Translate this page into:
Syringic acid, resveratrol and gallic acid compounds lipid metabolizing enzymes regulatory activity in isoproterenol-induced cardiac necrosis in rats
⁎Corresponding author at: Department of Cardiology, Xi’an International Medical Center Hospital, Xi'an, 710100, China. gaojiedr@sina.com (Jie Gao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
The present investigation aimed to analyze the in vivo study in support with in silico molecular docking mechanism of natural phenolic compounds Syringic acid (SA), Resveratrol (RV), Gallic acid (GA) and combination (COMB) with SA + RV against Isoproterenol (ISO) in myocardial necrotic rats. Methods: The compounds were tested for in vivo and in silico lipid metabolism enzymes HMG-CoA reductase and lipoprotein lipase (LPL) regulatory activities. Rats were orally pretreated with 50 mg/ kg of each compounds SA, RV, GA and COMB (SA 25 mg/ kg + RV 25 mg/ kg) for 30 days. GA has taken as positive control. After the treatment period, ISO (50 mg/ kg) in sub-cutaneous route was administered to the rats for two consecutive days. Then the rats sacrificed and the cardiac tissues were used for enzyme inhibitory analysis.
Results
SA, RV, GA and COMB exhibited HMG-CoA reductase enzyme inhibitory activity. Also, the compounds augmented the LPL enzyme activity in the rats administered with ISO. Furthermore, in silico molecular docking reports also supported to the activity of SA, RV, GA and COMB compounds towards the enzymes HMG-CoA reductase and LPL.
Conclusion
This report for the first time indicates the potential of phenolic compounds SA, RV, GA and COMB as excellent natural compounds in the therapeutic treatment of lipid metabolism disorders.
Keywords
Syringic acid
Resveratrol
Gallic acid
Isoproterenol
HMG-CoA reductase
Lipoprotein lipase
1 Introduction
Cardiovascular diseases (CVD) endure the prime cause of mortality worldwide. Among CVD the myocardial infarction (MI) is occurred by an interrupted blood supply to the demand of myocardium, which leads to the cardiac necrosis (Filho et al., 2011). MI exhibits edema, reduced cardiac output, abnormal cardiac rhythms that promotes to impair cardiac function. The reduced cardiac output stimulates baroreceptors that proceed to activate the compensatory mechanisms like sympathetic nerves system and renin-angiotensin-aldosterone system.
Isoproterenol (ISO) is a beta-adrenergic agonist chemical that induces severe cardiac stress which leads to the necrotic damage of heart muscle (Nwokocha et al., 2017). The mechanism of action of ISO is auto oxidation, generation of free radicals and hyper stimulation of beta adrenoceptors that causes to the myocardial injury (Haenen et al., 1990). ISO may also increase cardiac output by positive inotropic and chronotropic phenomena.
Lipid, lipoproteins and lipid metabolism are the crucial contributing factors for the genesis and progression of CVD. The lipid metabolism marker enzymes 3-hydroxy-3-methylglutaryl (HMG) CoA reductase (HMG-CoA reductase) and lipoprotein lipase (LPL) play a major role in the occurrence of CVD (Upadhyay, 2015a). The inhibition of these lipid metabolizing marker enzymes leads to the cardio protection and prevention of CVD.
CVD is prevented with the utilization of natural products. The ameliorative effect of these natural products may be due to phenolic compounds. These compounds reported various physiological activities like antioxidant and antibacterial (Lyu et al., 2020), anti-cancer (Hazafa et al., 2020), regulation of lipid metabolism (Toma et al., 2020) Toma and risk reduction of CVD (Lutz et al., 2019). The phenolic compounds like Syringic acid (SA), Resveratrol (RV) and Gallic acid (GA) have been exhibited many pharmacological activities and have been reported as anti-cancer (Pei et al., 2021)(Wu et al., 2019), anti-diabetic (Sabahi et al., 2021)(Jeyaraman et al., 2020) and cardio protective (Liu et al., 2020)(Li et al., 2022). In our previous study, we reported the cardio protective effect of these phenolic compounds via attenuating inflammatory marker enzymes (Manjunatha et al., 2020). The aim of this study is to examine the action of phenolic compounds such as SA, RV and GA in the management of myocardial necrosis by improving enzymes of lipid metabolism such as HMG-CoA reductase and LPL in ISO administered experimental animals by in vivo and in silico molecular mechanism analysis.
2 Materials and methods
2.1 Chemicals
SA, RV, GA and ISO were bought from Sigma Aldrich of USA. Other all chemicals used in the experimental analysis are of analytical grade. The activity of HMG-CoA reductase (Venugopala Rao and Ramakrishnan, 1975) was analyzed in cardiac tissue homogenate and also the activity of LPL (Fugman et al., 1984) was analyzed in heart homogenate. The protein (LOWRY et al., 1951) levels were measured in heart homogenate.
2.2 Animals and experimental method
Male Albino Wistar rats weighing around 100 to 120 gm were maintained to acclimatization of the laboratory conditions for one week and standard pellet diet with sufficient water provided. Animal house was ventilated well, dark and light cycles maintained continuously and the room temperature was maintained with 25 °C. All animal studies strictly followed animal ethical committee regulations. Preliminary dose dependent study was conducted to fix the dose of compounds and 50 mg/kg of SA, RV and GA was chosen. GA was taken as positive control.
The animals were categorized into 7 groups with 6 rats in each group as followed.
-
Control
-
ISO (50 mg/kg)
-
SA (50 mg/kg)
-
GA (50 mg/kg) + ISO (50 mg/kg)
-
SA (50 mg/kg) + ISO (50 mg/kg)
-
RV (50 mg/kg) + ISO (50 mg/kg)
-
COMB (SA 50 mg/kg + RV 50 mg/kg) + ISO (50 mg/kg)
The groups 3, 4, 5, 6 and 7 were orally pretreated with respective compounds for 30 days and ISO was sub-cutaneously injected on the last two consecutive days to the groups 2, 3, 4, 5, 6 and 7. There was no mortality of animals occurred due to the toxicity of ISO. Cervical dislocation employed to sacrifice the animals then blood and myocardial tissues were collected. Serum was separated from blood and homogenate was prepared from heart tissue to analyze various biochemical parameters.
2.3 Molecular docking mechanism analysis
2.3.1 In silico inhibition analysis of SA, RV and GA on HMG-CoA reductase and LPL
The structures of HMG-CoA reductase and LPL from Homo sapiens were acquired from the protein databank. Due to the unavailability of three-dimensional structures in database, the predicted models of PDB format were accumulated (Birrane et al., 2019; Istvan and Deisenhofer, 2001). To select the domain, HMG-CoA reductase and LPL from Homo sapiens were submitted to the server SBASE. Later, the stabilization of protein is achieved by adding hydrogens to the 3D structures by molecular dynamics simulation studies. NAMD (2.8) software and CHARMM (27) force field software were applied to perform the predicted models of MD simulations. The algorithm namely multiple-time-stepping was used in which, the calculation was conducted at every two steps for long range electrostatics and the calculation was conducted at every level step for short range forces. In this analysis, at new positions the new velocities were achieved by MD procedure with the support of equations of motion proposed by Hamilton. Advanced thermodynamic properties were employed to obtain the final model and followed by Root Mean Square Deviation (RMSD) to stabilize the information.
HMG-CoA reductase and LPL structures from Homo sapiens in molecular dynamics studies with lesser RMSD were procured. Ramachandran plot was employed by using PROCHECK server to test the protein structures stereo-chemical quality. Later ERRAT server for evaluation of structure was employed to examine the environment profile (Laskowski et al., 1993).
2.3.2 Identification of HMG-CoA reductase and LPL active sites from Homo sapiens
CASTp server (Tian et al., 2018) was used to build the possible binding sites of HMG-CoA reductase and LPL from Homo sapiens.
2.3.3 Docking analysis with SA, RV and GA
The software GOLD 3.0.1 was employed and the conformations of binding for SA, RV and GA were procured by executing docking studies (Jones et al., 1997). The compounds SA, RV and GA were docked to the HMG-CoA reductase and LPL enzymes active sites to analyze the probability of regulatory activity of these compounds. Later the process of docking, the protein–protein complex individual binding poses were selected and the binding energies were analyzed. Most energetic complex conformation was selected and employed for analysis of docking.
2.4 Statistical analysis
Duncan’s multiple range (DMR) test was performed to analyze the statistical study by considering p < 0.05. Result was expressed as means ± SD.
3 Results
3.1 In vivo activity of SA, RV, GA and COMB on lipid metabolizing enzymes
3.1.1 Effect of SA, RV, GA and COMB on HMG-CoA reductase
The Table 1 represents SA, RV, GA and COMB activity on lipid metabolizing enzyme HMG CoA reductase in heart homogenate. ISO administered animals showed a significant (p < 0.05) increase in the activity of enzyme in comparison to control animals. Pretreatment of SA, RV, GA and COMB to ISO animals revealed significant (p < 0.05) reduce in the activity of enzyme in comparison with ISO group. However, pretreatment with COMB in ISO administered animals ameliorated the enzyme activity to near normal. Animals pretreated with SA alone did not show significant difference when compared with control animals. Lower ratio of HMG CoA/Mevalonate denotes higher activity of HMG-CoA reductase and vice versa. Values are mean ± S.D. (n = six rats). Values not shared a common superscript (a, b, c and d) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
Groups
HMG CoA / Mevalonate ratio
Control
2.4 ± 0.2a
ISO (50 mg/kg)
0.6 ± 0.1b
SA (50 mg/kg)
2.5 ± 0.3a
GA (50 mg/kg) + ISO (50 mg/kg)
1.0 ± 0.2c
SA (50 mg/kg) + ISO (50 mg/kg)
1.6 ± 0.1d
RV (50 mg/kg) + ISO (50 mg/kg)
1.9 ± 0.1d
COMB (SA 50 mg/kg + RV 50 mg/kg) + ISO
2.3 ± 0.2a
3.1.2 Effect of SA, RV, GA and COMB on LPL
Table 2 shows the effect of SA, RV, GA and COMB on lipid metabolizing enzyme LPL in heart tissue. Rats injected with ISO demonstrated significant (p < 0.05) decrease of LPL activity in comparison with control rats. Administration of SA, RV, GA and COMB to ISO injected rats increased the LPL enzyme activity significantly (p < 0.05) when compared to ISO alone treated rats. COMB pretreatment to ISO treated rats markedly brought the LPL enzyme activity to near normal level. Pretreatment with SA showed no significant difference in comparison with control rats. Values are mean ± S.D. (n = six rats). Values not shared a common superscript (a, b, c and d) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
Groups
Lipoprotein lipase (LPL) U/mg protein
Control
38.3 ± 2.2a
ISO (50 mg/kg)
21.4 ± 3.1b
SA (50 mg/kg)
39.1 ± 1.8a
GA (50 mg/kg) + ISO (50 mg/kg)
24.8 ± 1.2c
SA (50 mg/kg) + ISO (50 mg/kg)
25.6 ± 1.5c
RV (50 mg/kg) + ISO (50 mg/kg)
29.5 ± 1.1d
COMB (SA 50 mg/kg + RV 50 mg/kg) + ISO
37.6 ± 1.4a
3.2 In silico activity of SA, RV and GA on HMG-CoA reductase and LPL lipid metabolizing enzymes by docking studies
The possible activity of SA, RV and GA on HMG-CoA reductase and LPL from Homo sapiens, a 3-dimensional structure was procured from protein databank (Fig. 1 A and B). Molecular dynamics of validation with Ramachandran plot was conducted by applying the program PROCHECK. The binding sites of HMG-CoA reductase and LPL were searched using CASTp server as well as comparing with template. The design and optimization of SA, RV and GA were carried out by the software Chemsketch and docking of compounds to the binding regions of enzymes HMG-CoA reductase and LPL was carried out by the GOLD (3.0.1) software. The particular docked conformations of SA, RV and GA with HMG-CoA reductase and LPL binding sites were selected.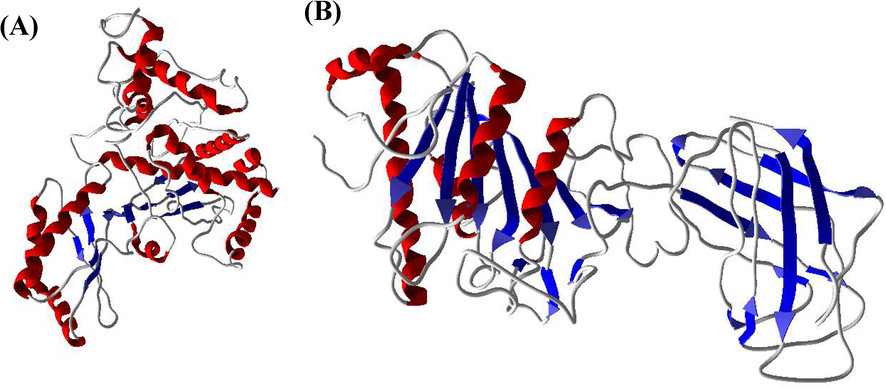
A and B. 3-Dimentional structures of lipid metabolizing enzymes HMG-CoA reductase and Lipoprotein lipase (LPL).
Figs. 2-4 revealed the molecular docking results of the compounds SA, RV and GA with HMG-CoA reductase enzyme. The molecular docking of SA with active of HMG-CoA reductase revealed one hydrogen bond between the LEU470 amino acid and H4 atom of SA (Fig. 2 A and B). In docking with RV with HMG-CoA reductase, three hydrogen bonds formed between amino acids LEU462, SER463 and ILE467 with atoms H14, H12 and H10 respectively (Fig. 3 A and B). The docking of GA with HMG-CoA reductase showed four hydrogen bonds between LYS460 with H18, LEU484 with H6, and CYS448 with H2 and H4 (Fig. 4 A and B) (Table 3).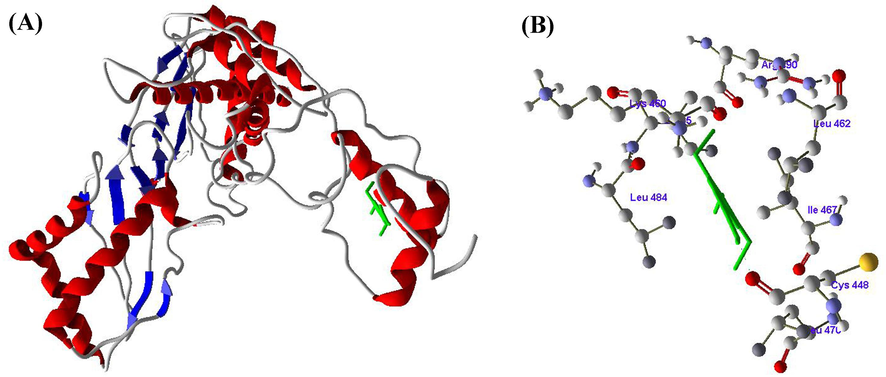
A and B. Molecular docking of Syringic acid (SA) with HMG-CoA reductase enzyme.
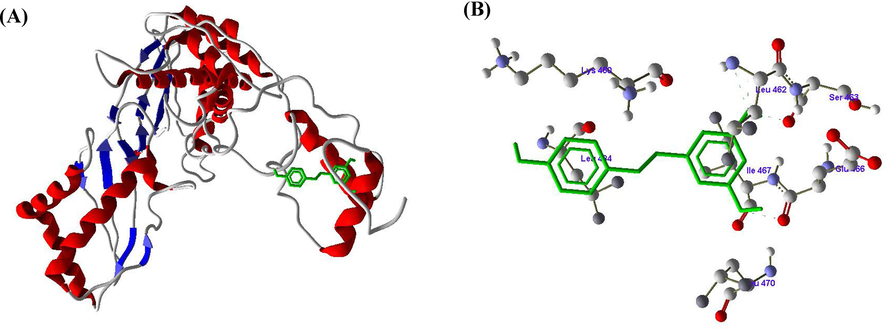
A and B. Molecular docking of Resveratrol (RV) with HMG-CoA reductase enzyme.
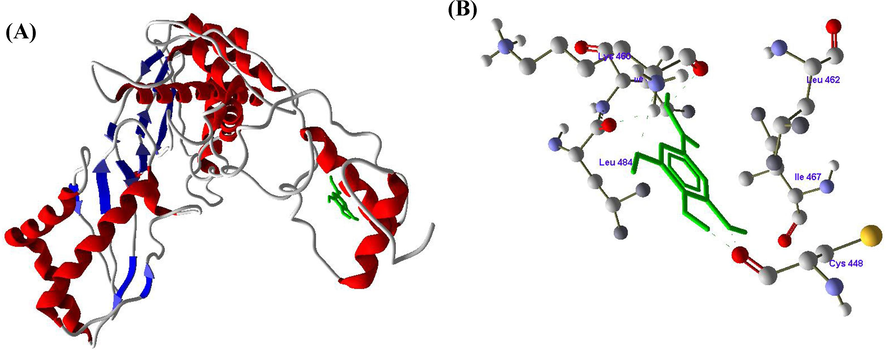
A and B. Molecular docking of Gallic acid (GA) with HMG-CoA reductase enzyme.
Enzyme
Compounds
No. of bonds
Atoms
Bond length (Å)
Docking score (KJ/mol)
Protein
molecule
HMG-CoA Reductase
Syringic acid
1
LEU470
H4
1.5
−55.27
Resveratrol
3
LEU462 SER463 ILE467
H14
H12
H101.6
1.8
1.4−39.27
Gallic acid
4
LYS460 LEU484 CYS448
H18
H6
H2
H41.7
1.5
1.4
1.5−48.23
LPL
Syringic acid
1
ILE37
H4
1.6
−41.25
Resveratrol
1
SER115
H8
1.4
–32.30
Gallic acid
5
ASP36 ILE37 LEU56
ASP134 ARG137H8
H10
H12
H14
H161.8
2.2
1.8
1.3
1.4−39.17
Figs. 5-7 represented the SA, RV and GA compounds molecular docking results with the enzyme LPL. From the molecular docking of SA into the active site of LPL, hydrogen bond noticed between the amino acid ILE37 and H4 atom (Fig. 5 A and B). RV also formed one hydrogen bond between the amino acid SER115 and H8 atom (Fig. 6 A and B), whereas GA formed five hydrogen bonds between ASP36 with H8, ILE37 with H10, LEU56 with H12, ASP134 with H14 and ARG137 with H16 amino acids and hydrogen atoms respectively (Fig. 7 A and B) (Table 3).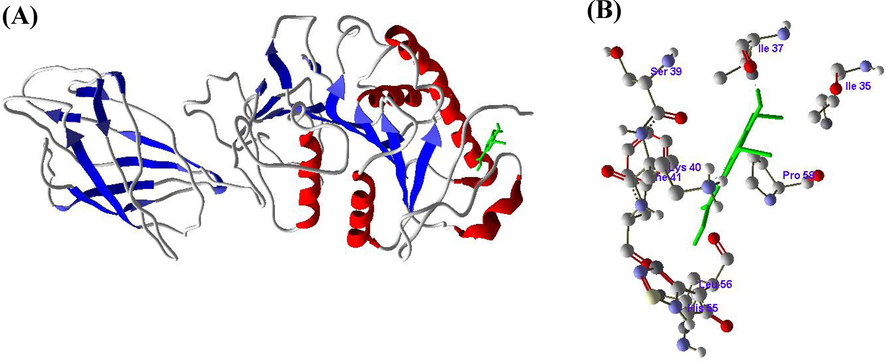
A and B. Molecular docking of Syringic acid (SA) with Lipoprotein lipase (LPL) enzyme.
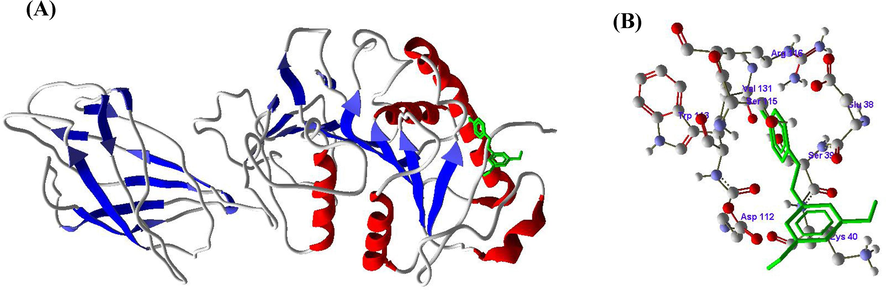
A and B. Molecular docking of Resveratrol (RV) with Lipoprotein lipase (LPL) enzyme.
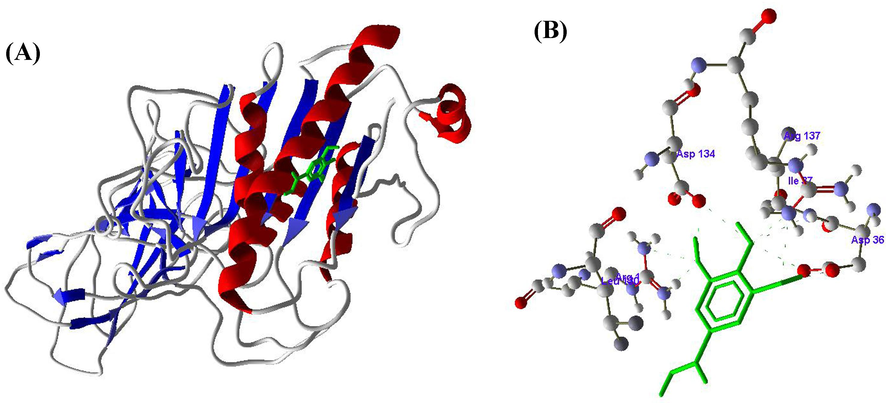
A and B. Molecular docking of Gallic acid (GA) with Lipoprotein lipase (LPL) enzyme.
Based on the above results, it is clearly revealed that HMG-CoA reductase inhibited and LPL enhanced due to binding of SA, RV and GA at active sites and forming the complexes with the active residues of HMG-CoA reductase and LPL.
4 Discussion
The present study, for the first time demonstrated the inhibitory activity of phenolic compounds SA, RV, GA and COMB on lipid metabolism enzymes HMG-CoA reductase and LPL in ISO administered cardiac necrotic rats.
ISO, an epinephrine induces oxidative stress in cardiomyocytes by stimulating adrenergic receptors that causes cell membrane destabilization and myocyte damage (Jagadeesh et al., 2016a). ISO also increases myocardial intracellular adenylyl cyclase levels, increases myocardial lipid deposition and increases MI (Dhawan et al., 1978). Administration of rats with ISO inhibited the lipid metabolizing enzymes, which was due to the oxidative damage caused by the free radicals generated by the autoxidation of ISO and the condition was reversed with the pretreatment of phenolic compounds SA, RV, GA and COMB.
CVD genesis and progression are mostly impacted by lipids and lipoproteins metabolism (Upadhyay, 2015b). The lipid metabolism responsible for elevated quantities of lipids and altered quantities of lipoproteins is connected with high risk of CVD (Jagadeesh et al., 2016b). The crucial mechanism to prevent the MI is homeostasis maintenance of cellular cholesterol. The elevated cholesterol in ISO treated animals is due to surged intake of LDL cholesterol by cardiac membrane from the blood stream. The maintenance of equilibrium of cholesterol is the main factor in the prevention of CVD. Excess cholesterol accumulation leads to hypercholesterolemia and atherosclerosis that protrudes to CVD. The raise in the levels of lipid biosynthesis play a crucial role for elevated levels of myocardial cholesterol in ISO administered MI (Nagoor Meeran et al., 2015). The increase in myocardial cholesterol induces phospholipid degradation and alters ion permeability, membrane bound enzymes stability, and membrane fluidity (Dhivya et al., 2017). The antilipidemic drugs targeting the positive effect on lipid profile are regulating through HMG-CoA reductase, which is the rate limiting enzyme of cholesterol metabolism. In our earlier report the beneficiary activities of SA, RV and COMB on lipid profile stimulated us to carry out this current research to investigate its effect on the activity of HMG-CoA reductase (Manjunatha et al., 2020). A significant increase in HMG-CoA reductase activity causes to increased synthesis of cholesterol resulting in foam cell formation, is the main step to initiate and accelerate atherosclerosis (Esterbauer et al., 1992). The available medications for treating hypercholesterolemia, statin inhibitors of HMG-CoA reductase are involved to maintain the cholesterol metabolism by downregulating the HMG-CoA reductase enzyme activity. Our results represented the pretreatment of SA, RV and COMB regulated the cholesterol biosynthesis by inhibiting HMG-CoA reductase in ISO treated experimental rats. Especially COMB showed the enhanced activity in the regulation of lipid metabolizing enzymes than individual compounds which may be due to the potent therapeutic antioxidant and anticholesterolemic activities of COMB. In our earlier report the COMB has been proved for its antioxidant activity (Sammeturi et al., 2019).
Triglycerides elevated levels involve in the activation of endothelial cells which produce 9-hydroxyoctadecadienoic acid by the enzyme LPL and promote atherosclerosis (Goldberg et al., 2011). Earlier it has been reported that elevated and reduced levels of LPL are considered as anti-atherogenic and atherogenic respectively (Reymer et al., 1995). In our study, ISO administration reduced the activity of LPL. Pretreatment of SA, RV, GA and COMB increased the activity of LPL in ISO treated rats. COMB enhanced the activity of LPL in ISO treated rats than SA and RV alone ISO treated rats, that clearly indicates the lipid metabolism regulation property of COMB. Our present reports are in accordance with earlier report which stated that Maslinic acid and GA protected lipid metabolizing enzymes by enhancing LPL enzyme in ISO administered cardiotoxicity (Shaik et al., 2020).
Thus, this investigation revealed the regulation of lipid metabolizing enzymes by analyzing in vivo and in silico molecular docking studies by phenolic compounds against Isoproterenol (ISO) in myocardial necrotic rats.
5 Conclusion
In conclusion, the phenolic compounds SA, RV, GA and COMB illustrated protective effect and regulation of lipid metabolism activities by inhibiting the HMG-CoA reductase and enhancing LPL enzymes. Furthermore, the molecular mechanism evidenced with molecular docking studies conformed that the compounds SA, RV, GA and COMB effectively regulated lipid metabolism enzymes and revealed the cardioprotection.
CRediT authorship contribution statement
Zhao Gao: Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Althaf Hussain Shaik: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Ming Lin: Software, Project administration, Methodology, Investigation, Formal analysis. Lei Jia: Software, Methodology, Investigation, Formal analysis, Data curation. Long Ma: Validation, Software, Methodology, Investigation, Data curation. Yanli Liu: Visualization, Software, Project administration, Methodology, Formal analysis. Jiuwei Shu: Validation, Resources, Methodology, Investigation, Formal analysis. Turki Mayudh Alrubie: Software, Methodology, Investigation, Formal analysis. Jayasimha Rayalu Daddam: Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. Jie Gao: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Acknowledgements
The authors express the gratitude for the support of funding by the Researchers Supporting Project (RSP2024R371) of King Saud University, Riyadh, Saudi Arabia. The author Dr. Jayasimha Rayalu Daddam thanks to Sahasra Gala, Texas, USA for her technical support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Structure of the lipoprotein lipase–GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc. Natl. Acad. Sci.. 2019;116:1723-1732.
- [CrossRef] [Google Scholar]
- Effect of isoprenaline on lipid profile & cardiac enzymes in rats. Indian J Exp Biol. 1978;16:376-378.
- [Google Scholar]
- Piperine modulates isoproterenol induced myocardial ischemia through antioxidant and anti-dyslipidemic effect in male Wistar rats. Biomed Pharmacother. 2017;87:705-713.
- [CrossRef] [Google Scholar]
- The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341-390.
- [CrossRef] [Google Scholar]
- Experimental model of myocardial infarction induced by isoproterenol in rats. Rev Bras Cir Cardiovasc. 2011;26:469-476.
- [CrossRef] [Google Scholar]
- Lipoprotein lipase- and phospholipase A2-catalyzed hydrolysis of phospholipid vesicles with an encapsulated fluorescent dye effects of apolipoproteins. Biochimica et Biophysica Acta (BBA)/Lipids and Lipid. Metabolism. 1984;795:191-195.
- [CrossRef] [Google Scholar]
- Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31:1716-1725.
- [CrossRef] [Google Scholar]
- Reduction of beta-adrenoceptor function by oxidative stress in the heart. Free Radic Biol Med. 1990;9:279-288.
- [CrossRef] [Google Scholar]
- The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr Cancer. 2020;72:386-397.
- [CrossRef] [Google Scholar]
- Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;1979(292):1160-1164.
- [CrossRef] [Google Scholar]
- Activation of β1-adrenoceptor triggers oxidative stress mediated myocardial membrane destabilization in isoproterenol induced myocardial infarcted rats: 7-hydroxycoumarin and its counter action. Eur J Pharmacol. 2016;777:70-77.
- [CrossRef] [Google Scholar]
- Protective effects of 7-hydroxycoumarin on dyslipidemia and cardiac hypertrophy in isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol. 2016;30:120-127.
- [CrossRef] [Google Scholar]
- Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;1
- [CrossRef] [Google Scholar]
- Development and validation of a genetic algorithm for flexible docking 1 1Edited by F. E. Cohen. J Mol Biol. 1997;267:727-748.
- [CrossRef] [Google Scholar]
- PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283-291.
- [CrossRef] [Google Scholar]
- Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene. 2022;808
- [CrossRef] [Google Scholar]
- Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3β signaling pathway. Biochem Biophys Res Commun. 2020;531:242-249.
- [CrossRef] [Google Scholar]
- Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275.
- [CrossRef] [Google Scholar]
- Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Phenolic compounds in extracts of hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules. 2020;25
- [CrossRef] [Google Scholar]
- Combined cardio-protective ability of syringic acid and resveratrol against isoproterenol induced cardio-toxicity in rats via attenuating NF-kB and TNF-α pathways. Sci Rep. 2020;10:3426.
- [CrossRef] [Google Scholar]
- Thymol attenuates altered lipid metabolism in β-adrenergic agonist induced myocardial infarcted rats by inhibiting tachycardia, altered electrocardiogram, apoptosis and cardiac hypertrophy. J Funct Foods. 2015;14:51-62.
- [CrossRef] [Google Scholar]
- Aqueous extract from leaf of Artocarpus altilis provides cardio-protection from isoproterenol induced myocardial damage in rats: Negative chronotropic and inotropic effects. J Ethnopharmacol. 2017;203:163-170.
- [CrossRef] [Google Scholar]
- Effects of syringic acid on apoptosis, inflammation, and AKT/mTOR signaling pathway in gastric cancer cells. Front Nutr. 2021;8
- [CrossRef] [Google Scholar]
- A lipoprotein lipase mutation (Asn291Ser) is associated with reduced HDL cholesterol levels in premature atherosclerosis. Nat Genet. 1995;10:28-34.
- [CrossRef] [Google Scholar]
- Syringic acid attenuates cardiomyopathy in streptozotocin-induced diabetic rats. Adv Pharmacol Pharm Sci. 2021;2021
- [CrossRef] [Google Scholar]
- Protective effects of syringic acid, resveratrol and their combination against isoprenaline administered cardiotoxicity in wistar rats. Saudi J Biol Sci. 2019;26:1429-1435.
- [CrossRef] [Google Scholar]
- Maslinic acid and gallic acid protective efficacy on lipids, lipoproteins and lipid metabolizing enzymes against isoproterenol administered cardiotoxicity: an in vivo and in silico molecular docking evidences. J King Saud Univ Sci. 2020;101230
- [CrossRef] [Google Scholar]
- CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res. 2018;46:W363-W367.
- [CrossRef] [Google Scholar]
- Phenolic compounds exerting lipid-regulatory, anti-inflammatory and epigenetic effects as complementary treatments in cardiovascular diseases. Biomolecules. 2020;10
- [CrossRef] [Google Scholar]
- Emerging risk biomarkers in cardiovascular diseases and disorders. J Lipids. 2015;2015:1-50.
- [CrossRef] [Google Scholar]
- Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem. 1975;21:1523-1525.
- [CrossRef] [Google Scholar]
- The cytotoxicity effect of resveratrol: Cell cycle arrest and induced apoptosis of breast cancer 4T1 cells. Toxins (basel). 2019;11
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103272.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







