Translate this page into:
Identification of antimicrobial compounds from the plant growth promoting bacteria (PGPR) tested against Fusarium wilt of tomato caused by Fusarium oxysporum f.sp. Lycopersici
⁎Corresponding authors. microcm1972@gmail.com (C. Mariappan), Sinouvassane@utar.edu.my (Sinouvassane Djearamane)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Infections and pests affecting plants pose significant threats to global food security. Over the top utilize of chemical pesticides is frequently utilized to diminish the impacts of plant illnesses caused by bacterial and parasitic pathogens. The greatest concern as we work towards more feasible horticulture is expanding agrarian efficiency for a developing populace. Microbial biocontrol operators (MBCAs) have illustrated their viability as a green procedure to oversee plant illnesses, invigorate edit development and execution, and increment yields. Other than their growth-promoting part, plant growth-promoting bacteria/fungi (PGPR/PGPF) can stifle plant illnesses by creating inhibitory chemicals and actuating a plant resistant reaction against plant pathogens. As biofertilizers and biopesticides, PGPR and PGPF are considered financially practical and appealing strategies for economical horticulture; This leads to a “win–win” circumstance. A few strains of PGPR and PGPF have been distinguished as compelling BCAs beneath controlled natural conditions. In common, any MBCA must overcome certain challenges some time recently it can be enrolled or broadly utilized for disease/pest control. Viable MBCA gives a down to earth arrangement to progress nursery edit execution with decreased fertilizer inputs and chemical pesticide utilization. This current audit points to fill the current crevice in information on plant growth-promoting microorganisms (PGPM), drawing consideration to the logical premise for arrangement, improvement proposals and extra investigate related to commercial utilize of PGPM.
Keywords
PGPR
Fusarium oxysporum
Biofertilizer
Tomato
Biopesticides
Biocontrol
Food security
Novelty of the work.
This research has the potential to lead to the development of new disease management strategies that are effective, environmentally friendly, and contribute to the long-term health and productivity of tomato crops. One novelty lies in the exploration of PGPR as a source of antimicrobial compounds for biocontrol of Fusarium wilt. Successful identification and validation of antimicrobial compounds from PGPR against Fusarium wilt of tomato may lead to the development of commercially viable biocontrol products for agricultural use. These products could benefit farmers by providing effective, affordable, and sustainable solutions for managing Fusarium wilt and other plant diseases. The novelty that lies in this work is the strain we isolated was found that it suppresses the pathogen growth above 50 percent which was evaluated in in vitro conditions.
1 Introduction
Tomatoes (Lycopersicon esculentum ) are the most developed ornamental vegetable created India is one of the greatest creators of the world with an advancement of approximately 1,87,35,900 metric tons (Verma et al., 2018). It features a put with the gather of Solanaceae (Petit-Houdenot and Fudal, 2017; Animashaun et al., 2017).
Tomato is an uncommonly nutritious natural item since of its colossal substance of vitamin A, C and regular cell fortifications masters, which are missing in several yields (Ellis et al., 2002; Maurya et al., 2019). It fills well in a by and large cool and dry environment; it is exceptionally much grasped to all climatic zones all over the planet. It has 95.3 % of water, 0.07 % of calcium and Niacin which have unimaginable centrality in metabolic works out of individuals (Petit-Houdenot and Fudal, 2017). Tomatoes are truly versatile but they grow well in warm conditions with perfect temperatures between 15 °C and 20 °C (Anitha and Rabeeth, 2009). Tomatoes grow well in a variety of medium and high-textured soils that are fully drained and have a pH of 5 to 7.5. Fusarium wilt disease was first described by G.E.Massee in England in 1895.It took center stage where no less than 32 countries discovered pollution, especially real in countries with hot climates. In India, it covers an area of 0.54 million hectares with a production growth of 7.60 million tons. (Mohammed et al., 2019). Moo abdicate of tomato is credited to its shortcoming to many pathogenic parasites, infinitesimal life forms, contaminations and nematodes which are noteworthy prerequisites of tomato advancement, for case, fusarium shrivel, dim shape, early revile, tomato leaf bend ailment, bacterial shrink and damping off. Fusarium shrink alone reason 30–40 % abdicate incident and in India, beneath the adversarial barometrical conditions, the hardships might reach as tall as 80 % (Bodah, 2017).
The Fusarium parasite is a known microbe of tomato plant which is available in extremely significant tomato developing locales of the world. Individuals from Fusarium species are pervasive soil-borne microbes of a wide scope of green and food crops which cause horrendous vascular withers, decays, and damping-off sicknesses. Fusarium shrivel alone reason 30–40 % yield misfortune and in India, under the antagonistic atmospheric conditions, the misfortunes might reach as high as 80 % (Hanson et al., 2016; Kalagatur et al., 2017). The side effect of brand contracture appears due to the actual weight of water, essentially due to vasodilation (Girhepuje and Shinde, 2011; Ramanathan and Black, 2010; McGovern, 2015). It produces 3 types of agamic spores. These are large-sized spores: fusiform, hyaline, mostly some septate and with an estimated size of 2.5–––3.3 x 3.5–5.5 µm; microspores - They are unicellular, transparent and ovoid to ellipsoidal and measure 2.5–4 x 6–15 µm; Chlamydospore- Formed into a more prepared mycelium. Fusarium oxysporum f.sp. lycopersici, a soil-borne plant pathogen of the Hyphomycetes family, causes fusarium, especially in tomatoes. (Maurya, 2019). Fusarium creates and gets by in all the sort of soil, however sandy soils are by and large great for advancement and progression. Defilement and ailment progression in fusarium wither is inclined toward by warm soil temperature and moo soil clamminess. Improvement of Fusarium is less in heavier mud soil.
The ailment impacts the tomato created at warm temperature 28 °C. Malady progression is slanted toward by warm temperatures 27 °C − 28 °C, dry climate and acidic soil pH 5–5.6 (Petit-Houdenot and Fudal, 2017). The efficacy of antimicrobial compounds against Fusarium oxysporum f. sp. lycopersici can indeed vary depending on several factors, including compound specificity, resistance development, bacterial interactions and environmental conditions. The development can be scattered by corrupted seeds or by exchanges filled in plagued soils. The earth can be brought into a field on contaminated equip, planning scales, bundling cartons or shoes (de Lamo et al., 2018). The most punctual side impacts appear up interior 48hrs after the area of the microbes. Within the sullied plants the clears out ended up yellow taken after by dropping of leaves which happens may be on one side of the plant or the two sides of shoot.
Parasite squares xylem vessels by assaulting the vascular tissues and reduces the advancement of water and causes extraordinary contracting. A longwise gritty colored streaks or vascular recoloring can be seen when the sullied cut stem is open (Petit-Houdenot and Fudal, 2017). Plant improvement progressing rhizobacteria offer an actually temperate way to bargain with increase trim creation and prosperity. They bestow productive results for plants by colonizing roots and quickening in common plant advancement by encourage creating seed germination, plant rise, root change, mineral food water utilization and biocontrol of plant organisms.
Isolation and screening of local sort of PGPR from tomato seedlings as plant development advancing and foes, which can be utilized as bioinoculants to extend the advancement and surrender of tomato. PGPR makes a difference in plant through diverse components. When striving to improve the accessibility of soil additives, it is crucial to synchronize defiant endeavors aimed at dissolving essential minerals like phosphorous, potassium, and zinc. Furthermore, the development of plant hormones such as indole-acetic acid, gibberellins, and ethylene must be taken into account. (Fadhil et al., 2018). Underhanded rebellious incorporate disease diminishes through anti-toxin and siderophore creation, challenge for supplements in space, and energy of secure frameworks (Verma et al., 2018).
Bacterial opponents were utilized as the biocontrol specialists. The auxiliary metabolites extricated from the bacterial opponents were subjected for the antifungal movement. The compounds which were extricated from the bacterial enemies repressed the mycelial development of the pathogen (Ramana et al., 2012). With this steady foundation data, the taking after goals have been defined to advance successful fluid based bioformulation for the administration of tomato early scourge and shrivel maladies.
2 Materials and methods
2.1 Isolation and identification of pathogen
The tomato plant, which exhibited typical symptoms of shrink disease, was acquired from the soil in the farmer's field. The pathogen was extracted from the brown discolored regions of the infected root fragments. These contaminated root fragments underwent a surface sterilization process using 0.1 % mercuric chloride for 30 min, followed by three rinses with sterile distilled water. Subsequently, they were placed in sterilized Petri dishes containing Potato Dextrose Agar (PDA) medium and incubated at a controlled laboratory temperature of 25 ± 2 °C for a duration of seven days. A pure culture of the pathogen was obtained utilizing the single hyphal tip technique. (Zinniel et al., 2002).
2.2 Isolation of bacterial antagonists
The endophytic bacterial strains were isolated by extracting a one cm section of solid tomato plant tissues from the root, shoot, and delicate stem of tomato plants. Subsequently, the extracted segments underwent surface sterilization for one minute using a 4 % sodium hypochlorite solution. The disinfectant was then removed by flushing the areas five times with sterile refined water. Following this, the segments were dried using sterile paper towels and crushed using a sterile pestle and mortar within a laminar flow chamber. The tissue extracts were diluted in 12.5 mM sodium phosphate buffer (pH 7.0) and plated on NA medium. The plates were incubated at 37 °C for two days. The rhizosphere was initially collected from the plant field (10 g) and suspended in 90 ml of sterile refined water. Serial dilutions were performed ranging from 10-2 to 10-6. Aliquots of 0.1 ml were then spread onto sterile Petri plates containing supplement agar medium. The Petri plates were incubated for 48 h at 30 °C. After two days of incubation, single colonies were streaked individually onto separate medium.
2.3 In vitro screening of bacterial antagonists
The in vitro screening of bacterial adversaries was conducted using the double culture method on PDA medium. In a single spot within the sterilized dish has been placed a 9-millimeter-diameter mycelial circle. A millimeter across the Petri dish sheet's boundaries, upon the other side of the mycelial sheet hosting the infectious agent, there was a streak of by its bacterial foes. Every course of therapy was tested in triplicate, with an untreated pathogenic sheet that did not include active antagonists. An incubation condition of 28 +/- 2 °C was maintained for the specimens. subsequently needs a week of incubated for evaluation of the bacteria's development in the direction of a bacterial community inside the inhibitory zones (Sasaki et al., 2015). In the same way to be is detailed below, we determined the level of inhibition (PI) relative to equation (1).
Where,
I – Inhibition per cent, C – Growth of pathogen in control, T – Growth of pathogen in treatment
2.4 Molecular characterization of bacterial antagonist
2.4.1 Isolation of genomic DNA
The CTAB method, recommended by (Weisburg et al., 1991), was employed to extract genomic DNA from the isolated bacteria that promote plant development. A culture of 25 ml volume, which had grown well, was subjected to centrifugation at 6,000 rpm for 5 min at 4 °C. Following the removal of the supernatant, in order to eliminate any extrinsic elements, the pellet was mixed with 1 ml of buffered TE and 0.5 ml of One-butanol. Afterwards, the entire mixture had been spun at 5,000 rpm for five minutes about 4 °C, while the fluid that was on top was removed. To make sure all traces of butanol were removed, the resulting pellet was reconstituted into 2 ml of buffered TE and centrifuged again. After that, the resultant pellet was mixed with 1 ml of TE buffer that included 100 µl of newly made lysozyme (10 mg per milliliter), and was allowed to stand at ambient temperature for five minutes. The entire mixture was then incubated at 37 °C for 1 h after 100 µl of 10 % SDS and 25 µl of 100 µg ml proteinase K were added and mixed well after the incubation time. Afterwards, 200 µl of 5 M NaCl was added after thorough mixing. After adding a 150 µl amount of CTAB solution and mixing it properly, it endured incubation at 65 °C for 10 min. After thoroughly mixing the two components, 1 ml of a phenol: chloroform extraction was added to the resultant. The resulting product was then centrifuged at 6,000 rpm for 15 min at 4 °C. Before incubation the mixture nightly at −20 °C, DNA from the mixture was expelled through the use of 0.6 vol of freezing isopropanol. The watery phase was gently transferred to a 2 ml microfuge container. Centrifugation was then used at 12,000 rpm for 15 min at 4 °C to pellet the DNA. Prior to being reconstituted in 50 µl of TE buffer, the resulting pellet was rinsed with 70 % alcohol, dehydrated over sweep for a period of ten minutes, and then. Furthermore, 1 µl of RNase-free solution (10 mg/ml) was swirled in and let stand around 37 °C to feed half an hour. Eventually, the genetic material was put away at −20 °C until it could be used again.
2.4.2 Molecular characterization of bacterial antagonist
To confirm the segregates, a specific forward primer (5′CGGGAGGCAGCAGTAGGGAAT3′) and reverse primer (5′CTCCCCAGGCGGAGTGCTTAAT3′) were used to amplify a 1.5 kb amplicon (Ramana et al., 2011). The amplification was carried out in a total reaction volume of 50 µl using the Eppendorf - Ace Cycler, Germany. The PCR conditions consisted of an initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 5 min. The PCR products were resolved on a 2 % agarose gel at 50 V, stained with ethidium bromide (0.5 µg/ml), and visualized using a gel documentation system. DNA sequencing was performed at Ticel Biopark Limited, Coimbatore, India. The rDNA homology searches were conducted using the Blast program via the internet.
2.4.3 Extraction of secondary metabolites produced by antagonistic bacteria
Bacterial disconnection was induced under controlled conditions at a temperature of 28 +/- 2 °C in Supplement broth for a duration of 5 days. Following hatching, the bacteria were separated by centrifugation at a speed of 5,000 rpm for 10 min, and the resulting supernatant was adjusted to a pH of 2.0 using concentrated hydrochloric acid. The bacteria were then allowed to hatch overnight. Subsequently, the mixture was extracted with an increased volume of ethyl acetate and incubated on a rotating shaker at a speed of 120 rpm at a temperature of 25 °C for a period of 3 h. The ethyl acetate layer was separated and subjected to evaporation using a vacuum jar evaporator at a temperature of 40 °C. After evaporation, the residues were re-suspended in one milliliter of methanol and subsequently tested (Senthilkumar et al., 2007).
2.4.4 Gas chromatography-mass spectrometry (GC/MS) analysis
Unstable components were detected using GC–MS analysis with an Elite-1 column (100 % Dimethyl polysiloxane) measuring 20 x 0.15 mm x 1 μm df. The gas chromatography Clarus 100 Perkin Elmer was used for preparation, and the turbo mass-gold-perkin-Elmer locator was employed. The carrier gas flow rate was set at one ml/min, with a split ratio of 10:1, and the injected volumes were 2 μl. Initially, the column temperature was held at 110 °C for 2 min, followed by gradual increments up to 200 °C at a rate of 5 °C/min, with a hold time of 9 min. The injector temperature was set at 250 °C and maintained for 36 min. The electron impact energy was set at 70 eV, the Julet line temperature was set at 200 °C, and the source temperature was also set at 200 °C. Electron impact (EI) mass scan (m/z) was recorded within the range of 45–450 aMU (Bhandari et al., 2009). The compounds present in the raw sample were identified by conducting computer searches on the NIST Ver.2.1 MS data library and comparing the spectrum obtained through GC/MS analysis.
2.5 Antifungal movement by agar plate strategy
The fully developed culture plate of Fusarium oxysporum on PDA was collected. Subsequently, the PDA was poured into the petri dishes and allowed to solidify. Once solidified, a nine mm corn borer mycelial plate of the pathogen was placed, and wells were created on three sides of the Petri plates. The potent extract from the effective isolate was poured into the wells at a rate of 50, 100, and 150 μl / mL per well. Each treatment was replicated three times. The growth and inhibition of mycelium were observed after 72 h of incubation (28 +/-2 °C). Sterile water without any potent extract was used as a control (Houterman et al., 2007).
2.6 Plant growth promotion assay
The impact of endophytic bacterial strains on plant growth was examined by utilizing the seedling energy list and the standard roll towel method (Abdul Baki and Anderson, 1973; Dennis and Webster, 1971). A total of twenty-five seeds were subjected to bacterial inoculation and placed on germination paper that had been pre-soaked. To ensure the seeds' stability, an additional strip of pre-soaked germination paper was delicately pressed on top. Subsequently, the seeds, along with the polythene sheet, were rolled and incubated in a growth chamber for a duration of 15 days. Two replications of this experiment were carried out. The root length and shoot length of each seedling were measured, and the germination rate was documented. The Vigor index was then calculated using the equation (2) as described by [2 9].
3 Results and discussion
3.1 Isolation and identification of pathogen
(Figs. 1–5) Represents the shrivel tainted tomato plants were collected from the field and pathogen was disconnected from tainted parcel utilizing PDA medium. After seven days of brooding, the pathogen created a white with pink colored soft mycelial development. The unadulterated culture of parasitic pathogen was kept up on PDA in inclines and after that protected in a fridge at 4 °C for advance characterization. The tiny perception uncovered that the disconnected pathogen created little and bullet molded microconidia and the chlamydospores which is created terminally and circular formed.
Healthy tomato plant.

Infected tomato plant.

Infected stem portion of the tomato plant.

Fusarium oxysorum f.sp.lycopersici.
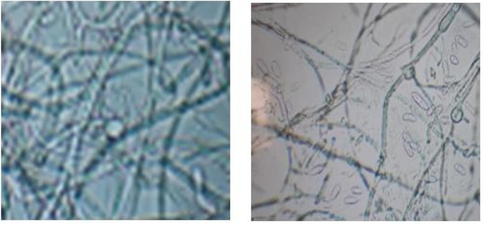
Microscopic observation of Fusarium oxysorumf.sp. lycopersici.
3.2 Isolation of bacterial antagonists
Fig. 6 shows plants growth promoting bacterial opponents were separated from the rhizosphere locale and root and delicate tissues of the solid tomato plant. Completely, 30 separates were separated out of which 8 segregates were separated from endophytes of solid tomato and 21 segregates were separated from rhizosphere soil of tomato. The tests were plated on NA medium and hatched at 28 ± 2 °C for 48 h. After hatching, little, circular, lifted, filamentous, unpredictable, harsh, smooth, dry, wrinkled and raised level colonies were watched. At that point, the colonies were sub-cultured, decontaminated, and put away at 4 °C on NA medium.
Plant Growth Promoting Rhizobacteria (PGPR).
3.3 In vitro screening of bacterial antagonists
Thirty bacterial confines were screened against F. oxysporum f. sp. lycopersici to test their adequacy in vitro. Among the confines, RB21 separated from rhizosphere soil recorded the least mycelial development of 4.26 cm compared to control which accounted 9.0 cm. This isolate recorded the minimum mycelial grow the by accounting 52.66 % reduction over control. Fig. 7 demonstrated that the isolated bacterial antagonist has the ability to suppress the grow of pathogen. The other bacterial antagonist was recorded the mycelial growth range from 4.83 cm to 8.50 cm. The least mycelial inhibition with growth of 8.10 cm was observed from isolate RB12 and accounted with low inhibition of 10.00 percent reduction over control.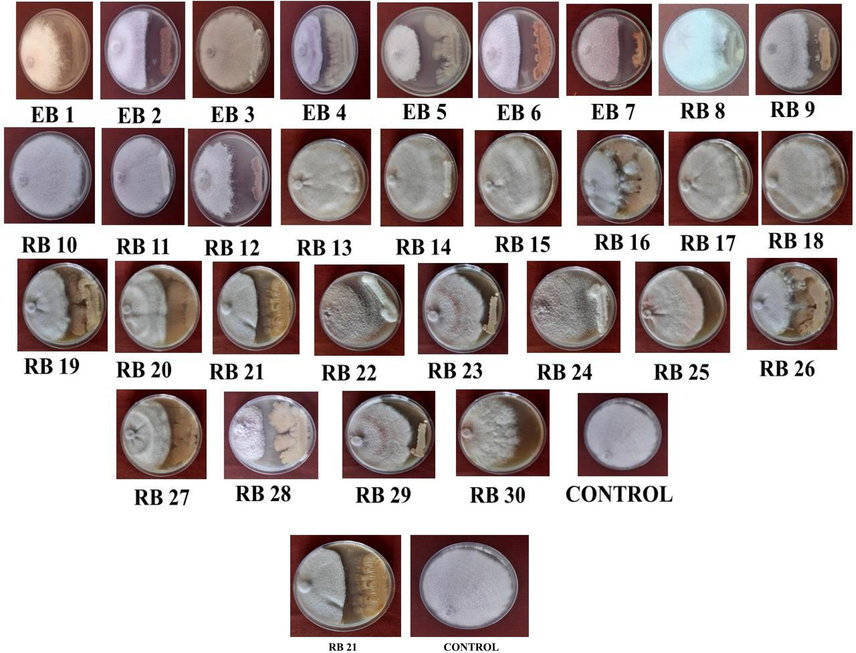
In vitro screening of bacterial antagonists.
3.4 Molecular characterization of bacterial antagonist
The DNA was separated from viable bacteria by CTAB strategy and subjected to PCR investigation utilizing widespread preliminaries comparing to 16S rDNA interceding arrangement. The successful bacterial adversary created amplicon measure of 1.5 kb, PCR item was settled on 1.2 per cent agarose at 50 V, recolored with ethidium bromide (0.5 µg/ml) and captured utilizing gel documentation framework. The PCR item was submitted for sequencing at Ticel Biopark Pvt. Ltd., Coimbatore. The gotten nucleotide groupings were altered to get full length sequenced and the arrangement homology look was performed utilizing NCBI Impact program. The nucleotide grouping our separated delivered 99.9 percent grouping closeness with Bacillus licheniformis accessible within the database. The nucleotide arrangement was submitted in NCBI database bearing the increase number ON626725.
3.5 Gas chromatography-mass spectrometry (GC/MS) analysis
The antimicrobial biomolecules display within the most viable enemy B. licheni form were dissected through the GC–MS investigation. The identified antimicrobial compounds from the isolate were 2-Piperidinone; 5-Aminovaleric acid; nonanoic acid; octyl ester; Propanamide;Uric acid;Tryptamine;Hydrazine;Uridine;Enalapril;LTryptophan;Ticlopidine;MethylUndecyl esters; N-Actyltyramine; Diethyl trisulphide; 1-Hexacosanol; Octadecanoic acid;Chanoclavine; Pheno3,5,-dimethoxy-;pentacanose13-phenyl;2-pyrrolidinone,1-methyl-;1pyrrolidinecarboxaldehyde;Benzeneethanol,4 hydroxy-;hydrazine,1-(3-hydroxybenzyl)-;Benzeneethanol,2-hydroxy-;Benzeneethanol,3 hydroxy-;AcetamideN (2-phenylethyl)-;Propanamide,3-phenyl-N-propyl-;1-Methoxy-2-indolinone;Hex-5-enamide, N-(2-phenylethyl); 3-Ethoxy-4-methoxyphenol; Formic acid, dodecyl ester; Glycyl-L-glutamic acid; 4-Methoxy-2,2,5-trimethylcyclopent-4-ene-1,3-dione;di-Alanyl-di-leucine;1H-Azepine,hexahydro-1- nitroso-;Thiophene,2-methoxy-5-methyl-; piperazine,2-methyl-1-nitroso; 1H- Indole, 2-methyl; 3Acetamidocoumarin;Glycyl-L-Proline;2H-Octahydropyrido[1,2-a]pyrazin-1-one; Cyclohexanone,2,6-diethyl;pentacanose,13-phenyl-;2Acetamidophenylpropionamide;2(O-Tolylazo)naphthalene; 1,7-Diphenyl-1,3,5-heptratriene.
3.6 Antifungal activity by agar plate
In vitro screening of bacterial enemy against the Fusarium oxysporum f.sp. lycopersici. B.licheniformis were successful in smothering the direct development of the mycelium of the pathogen when compared to the other confines. The unstable and non-volatile compounds extricated from the B.licheniformis disconnect hindered the mycelial development of the Fusarium oxysporum f.sp. lycopersici (Al‐Sadi et al., 2015). The mycelial development restraint was distinguished by the clear zone arrangement around the well. Zone formed around the well was measured. Size of zone formation around the wells were varied depending upon the concentration of extracted secondary metabolites added. Results were observed as 8 mm at 150 µl concentration, 5 mm at 100 µl concentration and 3 mm at 50 µl concentration (as can be seen Fig. 8).
Antifungal activity by agar plate.
3.7 Plant growth promotion
Tomato seeds were treated with plant growth promoting bacterial strain(B.licheniformis) to check the role of the strain by using roll towel method. After the incubation of 14 to 15 days, tomato seeds treated with effective bacterial strain was found to increase the vigor index when compared to untreated tomato seeds which serve as control. Fig. 9 shows that, the seed treatment with B. licheniformis has recorded the vigor index of 1270. The untreated control encompasses an exceptionally less power list of 1168.
Seed treatment of with Bacillus licheniformis has recorded the vigor index.
3.8 Discussion
3.8.1 Isolation of the pathogen, cultural characters of F. oxysporum f. Sp. Lycopersici isolates
In the present study, the pathogen was isolated from infected tomato plants collected from farmers field according to protocol described by (Rao and Rao, 1977). The pathogen was isolated using hyphal tip method to obtain pure culture of isolate on PDA. The isolate produced characteristic bullet shaped microconidia and round shaped chlamydospores. Several researchers noticed the same outcomes. The microconidia were straight or bent, single or bicelled, hyaline, pyriform, fusiform to ovoid, and one to two cells in number. The tiny, oval-shaped, hyaline-colored microconidia were either unicellular or had one or two septa. Chlamydospores are typically shaped singly or in sets, however they can occasionally be seen in clusters or short chains, according to (Katan and Katan, 1999). These are globular, thick-walled spores that are formed inside or at the tips of fungal hyphae that are thicker. In the soil, spores can live for a very long time. (Rekah et al., 2000; Delgado-Ramírez et al., 2021).
3.8.2 Isolation of bacterial antagonists
Plant development-promoting Rhizobacteria were isolated from the tomato plants' sound root tips in the exhibition case, and Endophytes were also isolated from the shoot, root, and fragile stem of the sturdy tomato plants. Similarly, a few labourers included bacterial adversaries. Bodah (2017) isolated the rhizobacteria out of the rhizosphere soil. Carmen (Bibi et al., 2019) confined the plant development advancing rhizobacteria from the rhizosphere locale of soil and endo rhizobacteria were disconnected from tomato soil and root tests developing in seven diverse locales of Solan and Sirmaur Locale of Himachal Pradesh, India (Bibi et al., 2019) disconnected the bacterial endophytes from the tissues of root of sound tomato plant.
3.8.3 In vitro screening of bacterial antagonists
The B. licheniformis disconnect recorded the inhibitory effect on the hyphal proliferation of F. oxysporum f.sp. lycopersici. This inhibitory effect can result from B. licheniformis producing non-volatile and unstable natural chemicals.
Additionally, (Mohsenian et al., 2012) detailed that the bacterial adversary Bacillus subtilis supress the development of parasitic mycelium. (Kamali et al., 2022) detailed that endophytic B. subtilis B28 disconnect appeared the most elevated restraint rates (51.16 %). This inhibitory impact may be due to the generation of unstable and non-volatile natural compounds by the B. licheniformis. Comparably, 30 recently separated B. inaquosorum strain KR2-7 impressively stifled Fusarium shrink of tomato plants. Fluorescent pseudomonads bacterial tomato shrivel pathogen R. solanacearum. PGPR able of restraining Alternaria solani. Table 1.
S. NO
ISOLATES
MYCELIAL GROWTH (cm)
PERCENTREDUCTION OVER
CONTROL
1.
EB 1
6.00
33.33
2.
EB 2
5.10
43.33
3.
EB 3
7.66
14.00
4.
EB 4
5.63
37.44
5.
EB 5
7.36
18.22
6.
EB 6
4.83
46.33
7.
EB 7
6.73
25.22
8.
RB 8
8.40
06.66
9.
RB 9
6.40
28.88
10.
RB 10
7.83
13.00
11.
RB 11
8.26
08.22
12.
RB 12
8.10
10.00
13.
RB 13
8.33
07.44
14.
RB 14
8.50
05.55
15.
RB 15
8.30
07.70
16.
RB 16
5.73
36.33
17.
RB 17
5.76
36.00
18.
RB 18
7.70
14.00
19.
RB 19
7.60
15.55
20.
RB 20
7.40
17.77
21.
RB 21
4.26
52.66
22.
RB 22
8.13
09.66
23.
RB 23
5.76
36.00
24.
RB 24
6.83
24.11
25.
RB 25
7.60
15.55
26.
RB 26
6.46
28.22
27.
RB 27
6.13
31.88
28.
RB 28
8.00
11.11
29.
RB 29
7.50
16.67
30.
RB 30
6.40
28.88
31.
Control
9.00
–
3.8.4 Biochemical characterization of bacterial antagonist
In the present study, the effective isolate was subjected to biochemical tests such as Gram’s staining, starch hydrolysis test, catalase and oxidase test, indole test, and citrate utilization test. All tests indicate positive results. Based on this, the identification of the isolate as a Bacillus species has been provisionally confirmed. Similarly, Cao et al. (2018) used these assays to potentially confirm the Bacillus isolate, and has also used similar tests to confirm the Bacillus isolate.
3.8.5 Molecular characterization of Bacillus sp
Within the display ponder, PCR intensification of 16S rDNA was intensified from the viable bacterial confine RB12.Based on results it was observed that our isolate was yielded 1500 bp amplicon size with primers and hence we confirmed the isolate as Bacillus licheniformis ON626725 by using blast software. Similarly, performed a PCR for the identification of the Pseudomonas and also identified the pseudomonas and Bacillus strains by using 16S rDNA sequencing conformed that the identified bacterial strain as Bacillus, Arthrobacter, Paeni bacillus by using the 16S rDNA Sequencing. identified the Bacillus sp., by 16srRNA sequencing.
3.8.6 Gas chromatography-mass spectrometry (GC/MS) analysis
The non-volatile natural compounds extricated from the separate B. licheniformis restrained the mycelial development of the pathogen and delivered greatest range of hindrance zone compared to the untreated control.The volatile organic compounds associated with B. licheniformis isolate responsible for the antifungal activity were Uric acid; Tryptamine; Hydrazine; Uridine; Enalapril; 5-Aminovaleric acid; nonanoic acid; octyl ester; Propanamide; Uric acid; Tryptamine; Hydrazine; Uridine; Enalapril; L-Tryptophan; Ticlopidine; Methyl Undecyl esters; N-Actyltyramine; Diethyl trisulphide; 1-Hexacosanol; Octadecanoic acid; Chanoclavine; Phenol 3,5,-dimethoxy-; pentacanose 13-phenyl;2-pyrrolidinone, 1-methyl-; 1- pyrrolidinecarboxaldehyde;Benzeneethanol,4-hydroxy-;hydrazine,1-(3 hydroxybenzyl)-; Benzeneethanol, 2-hydroxy-; Benzeneethanol, 3-hydroxy-;Acetamide N-(2-phenylethyl)-; Propanamide, 3-phenyl-N-propyl-; 1-Methoxy-2-indolinone; Hex-5-enamide, N-(2-phenylethyl)-; 3-Ethoxy-4-methoxyphenol; Formic acid, dodecyl ester; Glycyl-L-glutamic acid; 4-Methoxy2,2,5-trimethylcyclopent-4-ene-1,3-dione;di-Alanyl-di-leucine; 1H-Azepine, hexahydro-1- nitroso-; Thiophene,2-methoxy-5-methyl-; piperazine,2-methyl-1-nitroso; 1H- Indole, 2-methyl; 3-Acetamidocoumarin; Glycyl-L-Proline; 2H-Octahydropyrido[1,2-a]pyrazin-1-one; Cyclohexanone,2,6-diethyl-; pentacanose, 13-phenyl-; 2Acetamido-3-phenylpropionamide; 2-(O-Tolylazo)naphthalene; 1,7-Diphenyl-1,3,5-heptratriene. Comparably, compounds like 2-penten-1- ol, 2-methyl-; 2,2,4-Trimethyl-3-pentanol; Trans carvol; (-)-carvone; ethyl iso-allochate were identified through GC–MS analysis Benzene eicosyl, Benzyl isopropanol ester.
3.8.7 Antifungal activity by agar plate
In the present study, Bacillus licheniformis isolate was identified according to its morphological, cultural and 16srRNA gene sequence. Series of novel antifungal and antibacterial compounds has been isolated from the isolate of B. licheniformis such as Uric acid; Tryptamine; Hydrazine; Uridine; Enalapril; 5-Aminovaleric acid; nonanoic acid etc., may be responsible for pathogen inhibition which was visualized by the zone formation around the well and zone formation varies depending upon the concentration of the volatile and non-volatile compounds loaded into the wells. This Similarly, the antibacterial activity was attributed by the 3 chloropropionic acid 2 pentadecyl decyl ester (Ramana et al., 2012). However, nHexadecanoic acid was also demonstrated to possess antifungal activity. The antimicrobial compounds like 3,4- Furan diol, tetrahydro-, trans and 1,2-Benzenedicarboxylic corrosive, bis (2-methylpropyl) ester was moreover been detailed.
3.8.8 Plant growth promotion
In the demonstration, a B. licheniformis was exposed to the roll-towel method of plant development advancement in tomato seedlings (Knapp and Chandlee, 1996). In comparison to the untreated control, it was discovered to increase the tomato seedling's overall vigour list. Furthermore, distinguished those seeds treated with fluorescent pseudomonads enhanced the growth of roots and shoots of tomato plants compared to untreated seeds. Among the isolated 85 endophytic bacterial strains from roots, stems and shoots of cotton plants. EPBC 68 and EPBC 73 are said to prolong the life of cotton plants, mainly both under rolled towels and in potted culture.
4 Conclusion
Tomato plants which uncovered to the fusarium shrivel contamination were collected from the farmer’s field. Tomato plants exposed to Fusarium shrinkage are recognized by the bright yellow color of a caterpillar or bud, or by slightly shrinking and dangling lower flaps on a stem. Takes off or entire branches turn yellow. Parasitic pathogen was disconnected from the contaminated delicate tissues of the plant. Isolated pathogen was distinguished by the thick, creamish white with pink colored soft mycelial development by half plate culture procedure. Microconidia and chlamydospore arrangement by minuscule perception. 7 endophytic bacterial strains and 23 plant development advancing Rhizobacteria were confined from the root and delicate tissues and the rhizosphere locale soil of the sound tomato plants to control the fusarium shrivel in tomato plants. Distinctive colony morphologies such as little, circular, hoisted, filamentous, unpredictable, unpleasant, smooth, dry, wrinkled, raised, level colonies were watched. Isolated bacterial opponents were continued for the in vitro screening by double culture procedure against the Fusarium pathogen and the result was decided by the inhibitory zone around the mycelial development of the organism. Inhibitory zone was found to be 4.26 cm within the disconnect of RB 21 which could be a slightest mycelial development watched when compared to other segregates and control plate. The inhibitory rate was found to be 52.66 % decrease over control. Effective confine was taken and subjected for the biochemical tests such as Gram recoloring, Indole test, catalase and oxidase test, starch hydrolysis test, utilization of citrate test for the conditional distinguishing proof of a viable separate. Through this, biochemical considers, the compelling separate was probably affirmed as Bacillus species. With the 99.9 % closeness the compelling confine was distinguished as Bacillus licheniformis ON626725. Successful containment of B. licheniformis is due to the extraction of the unstable and non-volatile compounds it contains. This extract was subjected to GC–MS analysis for identifiable evidence of unstable and non-volatile compounds, it was then also tested for antifungal activity using an agar strategy and the results interpreted by the arrangement of regions. The compelling confine B. licheniformis was inspected for the Plant development advancement action in tomato seedlings by roll towel strategy in in vitro condition. Result was visualized by the increment within the root and shoot length of plant comparing to untreated seedlings of the tomato plant then, the viable disconnect was subjected to the atomic characterization by PCR method and PCR item was sequenced and submitted within the NCBI Impact program.
CRediT authorship contribution statement
S. Renga Sushma: Formal analysis, Data curation, Conceptualization. Amzad Basha Kolar: Validation, Resources, Formal analysis, Data curation. Shaik Azeem Taj: Visualization, Validation, Methodology, Formal analysis, Data curation. S.I. Beema Jainab: Methodology, Funding acquisition, Formal analysis. N.P.M. Mohamed Tariq: Formal analysis, Data curation. M.D. Saravanamoorthy: Writing – review & editing, Validation, Supervision. C. Mariappan: Writing – original draft, Supervision, Methodology. Abdulrahman I. Almansour: Writing – review & editing, Validation, Supervision, Software, Project administration. Sinouvassane Djearamane: Project administration, Methodology, Writing – review & editing. Ling Shing Wong: Project administration, Funding acquisition, Formal analysis. Saminathan Kayarohanam: Project administration, Investigation, Funding acquisition.
Acknowledgement
The project was funded by Researchers Supporting Project number (RSP2024R231), King Saud University, Riyadh, Saudi Arabia.
Ethical Statement
The author declares there is no ethical issues, because no experiment is conducted with human or other animals
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Vigour determination in soybean seed by multiple criteria. Crop Sci.. 1973;13:630-633.
- [Google Scholar]
- Population structure and development of resistance to hymexazol among Fusarium solani populations from date palm, citrus and cucumber. Journal of Phytopathology. 2015;163(11–12):947-955.
- [Google Scholar]
- Induced resistance to Fusarium wilt (Fusarium oxysporum) in tomato using plant growth activator Acibenzolar-S-methyl. Nigerian Journal of Biotechnology. 2017;32:83-90.
- [Google Scholar]
- Control of Fusarium Wilt of tomato by bioformulation of Streptomyces griseus in green house condition. African Journal of Basic & Applied Sciences. 2009;1(2):9-14.
- [Google Scholar]
- Tetrahydronaphthyl azole oxime ethers: the conformationally rigid analogues of oxiconazole as antibacterials. European Journal of Medicinal Chemistry. 2009;44(1):437-447.
- [Google Scholar]
- Study of population dynamics of Clavibacter michiganensis subsp. michiganensis in exposed and buried crop debris. Novel Research in Microbiology Journal. 2019;3(1):243-251.
- [Google Scholar]
- Root rot diseases in plants: a review of common causal agents and management strategies. Agric. Res. Technol. Open Access J. 2017;5:555661.
- [Google Scholar]
- The Fusarium oxysporum Avr2-Six5 effector pair alters plasmodesmatal exclusion selectivity to facilitate cell-to-cell movement of Avr2. Molecular plant. 2018;11(5):691-705.
- [Google Scholar]
- Xylem sap proteomics reveals distinct differences between R gene-and endophyte-mediated resistance against Fusarium wilt disease in tomato. Frontiers in microbiology. 2018;9:424561.
- [Google Scholar]
- Rhizobacteria associated with a native solanaceae promote plant growth and decrease the effects of fusarium oxysporum in tomato. Agronomy. 2021;11:579.
- [Google Scholar]
- Antagonistic properties of species groups of Trichoderma: Production of non-volatile antibiotics. Transactions of British Mycological Society. 1971;57:25-39.
- [Google Scholar]
- Fatty acid composition from an epiphytic strain of Fusarium oxysporum associated with algal crusts. Acta Microbiologica Polonica. 2002;51(4):391-394.
- [Google Scholar]
- Transesterification of non-edible oils over potassium acetate impregnated CaO solid base catalyst. Fuel. 2018;234(48):81-93.
- [Google Scholar]
- Transgenic tomato plants expressing a wheat endochitin gene demonstrate enhanced resistance to Fusarium oxysporum f. Sp. Lycopersici. Plant Cell, Tissue and Organ Culture. 2011;105:242-253.
- [Google Scholar]
- Conventional and molecular marker-assisted selection and pyramiding of genes for multiple disease resistance in tomato. Scientia Horticulturae. 2016;201:346-354.
- [Google Scholar]
- The mixed xylem sap proteome of Fusarium oxysporum‐infected tomato plants. Molecular plant pathology. 2007;8(2):215-221.
- [Google Scholar]
- Application of activated carbon derived from seed shells of Jatropha curcas for decontamination of zearalenone mycotoxin. Frontiers in pharmacology. 2017;8:308339.
- [Google Scholar]
- Perception of biocontrol potential of bacillus inaquosorum KR2-7 against tomato fusariumwilt through merging genome mining with chemical analysis. Biology. 2022;11:137.
- [Google Scholar]
- Vegetative compatibility grouping in Fusarium oxysporum f. sp. radicis – lycopersici from the UK, the Netherlands. Belgium and France. Plant Pathology. 1999;48:541-549.
- [Google Scholar]
- Rapid, small-scale dual isolation of RNA and DNA from a single sample of orchid tissue. Biotechniques. 1996;21:54-55.
- [Google Scholar]
- Management tactics for fusarium wilt of tomato caused by fusarium oxysporum f. sp. lycopersici (Sacc.): A review. International Journal of Research in Pharmacy and Pharmaceutical Sciences. 2019;4(5):01-07.
- [Google Scholar]
- Maurya, C (2019), Current seasonal variations in physicochemical and heavy metals parameters of sewage treatment plant effluent and suitability for irrigation.11,852-865.
- Management of tomato diseases caused by Fusarium oxysporum. Crop Protection. 2015;73:78-92.
- [Google Scholar]
- Biological control of Fusarium wilt in tomato by endophytic rhizobactria. Energy Procedia. 2019;157:171-179.
- [Google Scholar]
- Investigation of the ameliorating effects of eggplant, datura, orange nightshade, local Iranian tobacco, and field tomato as rootstocks on alkali stress in tomato plants. Photosynthetica. 2012;50(3):411-421.
- [Google Scholar]
- Complex interactions between fungal avirulence genes and their corresponding plant resistance genes and consequences for disease resistance management. Front. Plant. Sci. 2017;8:1072.
- [Google Scholar]
- Multiplex PCR‐based strategy to detect contamination with mycotoxigenic Fusarium species in rice and fingermillet collected from southern India. Journal of the Science of Food and Agriculture. 2011;91(9):1666-1673.
- [Google Scholar]
- A novel PCR–DNA probe for the detection of fumonisin-producing Fusarium species from major food crops grown in southern India. Mycology. 2012;3(3):167-174.
- [Google Scholar]
- Impact of Environmental Regulations on Innovation and Performance in the UK Industrial Sector. Management Decision. 2010;48:1493-1513.
- [Google Scholar]
- Gamma-ray induced meiotic chromosome stickiness in tomato. Theoretical and Applied Genetics. 1977;50(5):247-252.
- [Google Scholar]
- Disease development following infection of tomato and basil foliage by airborne conidia of the soilborne pathogens Fusarium oxysporum f. sp. radicis-lycopersici and F. oxysporum f. sp. basilici. Phytopathology. 2000;90(12):1322-1329.
- [Google Scholar]
- Genetic and pathogenic variability of Fusarium oxysporum f. sp. cepae isolated from onion and Welsh onion in Japan. Phytopathology. 2015;105(4):525-532.
- [Google Scholar]
- Role of antibiosis in suppression of charcoal rot disease by soybean endophyte Paenibacillus sp. HKA15. Current Microbiology. 2007;55:25-29.
- [Google Scholar]
- Plant growth promoting and antagonistic potential of indigenous PGPR from tomato seedlings grown in mid hill regions of Himachal Pradesh. Journal of Pharmacognosy and Phytochemistry. 2018;7(1):968-973.
- [Google Scholar]
- 16S ribosomal DNA amplification for phylogenetic study. J. Bacterial.. 1991;173:697-703.
- [Google Scholar]
- Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl. Environ. Microbiol.. 2002;68(5):2198-2208.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103227.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







