Translate this page into:
Hepatoprotective activity of Peltophorum pterocarpum leaf and bark in the isoniazid and rifampicin-induced hepatotoxic rats
⁎Corresponding author. avahgmb@buc.edu.in (Arumugam Vijaya Anand)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
In recent years, various phytomedicines have been used in the treatment of hepatic disorders. The aim of this study is to find out whether Peltophorum pterocarpum bark and leaves can help rats that have been exposed to isoniazid and rifampicin-induced hepatotoxicity.
Materials and Methods
The rats were divided into 10 groups each with 6 rats. The liver damage is induced by isoniazid and rifampicin. The leaf and bark of P. pterocarpum extracted with ethanol are freshly mixed in sterile water (100, 200 and 400 mg/kg body weight), and given to rats orally in the early morning as a single dosage per day until the study period. The animals were sacrificed and the tissue and serum samples were collected for further investigations.
Results
The liver damage induced by isoniazid and rifampicin altered the various biochemical parameters levels, but after the treatment of P. pterocarpum barks and leaves the levels were significantly altered when compared to the negative control rats. The drug silymarin was used as a standard.
Conclusion
The extracts have the protective effect of liver markers and membrane-bound enzymes against the toxin-treated rats. This result highlights the hepatoprotective properties of the leaves and barks of the plant in a similar manner and the formulation (Group 10) have high beneficial effects than other groups.
Keywords
Isoniazid
Rifampicin
Hepatoprotective
Liver enzymes
Phytomedicines
Mitochondrial enzymes
1 Introduction
Hepatic diseases are the most serious ailments including acute hepatitis, chronic hepatitis, hepatosis and cirrhosis. It is caused due to hepatotoxic agents, high consumption of alcohol, infections and autoimmune disorders. The improper or non-functional condition in the liver is caused by liver damage in which the majority of the liver cells die or turn to the fibrotic stage. In this condition the therapy can be considered, which would boost the growth of the liver cells, to retrieve the damaged cells into the normal cells (Kamble et al., 2008). The use of natural and herbal medicines to treat a wide range of serious illnesses that are currently plaguing people all over the world is becoming more and more popular. Many commercial medications are taken off the market due to their serious side effects and expensive cost. This is especially the case for their hepatotoxic adverse effects. As a result of this, a wide range of polyherbal formulations are currently advised for the management of several chronic illnesses, like, cancer, hypertension, heart disease, liver problems, and others.
Peltophorum pterocarpum is a well-known ornamental tree that grows along roadsides and is a member of the Fabaceae family. For their therapeutic benefits, including those that are neuroprotective and memory enhancing, antibacterial, anti-diabetic, cardiotonic, antioxidant, anti-inflammatory, hypocholesterolemic, anti-arthritic, and anti-cancer, all of the plant's parts have ethnobotanical and scientific records (Devi and Battu, 2018). Phenolic acids, flavonoids, terpenoids, alkaloids, steroids, carotenoids, tannins, monoterpenes, hexadecanoic acid, octadecanoic acid, saponins, etc., were found to be distributed in the plant, according to phytochemical studies (Amala and Poonguzhali, 2015). The P. pterocarpum was found to contain phenolic acids, flavonoids, tannins, saponins, coumarins, carotenoids, octadecanoic acid, β-sitosterol, [2–4] (-)-epicatechol, valeronone, isosatvene, hexadecanoic acid, octadecanoic acid, steroids, alkaloids, amino acids, carotenoids, coumarins, (+)-leucocynidin, quercetin, berginin, vitamins, etc. (Jain et al., 2012). The leaves and blossoms are employed to treat skin problems, sleeplessness, sprains, swellings, muscular pains and intestinal complaints behind pain during childbirth and are also comprehended to be acceptable sleep inducers (Jash et al., 2014). The goal of this investigation is to evaluate the beneficial hepatoprotective efficiency of bark and leaves of P. pterocarpum in rats intoxicated with isoniazid and rifampicin.
2 Materials and methods
2.1 Collection of plants and extraction
The P. pterocarpum barks and leaves were obtained from Tiruchirappalli, India, during the month of March to July. The plant specimen was recognized and placed at the Rapinat Herbarium, St. Joseph’s College, Tiruchirappalli, India. The traditional method was used for preparing ethanolic extract from the plant. The barks and leaves were dried in the shade and powdered mechanically then the Soxhlet apparatus was used to prepare the extract using ethanol. The filtrate obtained was dehydrated in a vacuum and then the extracts were stored at a cold temperature till further use.
2.2 Experimental design
The Wistar rats (adult male, 56 days old, and 150–165 g weight) were procured and maintained at 25°C with 12 h of alternative cycles of light and dark. The pellets were balanced with all essential nutrients and ad libitum water was given. For conducting the experimental protocols and handling of animals were authorized by the Institutional Animal Ethics Committee, Srimad Andavan College of Arts and Science, Tiruchirappalli, India (Registration Number: SAC/IAEC/BC/2016-/Ph.D.-007).
Every day during the experimental period, rats were given a single oral dosage of plant extracts (ethanolic extract) in the early morning. Animals used in experiments were distributed randomly into 10 groups and each group contained six rats housed individually. All the experimental animals were intoxicated with isoniazid and rifampicin except for the control group. Animals classified as control (G1) were given basal diet, negative control (G2) Intoxicated with isoniazid and rifampicin (untreated), G2 treated with P. pterocarpum (leaf extract) of 100 mg/kg (G3), G2 treated with P. pterocarpum (leaf extract) of 200 mg/kg (G4), G2 treated with P. pterocarpum (leaf extract) of 400 mg/kg (G5), G2 treated with P. pterocarpum (bark extract) of 100 mg/kg (G6), G2 treated with P. pterocarpum (bark extract) of 200 mg/kg (G7), G2 treated with P. pterocarpum (bark extract) of 400 mg/kg (G8), silymarin of 25 mg/kg and G2 administered with both extract (G10).
2.3 Analysis of the tissue and serum samples
Following the completion of the investigation period, the animals were fasted overnight for about 12 h and the samples were collected from treated rats by sacrificing them with standard protocol. The collected samples were tested for the following parameters of membrane-bound enzymes including, liver biomarkers like creatine kinase (CK), gamma glutaryl transferase (GGT), lactate dehydrogenase (LDH) and lipase and Na+K+ ATPase, Mg2+ ATPase, Ca2+ ATPase, total bilirubin and direct bilirubin in Turbo Chem 100 biochemical analyzer with commercially available kits from CPC Diagnostics.
2.4 Tissue sampling for histopathological study
For histopathological study, three rats from each group were perfused with cold physiological saline, followed by formalin (10% formaldehyde). The liver was excised immediately and fixed in 10% formalin. The classical paraffin sectioning, and haematoxylin eosin staining techniques were used for histopathological studies followed by (Slaoui and Fiette, 2011).
2.5 Statistical analysis
The results obtained from the biochemical analysis were statistically evaluated using one-way ANOVA followed by DMRT (Duncan's multiple range tests) using SPSS software 17 (Chicago, USA). The values given in the table are mean ± SD (standard deviation). P < 0.05 was considered statistically significant.
3 Result
The group intoxicated with isoniazid and rifampicin showed a reduction in body and liver weights. Table 1 represents the changes in the weight of the liver and body in experimental groups. Rats in all the groups except for the control showed a reduction in their body and liver weights. A steady increase in body weight was noted in the groups treated with ethanolic extract of P. pterocarpum leaves and barks and the results were compared to the silymarin-treated rats. Compared to the control, rats which received standard drug didn’t show an increase in body weight. Values are expressed in mean ± SD (n = 6). Mean values in the same column with different superscript from a to h are different P values.
Groups
Bodyweight (g)
Liver weight (g)
G1
160.33 ± 0.88a
5.86 ± 0.09a
G2
113.50 ± 0.76b
3.51 ± 0.12b
G3
123.00 ± 0.82c
4.33 ± 0.02c
G4
138.67 ± 0.92d
4.88 ± 0.05d
G5
153.83 ± 0.79e
5.40 ± 0.0e
G6
118.17 ± 0.54c,f
4.16 ± 0.04f
G7
130.83 ± 0.70 g
4.41 ± 0.04c
G8
146.67 ± 0.88 h
5.05 ± 0.06d
G9
156.67 ± 1.48e
5.54 ± 0.06e
G10
156.83 ± 0.48e
5.73 ± 0.01a,e
Table 2 represents the results of LDH, GGT, lipase and CK. Increases in levels of LDH, GGT, lipase and CK were observed in groups induced with isoniazid and rifampicin. After the treatment with plant extracts and standard drug, the concentrations were normalized in the rats. Table 3 represents the levels of liver protein, direct bilirubin and total bilirubin in control and experimental rats. The liver protein decreased and bilirubin levels elevated potentially (P < 0.05) in toxin-induced animals. After, the treatment with silymarin, leaves and bark extracts, there was a significant improvement in the liver parameters was observed when compared to the control (P < 0.05). Table 4 represents the Na+K+ ATPase, Mg2+ ATPase and Ca2+ ATPase levels in the experimental and control groups. The activities of the mentioned enzymes were reduced significantly (P < 0.05) in toxin-induced animals. The silymarin and ethanolic leaves and bark extract of the plant treatment significantly normalized the altered levels in comparison with the normal control (P < 0.05). Values are expressed in mean ± SD (n = 6). Mean values in the same column with different superscript from a to f are different P values. LDH: lactate dehydrogenase; GGT: gamma glutaryl transferase; CK: creatine kinase. Values are expressed in mean ± SD (n = 6). Mean values in the same column with different superscript from a to f are different P values. Values are expressed in mean ± SD (n = 6). Mean values in the same column with different superscript from a to g are different P values. NA+K+ ATPase: sodium–potassium ATPase; Ca2+ ATPase: calcium ATPase; Mg2+ ATPase: magnesium ATPase.
Groups
LDH (U/L)
GGT (U/L)
Lipase (U/L)
CK (U/L)
G1
167.62 ± 4.30a
10.07 ± 0.43a
56.02 ± 1.19a
169.50 ± 3.13a
G2
319.68 ± 8.16b
19.98 ± 1.23b
89.11 ± 1.92b
909.67 ± 9.17b
G3
280.36 ± 4.82c
15.51 ± 0.26c
80.11 ± 1.35c
704.83 ± 7.90c
G4
224.92 ± 6.31d
13.47 ± 0.16d
69.43 ± 1.17d
531.67 ± 8.86d
G5
170.85 ± 2.98a
11.57 ± 0.38a,e
59.33 ± 0.70e
180.17 ± 1.92a,e
G6
297.15 ± 7.29e
16.58 ± 0.42c
82.21 ± 0.68c
763.33 ± 6.79f
G7
236.43 ± 5.68d
15.00 ± 0.28c
73.26 ± 1.04d
564.17 ± 19.21d
G8
179.44 ± 2.11f
12.64 ± 0.36a,e
62.52 ± 1.95e
205.83 ± 10.19e
G9
168.32 ± 1.68a
11.10 ± 0.31a
57.14 ± 0.98a
174.67 ± 3.92a
G10
167.91 ± 2.20a
10.10 ± 0.52a
56.92 ± 0.91a
168.81 ± 3.19a
Groups
Protein (mg/dl)
Direct Bilirubin (mg/dl)
Total Bilirubin
(mg/dl)
G1
9.42 ± 0.04a
0.73 ± 0.01a
0.88 ± 0.01a
G2
4.71 ± 0.40b
1.32 ± 0.01b
1.62 ± 0.03b
G3
5.64 ± 0.18c
1.11 ± 0.10c
1.37 ± 0.01c
G4
7.39 ± 0.19d
1.01 ± 0.01d
1.16 ± 0.00d
G5
9.02 ± 0.04a
0.77 ± 0.01a
0.95 ± 0.00a
G6
5.10 ± 0.05c
1.20 ± 0.02e
1.44 ± 0.01e
G7
6.86 ± 0.08e
1.08 ± 0.02d
1.29 ± 0.04c,d
G8
8.79 ± 0.11a
0.84 ± 0.02a
1.04 ± 0.03a
G9
9.20 ± 0.03a
0.76 ± 0.00a
0.92 ± 0.00a
G10
9.48 ± 0.05a
0.71 ± 0.01a
0.89 ± 0.01a
Groups
NA+K+ ATPase
Ca2+ ATPase
Mg2+ ATPase
(µg of pi liberated/g tissue/min)
G1
61.2 ± 1.4a
78.04.7a
100.3 ± 0.9a
G2
37.2 ± 1.0b
49.0 ± 0.4b
73.4 ± 0.6b
G3
45.4 ± 0.6c
58.0100.7c
83.0 ± 2.6c
G4
51.8 ± 0.7d
63.6 ± 0.4d
87.4 ± 0.4d
G5
53.2 ± 0.5d
73.0 ± 1.4e
93.4 ± 0.4e
G6
43.1 ± 0.5c
55.2 ± 0.7c
80.2 ± 1.2c
G7
49.0 ± 0.7d,e
61.4 ± 0.7d
84.8 ± 0.8f
G8
52.4 ± 0.6d
71.2 ± 0.8e
89.0 ± 0.7d
G9
57.0 ± 0.3f
75.2 ± 0.4f
97.4 ± 0.4 g
G10
60.0 ± 0.3a,f
76.4 ± 0.5a,f
98.4 ± 0.4a,g
Fig. 1 represents that the histopathological evaluation of rat livers of the experimental and control groups. The liver of (Fig. 1A) rats in the control group was found to be normal with a central vein and predominant nucleus. The liver of the rats intoxicated with isoniazid and rifampicin (Fig. 1B) showed hepatocellular alteration, irregular hepatocytes, hydropic degeneration, nuclear congestion, blood spot appearance and vacuolization observed in histoarchitectural examinations, compared with control. The (Fig. 1J) rats treated with P. pterocarpum leaves and bark (400 mg/kg) show normal histological architecture respectively. The isoniazid and rifampicin treated (Fig. 1C and Fig. 1D) rats supplemented with P. pterocarpum (100, 200 mg/kg) of leaves showed partial recovery of hepatocytes with mild degeneration.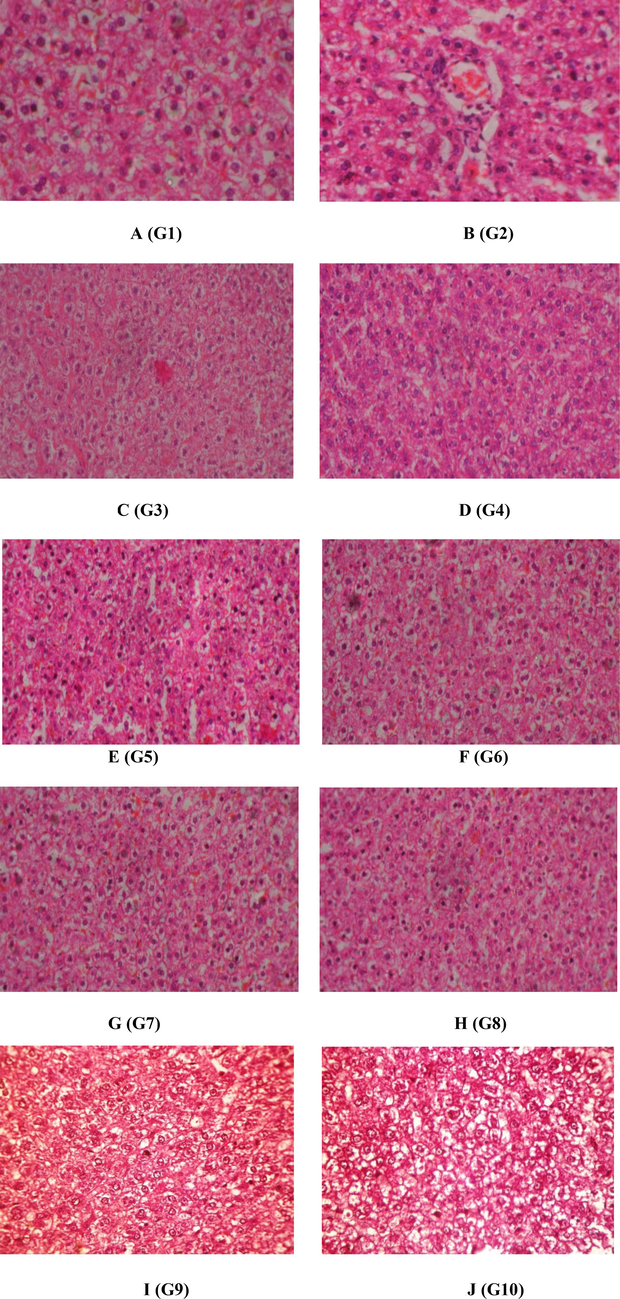
(A to J/G1 to G 10). The histopathological examination of liver of the control and experimental rats. 1A) Control (basal diet and saline water, G1-group 1), 1B) untreated- Isoniazid and rifampicin induced as negative control (G2), 1C) G2 administered with 100 mg/kg of leaf extract of P. pterocarpum (G3), 1D) G2 administered with 200 mg/kg of leaf extract of P. pterocarpum (G4), 1E) G2 administered with 400 mg/kg of leaf extract of P. pterocarpum (G5), 1F) G2 administered with 100 mg/kg of bark extract of P. pterocarpum (G6), 1G) G2 administered with 200 mg/kg of bark extract of P. pterocarpum (G7), 1H) G2 administered with 400 mg/kg of bark extract of P. pterocarpum (G8), 1I)25 mg/kg of silymarin (G9) and 1 J) G2 administered with both leaf and bark extract (G10).
The isoniazid and rifampicin treated (Fig. 1F and G) rat supplemented with P. pterocarpum (100, 200 mg/kg) of barks noted signs of protection and considerable extent as obvious from the formation and regeneration of normal hepatic cells as well as preserved central vein and vacuoles in the histology of the liver. The higher doses of ethanolic extracts of leaves and barks of P. pterocarpum (400 mg/bw) treated rats (Fig. 1E and H) rescued the normal morphology of the liver as similar to the control. The standard silymarin-treated rats (Fig. 1I) showed normal liver cells with a well-conserved central vein, cytoplasm and prominent nucleus. The plant extracts of leaves and barks of P. pterocarpum alone treated group (Fig. 1J) showed the normal hepatic cell architecture, this may prove the harmless nature of the plant extracts.
4 Discussion
The body and liver weight are important indicators of the toxicity of the drug, because of the morphological changes occurring in the toxic conditions. Several factors influence the weight changes including the animal, gender, sex, toxin and environmental conditions (Gur and Waner, 1993). In the current study, the weight of the body and liver is decreased in the isoniazid and rifampicin-treated groups but the weight was significantly increased in the treatment with the leaves and barks extracts of P. pterocarpum and the results were compared to the silymarin treated rats. Tersitore et al. (1987) have revealed that a decrease in body weight is noted in ascitic hepatoma-bearing rats with tumour growth. This is due to a reduction in food consumption and its absorption may contribute to muscle wastage in the tumour (Pain et al., 1984).
From the study of Vijayakumar et al. (2018) suggested that the ethanolic extract of Psidium guajava and quercetin fraction from P. guajava had a protective effect against the oxidative stress in the HepG2 cell line induced by the carbon tetrachloride (CCl4). The findings of the current investigation also prove the protective effect of the ethanolic extract of leaves and bark on liver cells. In a dosage-dependent manner, the P. pterocarpum leaves have higher activity when compared to the bark extract. The plant extract alone treated group has a similar value when compared with the normal control rats. This proves the protection of the plant without side effects. The LDH is an important enzyme in the glycosylase family; it occurs in the cytoplasm of tissues and is mainly present in the kidney. In the current study, in the toxin-treated rats, the concentration of LDH is highly elevated. Then the treatment of leaves and barks of the extract of the plant reduces the level, which is nearer to the standard drug-treated animals. The GGT is a peptidase, that catalyses the transfer of gamma glutaryl group from peptides. The level of GGT is increased in the hepatic damage condition (Carla et al., 2016). The findings of the current study highlighted that the GGT were elevated in the toxin-induced animals, and the treatment with plant leaves and bark extract the content was restored. This may prove the recovery of the hepatic damage. The CK is a cytosolic enzyme. It is the most sensitive enzyme to muscle damage. The administration of leaves and bark of plant extract may reduce CK. The lipase enzyme helps to digest fats present in the diet up to 50–70% and also increases the risks of vitamin deficiencies and liver diseases. The findings of the current study presented that the toxin-treated rats showed an increase in lipase. After the treatment of leaves and bark extracts, the level of lipase is considerably decreased. A similar effect is noted in the earlier study of Maqsood et al. (2017).
The liver regulates the synthesis of protein. Hepatic damage due to the toxins such as isoniazid and rifampicin alters the synthesis and metabolism of liver glycogen and protein (Andrea and Harugara, 1987). The levels of protein and glycogen were stimulated by the administration of ethanolic leaves and bark extract of the plant. This may be due to the hepatoprotective property of the leaves and bark extract. In this study, the extract prevents the deamination process and retains the protein level, as well as some enzymes, that regulate the glucose conversion into glycogen. The previous study of the treatment of Tricholepis radicans in toxin-induced hepatic rats increases the protein and glycogen levels. John et al. (2011). Gracilaria corticata alters the liver enzymes in AFB1-induced hepatotoxicity (Manoharan et al., 2008). This may be proven by the present study also.
According to the study of Friedman et al. (1978) stated that bilirubin is the end product of haemoglobin; it binds to albumin and migrates to the liver, where it is bound to glucuronic acid and excreted in bile. The rise in bilirubin concentration is directly proportional to hepatic dysfunction due to the toxin. In the current study, the level of bilirubin is elevated because of the toxicity induced by isoniazid and rifampicin. In this investigation, after the administration of leaves and bark extract of the plant, the level of direct and total bilirubin was decreased. Hepatotoxins can decrease the protective effect of the hepatoprotective drug that can preserve the normal activity of the liver. The ATPase is mainly involved in the translocation process of sodium, potassium, calcium and magnesium. The enzyme ATPase regulates the electrolytes in cells and cellular membranes. In liver toxic conditions, alterations occur in the structure of the membrane and it reduces the enzyme level in the blood (Premalatha and Sachidanandam, 1998). Ionic homeostasis and the physiological function of the liver are dependent on trans membranous enzymes like Ca2+ ATPase and Na + K+ ATPase (Frolkis et al., 1999). The study of Chandramohan et al. (1996), who stated that the enzyme Na+K+ ATPase regulates the intracellular Na+ concentration and water content in the cell. The Na+K+ ATPase inhibition has been reported as a key regulator to minimize cellular metabolism. Ca2+ ATPase is mainly involved in the regulation of Ca2+ pumping action and it is also involved in the contraction of the muscles and also the neurosecretion process. The decreasing concentration of the levels of protein is associated with the decreasing level of Ca2+ ATPase (Hemmings et al., 2002). In the current study, the protein content is reduced and it may reduce the Ca2+ ATPase level. Mg2+ ATPase is pivotal in the electrolyte transport across the biological membrane. In toxic conditions, the membrane permeability is disturbed and this may be due to the lower concentrations of Mg2+ ATPase. Overall, the protective nature of the plant extract may prevent the cellular membrane from toxin-induced damage and it restored the level of membrane-bound enzymes. In the current investigation, the histopathology changes also support the hepatoprotective efficacy of P. pterocarpum in the toxin-induced animal model. The changes seen are supportive of the studies by Adedapo et al. (2005) who have described the plant extracts of Phyllanthus amarus restored the liver histology against toxin-induced hepatotoxicity. This also explains the restoration of enzymes shown by the groups in the current investigation.
5 Conclusion
The findings of this investigation suggest the protective effect of the P. pterocarpum leaves and bark extract on the liver markers and membrane-bound enzymes against the toxin-treated rats. This result demonstrates that P. pterocarpum leaves and barks are hepatoprotective. However, further identification of effective bioactive compounds from the extract needs to be done. The limitation of the present investigation is the lack of molecular studies which may provide the mechanism of action of the lead compound at the cellular level.
CRediT authorship contribution statement
Maria Jerline Babu: Writing – original draft, Methodology, Formal analysis, Data curation. Ramasamy Manikandan: Writing – original draft, Resources, Methodology, Formal analysis. Balasubramanian Balamuralikrishnan: Writing – review & editing, Writing – original draft, Resources, Investigation. Bharathi Kathirvel: Resources, Methodology, Formal analysis. Rajakrishnan Rajagopal: Writing – review & editing, Visualization, Validation. Ahmed Alfarhan: Writing – review & editing, Visualization, Validation, Resources. Arun Meyyazhagan: Writing – review & editing, Visualization, Resources, Formal analysis. Arumugam Vijaya Anand: Writing – review & editing, Validation, Supervision, Resources, Project administration, Funding acquisition.
Acknowledgement
The authors record their gratitude to the Institution and laboratory fellow mates for providing constant support during the work. The authors extend their appreciation to the Researchers Supporting Project Number: RSP2024R465, King Saud University, Riyadh, Saudi Arabia.
Funding sources
The authors extend their appreciation to the Researchers Supporting Project Number: RSP2024R465, King Saud University, Riyadh, Saudi Arabia.
Institutional review board statement
The experimental protocols and handling of animals were authorized by the Institutional Animal Ethics Committee, Srimad Andavan College of Arts and Science, Tiruchirappalli, Tamil Nadu, India (Approval No.: SAC/IAEC/BC/2016-/Ph.D.-007).
Data availability statement
The authors confirm that the data findings that support this study are available upon request with corresponding author.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Some clinicopathological changes associated with the aqueous extract of the leaves of Phyllanthus amarus in rats. Phytother. Res... 2005;19(11):971-976.
- [CrossRef] [Google Scholar]
- Assessment of total phenolic, falvonoid content and anti-oxidant potential of Peltophorum pterocarpum. (DC.) Baker Ex-Heyne flower extract. IJAR. 2015;1(12):105-107.
- [Google Scholar]
- Comparative changes in hepatic DNA, RNA, protein, lipid and glycogen induced by sub toxic dose of CC14 in chlordecone, mirex, and phenoharbital pretreated rats. Toxicol. Lett.. 1987;35(2–3):191-200.
- [CrossRef] [Google Scholar]
- In vivo effect of L–cysteine on ethanol-induced alterations in membrane–bound ATPases of liver and kidney of experimental rats. J. Clin. Biochem. Nutr.. 1996;20:225-230.
- [Google Scholar]
- A phytochemical andpharamacological review on Peltophorum pterocarpum (Dc.) Baker Ex Heyne. WJPPS.. 2018;7(6):166-176.
- [CrossRef] [Google Scholar]
- Factors influencing the incidence of neonatal jaundice. Br. Med. J.. 1978;1(6122):1235-1237.
- [CrossRef] [Google Scholar]
- Effects of partial hepatectomy on the plasma membrane status and the invertor mechanism of the hepatocyte Na, K-ATPase activity regulation in rats of various age. Aging-Clin. Exp. Res.. 1999;11:130-134.
- [Google Scholar]
- The variability of organ weight background data in rats. Lab Anim.. 1993;27(1):65-72.
- [CrossRef] [Google Scholar]
- Differential inhibitory effects of carbon tetrachloride on the hepatic plasma membrane, mitochondrial and endoplasmic reticular calcium transport systems: implications to hepatotoxicity. Cell Biochem. Funct.. 2002;20(1):47-59.
- [CrossRef] [Google Scholar]
- Antimicrobial, free radical scavenging activities and chemical composition of Peltophorum pterocarpum Baker ex K. Heyne Stem Extract. Der Pharma Chemica. 2012;4(5):2073-2079.
- [Google Scholar]
- Peltophorum pterocarpum: chemical and pharmacological aspects. Int. J. Pharm. Sci. Res.. 2014;5(1):26-36.
- [Google Scholar]
- Hepatoprotective efficacy of Tricholepis radicans DC. against CC14 induced liver toxicity in albino rats. J. Pharm. Res... 2011;4(4):1073-1075.
- [Google Scholar]
- Hepatoprotective activity studies of herbal formulations. Int. J. Green Pharm.. 2008;2(3):147-151.
- [Google Scholar]
- Potential hepatoprotective effect of aqueous extract of Gracilaria corticata inAFB1 induced hepatotoxicity in wistar rats. J. Biol. Sci.. 2008;8:1352-1355.
- [CrossRef] [Google Scholar]
- Lipase inhibitory activity of Lagenaria siceraria as a strategy to treat obesity. Asian Pac. J. Trop. Med.. 2017;10(3):305-310.
- [CrossRef] [Google Scholar]
- Protein synthesis in liver and skeletal muscle of mice bearing an ascites tumor. Cancer Res.. 1984;44(3):1054-1057.
- [Google Scholar]
- Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric.. 2016;96(4):1068-1084.
- [CrossRef] [Google Scholar]
- Immunomodulatory activity of Semecarpusanarcadium Linn. nut milk extract in aflatoxin B-induced hepatocellular carcinoma in rats. Pharm. Pharmacol. Commun.. 1998;4(10):507-510.
- [CrossRef] [Google Scholar]
- Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol. Biol.. 2011;691:69-82.
- [Google Scholar]
- Early development of protein metabolic perperbation in the liver and skeletal muscle of tumours leasing rats. Biochem. J.. 1987;241(1):153-159.
- [CrossRef] [Google Scholar]
- Hypolipidemic effect of Psidium guajava leaf extract against hepatotoxicity in rats. Pharmacogn. Mag.. 2018;14(53):4-8.
- [CrossRef] [Google Scholar]







