tube and add an appropriate extraction buffer (phosphate buffer) to extract the enzymes (SOD, POD, CAT, and GR). Homogenize the mixture thoroughly and centrifuge it to separate the soluble fraction. Spectrophotometer was used to record optical values of enzymes in pansy leaves at different specific wavelengths. A method of Nakano and Asada (1981) was successfully employed for the determination of enzymes in the pansy plants.
2.3.5 Measurements of GB and APX
Fresh pansy leaf samples of 0.3 g were homogenized in a suitable extract buffer. Pansy leaf samples were centrifuged and the supernatant was collected for the determination of optical value via spectrophotometer at specific wavelengths according to the earlier described method (Grieve and Grattan, 1983).
2.4 Statistical analysis
The tagged pansy plants data were analyzed using the analysis of variance (ANOVA) technique under completely randomized design (CRD) with two factors (salinity and melatonin) through the computation software Statistix 8.1. However, treatment means were separated by using the least significant difference (LSD) test at 0.05 probability.
3 Results
3.1 Melatonin improves pansy growth and flowering yield
Pansy growth and yield were reduced significantly higher at 100 mM NaCl than in other treatments of salinity. However, melatonin 100 µM improved significantly all the growth and yield traits than 50 µM and control under salinity. Pansy plants treated with melatonin of 100 µM exhibited increased plant height and canopy, number of leaves, and plant fresh biomass under salinity (Fig. 1). The maximum reduction in the number of flowering buds and flowers per plant and flowers fresh weight were recorded in pansy under salinity of 100 mM. The number of flowering buds and flowers per plant and flowers fresh weight were significantly higher in melatonin-treated (100 µM) pansy under salinity (Fig. 2).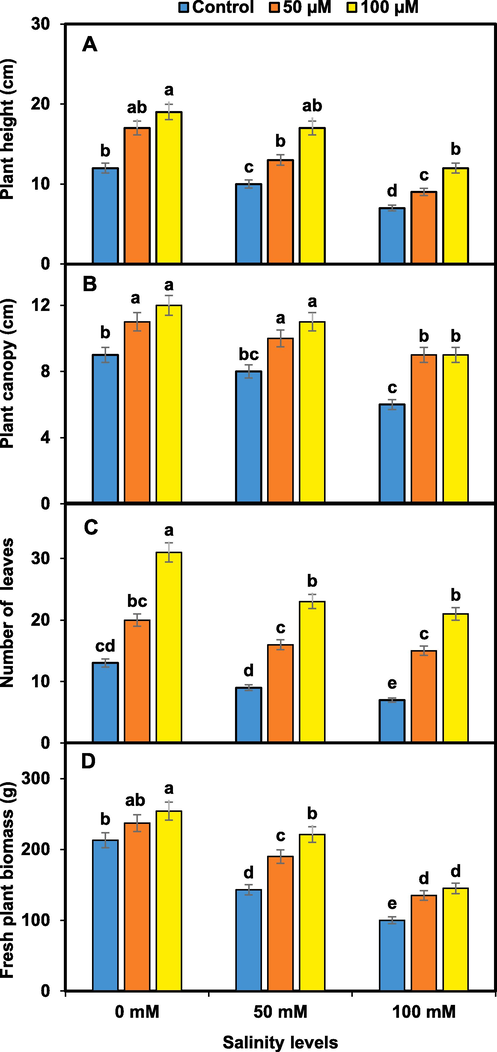
Melatonin spray improves pansy growth i.e. plant height (A), plant canopy (B), number of leaves (C), and fresh plant biomass (D) under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).
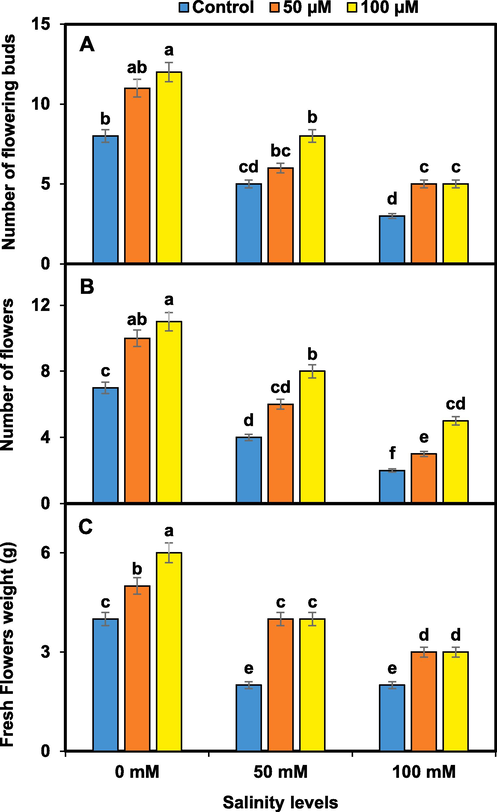
Melatonin effects on pansy yield i.e. number of flowering buds (A), number of flowers (B), and fresh flowers weight (C) under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).
3.2 Melatonin regulates the photosynthetic apparatus of pansy
Salinity stress caused a significant reduction in the content of photosynthetic pigments in pansy plants compared to the control group. However, the application of melatonin mitigated the adverse effects of salinity on photosynthetic pigments. The chlorophyll ‘a’, ‘b’, and total chlorophyll showed a noticeable improvement with melanin at 100 µM under both salinity concentrations. The increase in chlorophyll content was recorded with melatonin (50 and 100 µM) (Fig. 3).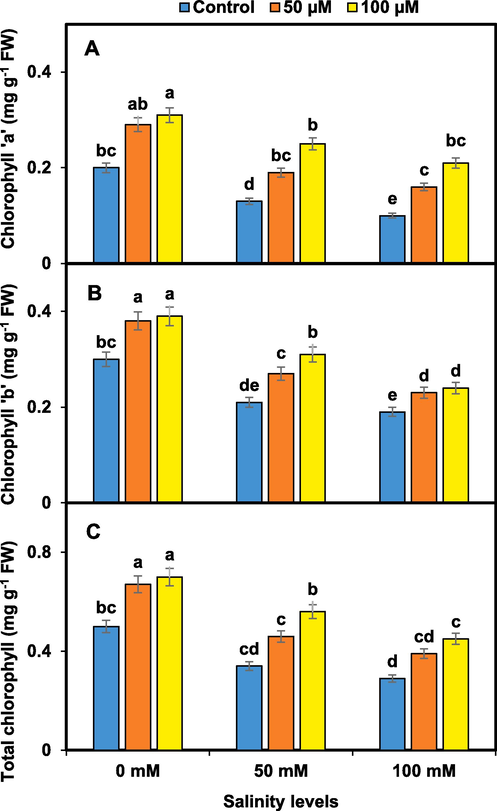
Melatonin effects on pansy photosynthetic pigments i.e. chlorophyll ‘a’ (A), chlorophyll ‘b’ (B), and total chlorophyll (C) under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).
3.3 Melatonin normalizes oxidative injury in pansy
Salinity stress significantly increased the levels of MDA and H2O2 compared to non-stressed pansy plants. The application of melatonin at concentrations of 50 and 100 µM effectively mitigated the salinity-induced elevation of MDA and H2O2 levels in pansy plants. Both melatonin treatments resulted in a significant decrease in MDA and H2O2 levels compared to the salinity-stressed plants without melatonin treatment. Remarkably, melatonin (100 µM) exhibited a more pronounced effect in reducing MDA and H2O2 levels compared to the lower concentration (50 µM) (Fig. 4).![Melatonin effects on oxidative [MDA (A) and H2O2 (B)] stress indicators in pansy under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).](/content/185/2024/36/8/img/10.1016_j.jksus.2024.103286-fig4.png)
Melatonin effects on oxidative [MDA (A) and H2O2 (B)] stress indicators in pansy under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).
3.4 Melatonin enhanced antioxidant activities in pansy
The effect of melatonin was more pronounced at a concentration of 100 µM. The antioxidant activities of SOD, POD, CAT, and GR were enhanced compared to both the salinity-stressed plants without melatonin treatment and the plants treated with 50 µM melatonin. Current findings revealed that higher concentrations of melatonin (100 µM) exerted a more potent protective effect on the antioxidant defense system of pansy plants under salinity stress (Fig. 5).![Melatonin effects on antioxidants [SOD (A), POD (B), CAT (C), and GR (D)] activities i.e. in pansy under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).](/content/185/2024/36/8/img/10.1016_j.jksus.2024.103286-fig5.png)
Melatonin effects on antioxidants [SOD (A), POD (B), CAT (C), and GR (D)] activities i.e. in pansy under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).
3.5 Melatonin boosted osmolytes accumulation in pansy
Salinity stress alone led to a significant reduction in GB and APX accumulation in pansy plants. However, melatonin treatment at both 50 and 100 µM concentrations resulted in a substantial increase in GB and APX levels compared to the only salinity-stressed plants. Results indicated that melatonin can enhance the accumulation of GB, which helps in osmotic adjustment and protection against salinity-induced stress (Fig. 6).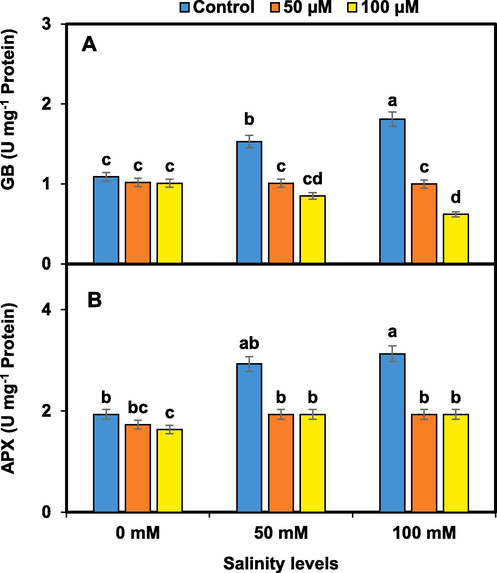
Melatonin effects on GB (A) and APX (B) accumulation in pansy under salinity. Mean values sharing different lettering showed significant effects on traits at 0.05 probability. Data presented are means ± SEs (n = 3).
4 Discussion
Melatonin application can increase plant height, canopy size, leaf number, and fresh plant biomass in the present study (Fig. 1). Moreover, melatonin has a positive impact on flowering-related factors such as raising the number of blooming buds and flowers and improving their fresh weight (Fig. 2). These improvements are important to enhance growth traits and floral yield of plants subjected to salinity stress (Shahzad et al., 2022). Melatonin supplementation had a favorable impact on plant growth and fresh and dry biomass under salinity stress. Current results showed that melatonin spray improved leaf size, number of leaves, and overall plant health. Current findings are in accordance to earlier researchers because melatonin had potential to cope with salt toxicity in crops such as Indian jujube (Ahmad et al., 2023) and tomato (Altaf et al., 2020).
Present results revealed that melatonin plays a crucial role in enhancing plant resilience. Melatonin significantly increased the chlorophyll content in pansy under salinity stress. Under salinity, pansy treated with melatonin showed an increase in photosynthetic pigments (Fig. 3). Earlier research work suggested that supplementing with melatonin can lessen the negative effects of salinity on chlorophyll formation (Mishra et al., 2023). Melatonin encourages higher photosynthetic efficiency by increasing the production of chlorophyll content, which improves plant growth salinity stress (Ayyaz et al., 2022). These results demonstrate melatonin is an efficient strategy to increase salinity tolerance in plants (Khan et al., 2022).
Melatonin has been found to increase plants resilience to salinity by lessening oxidative damage as revealed from the present results (Fig. 4). According to the current study, melatonin treatments increased levels of H2O2 and MDA, two signs of oxidative stress under salinity in pansy plants. Salinity causes an increase in ROS production, which damages cells (Moustafa-Farag et al., 2020). Melatonin functions as a powerful antioxidant, scavenging ROS and reducing oxidative stress (Bose and Howlader, 2020). Melatonin contributes to cellular homeostasis maintenance and membrane integrity preservation by lowering H2O2 and MDA levels, eventually enhancing pansy tolerance to salinity stress (Yavaş and Hussain, 2022). Hence, these results demonstrate the potential of melatonin to improve plant tolerance against salinity stress.
The impact of melatonin on boosting antioxidant enzyme activities in plants under salinity stress is also studied by Debnath et al. (2019). Important enzymes involved in preventing oxidative stress include SOD, POD, CAT, and GR activation (Sheikhalipour et al., 2022; Altaf et al., 2023). Stress from salinity frequently triggers excessive ROS production, which harms plant tissues (Saddhe et al., 2019). Hence, current research has shown that melatonin supplementation can enhance SOD, POD, CAT, and GR activity, hence lowering oxidative damage (Fig. 5). These enzymes are essential for neutralizing ROS and preserving the cellular redox balance (Arnao and Hernández-Ruiz, 2021). Melatonin has the potential to be an effective phytohormone in harsh environment because of its capacity to strengthen the antioxidant defense system in plants under saline stress (Esboei et al., 2022).
The pansy tolerance to salinity was increased by melatonin (Fig. 6). Previous research has shown that melatonin application raises the levels of two crucial substances, GB and APX (Li et al., 2019b). So, APX and GB scavenges potentially hazardous ROS (Huang et al., 2022). Melatonin helps to lessen the harmful effects of salt stress on pansy plants by raising the amounts of these signaling compounds (Debnath et al., 2019; Kamiab, 2020). The current findings show that melatonin has the potential to be a useful tool for enhancing plant productivity and resilience under saline conditions.
5 Conclusion
In the light of current findings, pansy plants may benefit from melatonin spray by reducing the harmful effects of salinity stress. The protective impact of melatonin against salt stress considered as a powerful antioxidant and its potential to regulate several physiological processes such as osmotic balance, photosynthesis, and antioxidant defense mechanisms. Conclusively, melatonin had potential to contribute for the improvement of pansy growth, and floral yield under salinity stress.
Consent to participate
All authors consent to participate in the manuscript publication.
Consent for publication
All authors approved the manuscript to be published.
Ethics approval
Not applicable.
Funding
This research received no external funding.
CRediT authorship contribution statement
Hafiza Muniba Din Muhammad: Writing – original draft. Safina Naz: Conceptualization. Riaz Ahmad: Data curation, Formal analysis, Writing – review & editing. Ehsan Ali: Resources, Writing – review & editing. Muhammad Anwar: Formal analysis, Resources, Writing – review & editing. Muhammad Ahsan Altaf: Formal analysis, Writing – review & editing. Saleh Alansi: Funding acquisition, Resources. Abdulaziz A. Alsahli: Funding acquisition, Resources, Writing – review & editing. Sami Abou Fayssal: Writing – review & editing.
Acknowledgements
Thanks to Higher Education Commission, Pakistan for funding to conduct the research. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R236) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Exogenous melatonin spray enhances salinity tolerance in Zizyphus germplasm: a brief theory. Life.. 2023;13:493.
- [CrossRef] [Google Scholar]
- Combined application of methyl salicylate and l-arginine alleviates chilling injury, potentiates antioxidant system and maintains quality of sweet pepper (Capsicum annum L.) fruits cv. ‘Winner’. J. Hort. Sci. Technol.. 2021;4:68-75.
- [CrossRef] [Google Scholar]
- Exogenous melatonin enhances salt stress tolerance in tomato seedlings. Biol. Plantar.. 2020;64:604-615.
- [CrossRef] [Google Scholar]
- Phytomelatonin: an overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plantar.. 2021;172:820-846.
- [CrossRef] [Google Scholar]
- Phytohormones mediated modulation of abiotic stress tolerance and potential crosstalk in horticultural crops. J. Plant Growth Regul.. 2023;42:4724-4750.
- [CrossRef] [Google Scholar]
- Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol.. 2021;23:7-19.
- [CrossRef] [Google Scholar]
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol.. 1949;24:1.
- [CrossRef] [Google Scholar]
- Uncovering the role of melatonin in plant stress tolerance. Theor. Exp. Plant Physiol.. 2022;34:335-346.
- [CrossRef] [Google Scholar]
- Melatonin plays multifunctional role in horticultural crops against environmental stresses: a review. Environ. Exp. Bot.. 2020;176:104063
- [CrossRef] [Google Scholar]
- Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci.. 2019;20:1040.
- [CrossRef] [Google Scholar]
- Exogenous application of melatonin alleviates drought stress in ranunculus asiaticus by improving its morphophysiological and biochemical attributes. Horticulturae.. 2023;9:262.
- [CrossRef] [Google Scholar]
- Melatonin confers fenugreek tolerance to salinity stress by stimulating the biosynthesis processes of enzymatic, non-enzymatic antioxidants, and diosgenin content. Front. Plant Sci.. 2022;13:890613
- [CrossRef] [Google Scholar]
- Melatonin: a multifunctional factor in plants. Int. J. Mol. Sci.. 2018;19:1528.
- [CrossRef] [Google Scholar]
- Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant and Soil. 1983;70:303-307.
- [Google Scholar]
- The effect of the pre-transplant pot media quality on pansy garden performance. Int. J. Plant Soil Sci.. 2017;19:1-12.
- [CrossRef] [Google Scholar]
- Pansy floral development and nutrient absorption as influenced by temperature, nitrogen form, and stage of plant development. J. Plant Nutr.. 2001;24:1975-1985.
- [CrossRef] [Google Scholar]
- Melatonin as a regulator of plant ionic homeostasis: implications for abiotic stress tolerance. J. Exp. Bot.. 2022;73:5886-5902.
- [CrossRef] [Google Scholar]
- Salinity affects the growth and quality of rose (Rosa damascena) J. Hort. Sci. Technol.. 2021;4:30-35.
- [CrossRef] [Google Scholar]
- Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr.. 2020;43:1468-1484.
- [CrossRef] [Google Scholar]
- Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020;168(2):256-277.
- [Google Scholar]
- Melatonin function and crosstalk with other phytohormones under normal and stressful conditions. Genes. 2022;13:1699.
- [CrossRef] [Google Scholar]
- Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signalling molecules. Antioxidants. 2019;8(12):641.
- [Google Scholar]
- Abiotic and biotic stress in horticultural crops: insight into recent advances in the underlying tolerance mechanism. Front. Plant Sci.. 2023;14:1212982
- [CrossRef] [Google Scholar]
- The role of melatonin in salt stress responses. Int. J. Mol. Sci.. 2019;20:1735.
- [CrossRef] [Google Scholar]
- Enhanced CO2 capture for photosynthetic lycopene production in engineered Rhodopseudomonas palustris, a purple nonsulfur bacterium. Green Chem.. 2022;24:7500-7518.
- [CrossRef] [Google Scholar]
- Exogenous melatonin improves seed germination in Limonium bicolor under salt stress. Plant Signal. Beh.. 2019;14:1659705
- [CrossRef] [Google Scholar]
- Reactive oxygen species in plants: from source to sink. Antioxidants (Basel).. 2022;11(2):225.
- [CrossRef] [Google Scholar]
- Smart reprogramming of jujube germplasm against salinity tolerance through molecular tools. Funct. Integrat. Genomics. 2023;23:222.
- [CrossRef] [Google Scholar]
- Signal crosstalk of phytomelatonin during salinity stress tolerance in plants. Plant Growth Regul.. 2023;101:35-51.
- [CrossRef] [Google Scholar]
- Role of melatonin in plant tolerance to soil stressors: salinity, pH and heavy metals. Molecules. 2020;25:5359.
- [CrossRef] [Google Scholar]
- Foliar application of micronutrients enhances growth, flowering, minerals absorption and postharvest life of tuberose (Polianthes tuberosa L.) in calcareous soil. J. Hort. Sci. Technol.. 2021;4:41-47.
- [CrossRef] [Google Scholar]
- Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol.. 1981;22:867-880.
- [Google Scholar]
- Evaluation of capsicum annum L. genotypes against salinity induced by NaCl. J. Hort. Sci. Technol.. 2021;4:62-67.
- [CrossRef] [Google Scholar]
- The bacterial MtrAB two-component system regulates the cell wall homeostasis responding to environmental alkaline stress. Microbiol. Spect.. 2022;10:e02311-e02322.
- [CrossRef] [Google Scholar]
- Growth and yield response of different brinjal cultivars to irrigation deficit conditions. J. Hort. Sci. Technol.. 2019;2:78-84.
- [CrossRef] [Google Scholar]
- Melatonin distribution reveals clues to its biological significance in basal metazoans. PLoS One. 2012;7:e52266
- [CrossRef] [Google Scholar]
- Reactive nitrogen species: paradigms of cellular signaling and regulation of salt stress in plants. Environ. Exp. Bot.. 2019;161:86-97.
- [CrossRef] [Google Scholar]
- Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes. BMC Plant Bio.. 2022;22:1-17.
- [CrossRef] [Google Scholar]
- Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J Hazard Mat. 2020;398:122882
- [CrossRef] [Google Scholar]
- Effect of heat stress on root architecture, photosynthesis, and antioxidant profile of water spinach (Ipomoea aquatica Forsk) seedlings. Horticulturae.. 2023;9:923.
- [CrossRef] [Google Scholar]
- Recent progress on melatonin-induced salinity tolerance in plants: an overview. Turkish J. Agri-Food Sci. Technol.. 2022;10:1447-1454.
- [CrossRef] [Google Scholar]
- Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci. Total Environ.. 2021;771:144751
- [CrossRef] [Google Scholar]







