Translate this page into:
Zinc oxide nanoparticle: An effective antibacterial agent against pathogenic bacterial isolates
⁎Corresponding authors. imahmood@kku.edu.sa (Irfan Ahmad), Mo.saeed@uoh.edu.sa (Mohd Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Zinc oxide nanoparticle (ZnO NP) was investigated to find out the next generation nano antibiotics that can be developed from them to counter pathogenic microorganisms responsible for multi-drug resistance. It has been seen that they have unique physicochemical properties that can be utilized against toxicological and biological properties of microorganisms.
Methods
Application of well diffusion and the procedure of micro broth dilution utilizing Alamar blue were done to evaluate the inhibition zone, minimum bactericidal concentration (MBC), and the minimum inhibitory concentration (MIC). Also, the antibacterial effectiveness of ZnO NP that constitutes the genesis of biofilms was analyzed by crystal violet formation assay. Inhibition of bacteria was ascertained through the percentage of inhibition of bacterial colonies upon treating with ZnO NP.
Results
In this study, we scrutinized the efficacy of ZnO NP on Gram-negative as well as Gram positive infectious strains. Ultimate inferences demonstrated the consequences that ZnO NP possessed statistically significant antibacterial efficacy with the significant inhibitory zone (16–21 mm), minimum inhibitory concentration (15.625–125 μg/ml) and minimum bactericidal concentration (62.5–250 μg/ml). Quantitative estimation of biofilms formed by Streptococcus pyogenes, Salmonella and Klebsiella pneumoniae at MIC × 2 of ZnO NP showed 2.3, 2.83 and 2.72-fold decrease, respectively.
Conclusions
The results imply that ZnO NP can be utilized as substitute antibacterial agents especially against Gram positive bacterial isolates, which can help develop various antibiotic agents in the clinical setup.
Keywords
Zinc oxide nanoparticles
Antibacterial
Biofilm
Gram-positive bacteria
Gram-negative bacteria
1 Introduction
Zinc oxide nanoparticle (ZnO NP) is renowned for its antimicrobial properties and demonstrated the highest toxicity against a wide range of microorganisms (Hu et al., 2009). ZnO NP is found to be more efficient as antimicrobials than the powder form (Tayel et al., 2011). ZnO produces a noteworthy antimicrobial effect when the particle's size is shortened to the span of nanometer, enabling these particles to interact and infiltrate to the interior of the cell and eventually demonstrate a noticeable bactericidal effect (Seil and Webster, 2012). It is a proven fact from scanning electron microscope (SEM) as well as transmission electron microscope (TEM) pictures of the microbial cells that nanoparticles constituting of zinc oxide disrupt the membrane of microbial cell leading to intracellular accumulation and interaction with biomolecules in the cytoplasm causing cell apoptosis (Siddiqi et al., 2018).
It has been observed that 90% of bacterial species disappeared after exposure to ZnO NP (Siddiqi et al., 2018). Also, research have suggested that ZnO NP was responsible for 100% impediment of bacterial growth at a concentration of between 3 mM and 10 mM (Brayner et al., 2006). ZnO NP has demonstrated effectiveness versus different microorganisms including both bacteria and fungi such as Bacillus species, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Pseudomonas vulgaris, Klebsiella pneumoniae, as well as Aspergillus niger and Candida albicans (Siddiqi et al., 2018).
In a study by Nicolle, 2008, when six various nanoparticles were analyzed, it was seen that nanoparticles of ZnO NP having comparatively smaller particle size displayed a broader span of antibacterial outcomes on different types of Gram-positive bacteria, i.e., Staphylococcus epidermidis, Enterococcus faecalis, Staphylococcus aureus, Streptococcus pyogenes, Bacillus subtilis, including Gram-negative types such as E.coli (Jones et al., 2008). Also, it was seen that Gram-positive isolates were more vulnerable to ZnO when contrasted with different Gram-negative isolates (Tayel et al., 2011). In an investigation, Roselli et al., have found that zinc oxide may protect intestinal cells from enterotoxigenic Escherichia coli due to antibacterial activity and obstruct the adhesion and internalization of bacteria (Roselli et al., 2003).
ZnO NP has been suggested as an alternative to antibiotics as it is harmless to normal body cells even at the maximum amount of 100 μg/ml (Siddiqi et al., 2018). Growth-inhibiting dose containing 15 μg/ml of zinc oxide has demonstrated antibacterial action versus both Gram-positive and Gram-negative isolates, specifically E. coli, Salmonella typhimurium, and Staphylococcus aureus. Antibacterial activity at a dose as little as 5 μg/ml has been observed against Klebsiella pneumoniae (Brayner et al., 2006; Stoimenov et al., 2002). Due to the antibacterial property of zinc, it could be effectively used in the products like lotions, cream, toothpastes and ointments (Almoudi et al., 2018). Antibacterial properties of ZnO NP are for the most part due to the disruption of membrane or intracellular reactive oxygen species which are induced in bacterial cell (Jones et al., 2008).
In the current era of global human health threats due to the emergence of microbial antibiotic resistance, emergence of new bacterial strains, and lack of vaccines, there is a dire requirement for introducing novel antimicrobial agents that can control multi-drug resistant pathogenic microbes. Zinc oxide nanoparticles property of antimicrobial action has received tremendous attention in the whole world due to its remarkable antimicrobial properties. Therefore, it was of considerable importance to scrutinize the role of ZnO NP, emphasizing its antimicrobial toxicity against well-known human pathogens encompassing both Gram negative and Gram-positive pathogenic bacterial isolates.
2 Materials and methods
2.1 Nanoparticle suspension preparation
ZnO NP (constituting < 100 nm) were procured from the Sigma Chemical Co. Ltd. company (St. Louis, MO, USA) and preparation of the main stock was achieved by suspension of 500 μg in 1 ml of DMSO. Sonication of the stock was carried out (Sonics Vibra cell; Sonics & Material, Newtown, CT, USA) at 40 °C for a period 10 min.
2.2 Strains of bacteria and growth conditions
Gram-negative and Gram-positive bacterial isolates consist of Pseudomonas aeruginosa, Salmonella, Klebsiella pneumonia, Escherichia coli, Streptococcus pyogenes, Staphylococcus saprophyticus, Enterococcus faecalis, Staphylococcus aureus, respectively were utilized in this study. Muller Hinton (MH) broth was used for the promising growth of these pathogenic strains.
2.3 Evaluation of antibacterial vulnerability by the well diffusion method
For evaluation of the tested bacteria for testing vulnerability to the ZnO NP, growth of the bacterial isolates till the logarithmic phase, i.e., O.D.610 of 0.4–0.6, was done in MH broth. Further dilution of the tested strains were done in MH broth till a theoretic O.D.610 of 0.01. In order to find out the antibacterial effectiveness of the compound ZnO NP, the application of the agar well diffusion process was continued (Arora et al., 2021). 6 mm diameter wells in the nutrient agar were achieved by sterile syringe cap and lawn culture were ultimately done on the agar utilizing the sterile cotton swab of the diluted culture. Subsequently, DMSO and 20 μl of ZnO NP (500 μg/ml) were put in the wells of the petridish and were subjected to further aerobic incubation for 24 h at a temperature of 37 °C. Estimation of the zone of inhibition (ZOI) of the bacterial growth along with the well was considered in millimeters. The actual ZOI was confirmed by subtracting the average ZOI by ZnO NP from the average ZOI by the DMSO.
2.4 MIC & MBC ascertainment
The minimum inhibitory concentration (MIC) as well as minimum bactericidal concentration (MBC) of ZnO NP were ascertained. Amounts of the ZnO NP utilized for MBCs and MICs on the bacterial isolates were treated with 2-fold dilution of ZnO NP spanning from 500 μg/ml to 7.812 μg/ml. In order to find out the MIC, the bacterial isolates were developed to the logarithmic phase (0.4–0.6 at O.D.610) followed by subjected to more dilution in MH broth to a hypothetical level O.D.610 of 0.01. Subsequently, 180 μl culture containing all bacterial strains was put inside the polystyrene sterile flat-bottom wells of 96-well plates. The wells were further subjected with 20 μl of 2-fold dilution of ZnO NP. The wells loaded with 20 μl of DMSO was deliberated as control. Further incubation of the plates was done aerobically for almost 24 h at 37 °C (Ahmad et al., 2020). Then 20 μl of alamar blue dye (Thermo Fisher, USA) was put into each particular well after subjecting to an incubation period of 24 h, and after each hour, the development of pink color was checked. The lowest concentration of the NP in a particular well where the color was unchanged was considered as MIC.
For finding out the MBC of ZnO NP of the bacteria isolates, 10 μl of culture was taken out from the wells containing unchanged color of the Alamar blue, was again sub-cultured on nutrient agar and further aerobically incubated for 24 h at a temperature of 37 °C. Afterwards, the least concentration of the ZnO NP where the bacterial growth was not found, was taken as MBC for the examined strains.
2.5 Growth inhibition test
In order to find the bactericidal actions of ZnO NP, colony count method was done. An approximate 5 μl of ZnO NP (at MBC concentration), which was treated with suspension of bacteria for 1 day, were plated on nutrient agar utilizing the spread plate method and the negative control. After a period of 24 h of incubation, the plates were checked to find out the inhibition of growth of the bacterial cells through the process of colony counting. Determination of the percentage lost in viable bacterial cells was done by comparing with the control utilizing the given equation: where I% = percentage inhibition of bacterial growth; μC = mean value of O.D.610 in control, μT = mean value of O.D.610 in treatment.
2.6 Antibiofilm effect of ZnO NP
The performance of the biofilm formation containing the bacterial isolates was done as per a earlier study with slight alterations (Zhang et al., 2014). In brief, 180 μl of the fresh suspension of bacteria (O.D.610 = 1.0) was added to polystyrene 96-well microtiter plates and 20 μl of varying concentrations of ZnO NP (MIC × 1, MIC × 0.5, MIC × 2) was added in 96 wells plate and incubation of the suspension was undertaken for a period of 24 hrs at temperature of 30 °C without any shaking in order to form the biofilm. Bacterial cells, which remained untreated and were utilized in every investigations set, were considered as negative control. After 24 hrs of incubation, the addition of crystal violet was undertaken into these wells for the purpose of staining. Absorbance was measured at 488 nm and repetition of each particular experiment was done for 3 times.
2.7 Performance of time killing kinetic assay
To find out the efficacy of ZnO NP on the tested bacterial cells, 180 μl bacterial culture (O.D.610 of 0.01) was exposed with 20 μl of ZnO NP (MIC × 0.5, MIC × 1, MIC × 2). The culture wells containing 20 μl of DMSO was taken as the control. Afterwards, incubation of the plates were done aerobically at a temperature of 37 °C and measurement of optical density (OD) was taken at 610 nm continuously every 2 h in FLUOstar Omega plate reader (BMG Labtech, Allmendgrun, Ortenberg, Germany). Mean of OD was further plotted versus the time to find out the efficacy.
2.8 Statistical analysis
All investigations were done thrice and the final results were taken as the mean ± SD. Graphpad prism software – 6.0 (La Jolla, USA), was utilized to do the statistical procedures. Disparity between two groups were scrutinized by the two-tailed Student’s t test and the value of p < 0.05 was taken as statistically noteworthy.
3 Results
3.1 Antibacterial properties of ZnO NP
In order to find out the antibacterial efficacy of ZnO NP, clinically prepared isolates of Gram negative and Gram positive bacterial such as Pseudomonas aeruginosa, Salmonella, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, Staphylococcus saprophyticus and Streptococcus pyogenes, respectively were treated. Susceptibility studies were done which showed that ZnO NP has prominent antibacterial efficacy to Gram positive isolates than Gram negative (Fig. 1A). Greater than 8 mm zone dimensions was considered as noteworthy when concerning sensitivity of the various strains of bacteria to ZnO NP investigated. ZOI to Gram negative bacteria ranged from 16 to 18 mm in diameter while 18 to 21 mm in diameter was found against Gram positive bacteria. Distinctive amounts of ZnO NP was utilized to treat all the strains of bacteria to decide the MBC and MIC.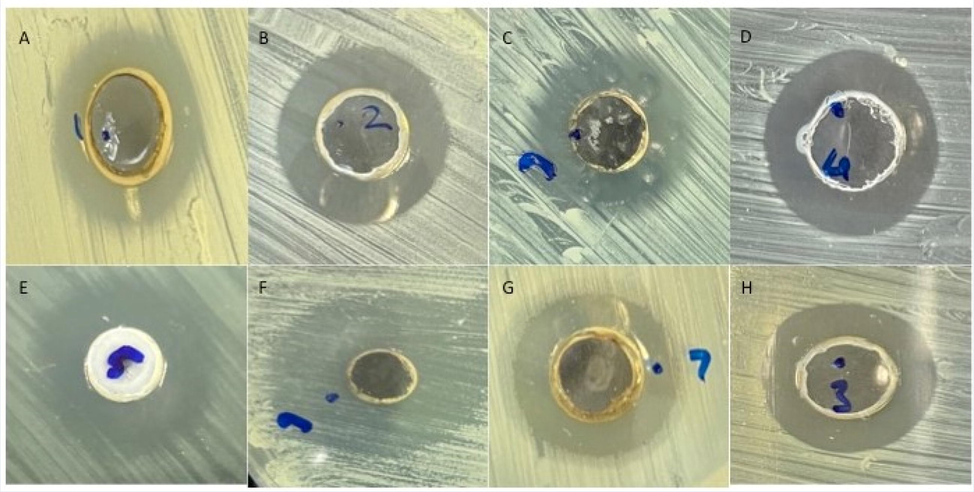
Effect of ZnO NP against Gram negative and Gram positive bacterial strains. Formation of zone of inhibition (A) Pseudomonas aeruginosa (B) Escherichia coli (C) Klebsiella pneumoniae (D) Salmonella (E) Staphylococcus aureus (F) Staphylococcus saprophyticus (G) Streptococcus pyogenes and (H) Enterococcus faecalis.
In order to investigate the MIC as well as MBC, the selected bacterial isolates were exposed with the aforementioned volume of ZnO NP, which was ensued by period of incubation of 24 h. The persistence of color of alamar blue was considered the least concentration of the ZnO NP and considered as MIC. It is apparent from Fig. 1 that entire bacterial isolates were notably susceptible to the compound ZnO NP. Inhibition of bacterial growth was achieved at MIC ranging from 15.625 to 125 μg/ml and MBC ranging from 62.5 to 250 μg/ml. The above results were achieved with subsequent higher inhibition zones ranging from 16 to 21 mm (Fig. 1B).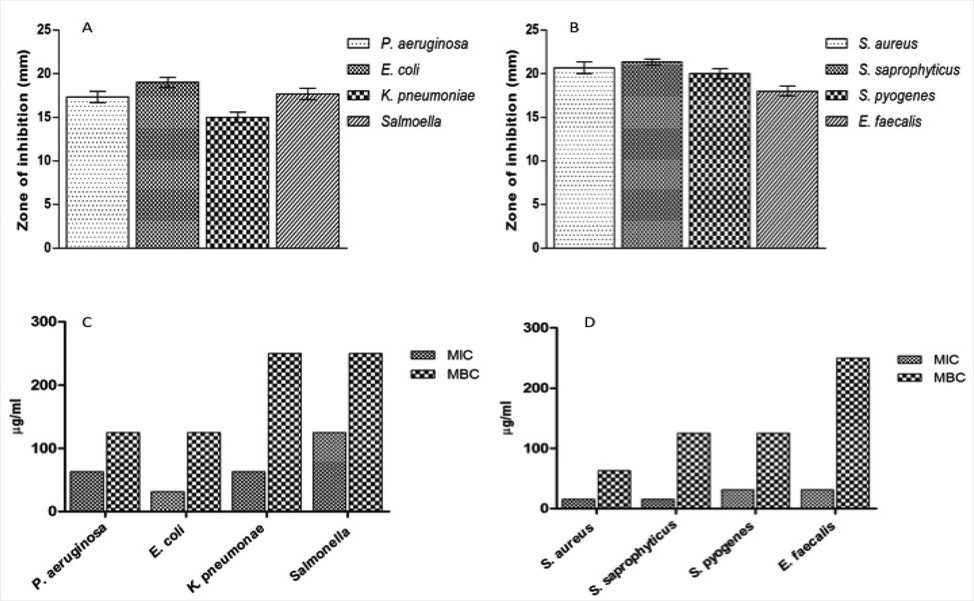
Effect of ZnO NP against Gram negative and Gram positive bacterial strains. MIC and MBC values of ZnO NP against Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Salmonella, Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus pyogenes and Enterococcus faecalis.
3.2 ZnO NP inhibits the viability of bacterial cells
Determination of the growth repressive properties of ZnO NP on the tested strains of bacteria were done by counting the bacterial colony after treating with NP. The consequences of colony count on tested bacterial strains showed depletion in colony numbers at the MBC concentration of ZnO NP (Fig. 2A & B). The colony count results depicted a notable (p < 0.0001) disparity in growth inhibition of all the tested strains of bacteria for the said concentrations of ZnO NP when compared to the negative control. Our study demonstrated the highest growth inhibition against S. saprophyticus (Fig. 2B) and the lowest growth inhibition against P. aeruginosa (Fig. 2A). The inhibition of bacterial growth was ranged from 97% to 82%. It can be concluded that the growth inhibitions demonstrated by ZnO NP showed that it possessed a strong antibacterial activity.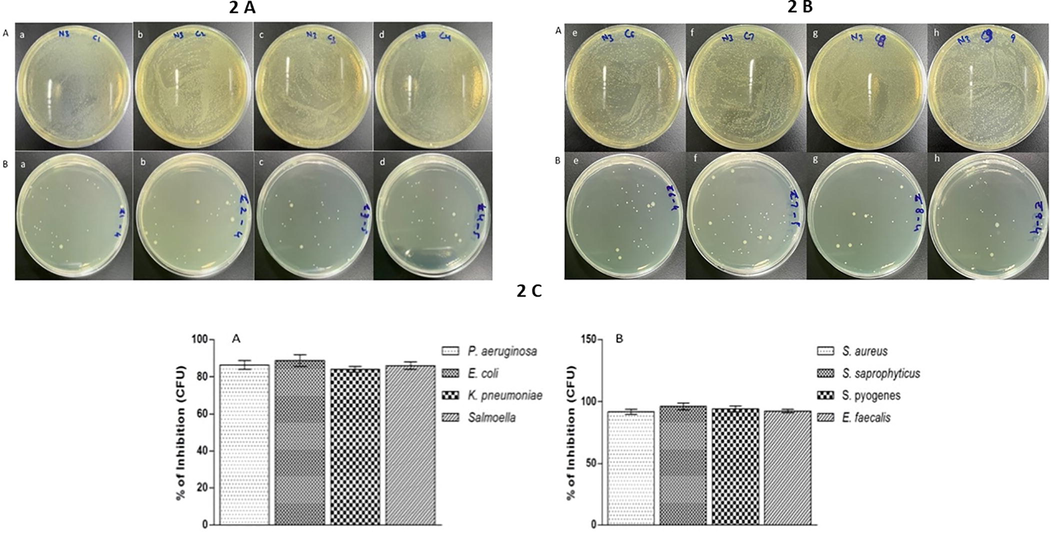
Percentage of growth inhibition formed by ZnO NP. The bacterial colony image (A) without any treatment (B) with ZnO NP at MBC concentration and (2C) Percentage of growth inhibition by ZnO NP at MBC concentration for 24 h at 37 °C. (a) Pseudomonas aeruginosa (b) Escherichia coli (c) Klebsiella pneumoniae (d) Salmonella (e) Staphylococcus aureus (f) Staphylococcus saprophyticus (g) Streptococcus pyogenes and (h) Enterococcus faecalis. Results are displayed from the three independent tests using means ± SD.
3.3 ZnO NP inhibits bacterial biofilm formation
We attempted to explore how ZnO NP could affect the biofilm formation of the tested Gram negative and Gram positive bacteria such as Pseudomonas aeruginosa, Salmonella, Klebsiella pneumoniae, Escherichia coli Staphylococcus aureus, Enterococcus faecalis, Staphylococcus saprophyticus and Streptococcus pyogenes, respectively. It was interesting to note that biofilm formation was affected entire tested strains of bacteria after subsequent treatment with different amounts of ZnO NP for a period of 24 h (Fig. 3A & B). The bacterial strains were incubated at 37 °C with MIC × 0.5, MIC × 1, MIC × 2 of ZnO NP for 24 h. The strains of bacteria which did not have any concentration of NP were taken as control. Moreover, the ZnO NP's inhibition of biofilm formation rate was established on the amount and the treatment time.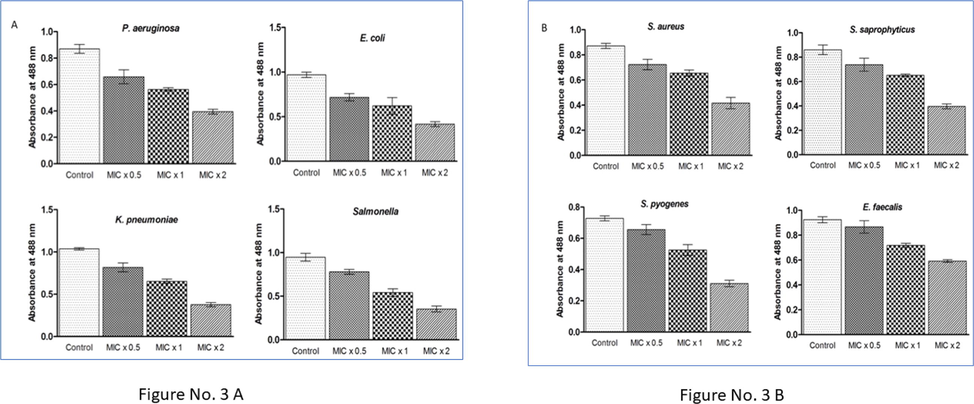
ZnO NP reduces the biofilm formation. (3A) Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Salmonella (3B) Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus pyogenes, Enterococcus faecalis were treated with different concentrations (MIC × 0.5, MIC × 1 and MIC × 2) of ZnO NP under biofilm developing circumstances for 24 hrs. Results are displayed from the three independent tests using means ± SD.
Inhibition of biofilm formation was found significant against all the tested bacteria but it was drastically reduced against S. pyogenes, Salmonella and K. pneumoniae in the wells treated with MIC × 2 of ZnO NP. Quantitative estimation of biofilms formed by S. pyogenes, Salmonella and K. pneumoniae at MIC × 2 of NP showed 2.3, 2.83 and 2.72-fold decrease, respectively, that of the biofilm formed in absence of NP (control) (Fig. 3A & B). These inferences indicated that ZnO NP inhibited the formation of the biofilm by all the tested bacteria.
3.4 Bacterial growth repression
Real time investigation was executed on the efficacy of ZnO NP on the growth of bacteria at different timings. In order to find out the time killing kinetic, treatment of 180 μl of bacterial culture (0.01 at OD610) was done with an amount of 20 μl of ZnO NP at MIC × 0.5, MIC × 1 and MIC × 2 concentration. Bacterial growth was monitored at the period intervals of 2 h (Fig. 4). It was seen that the growth of the bacterial isolates were depleted after treatment of ZnO NP at different concentration. The dose-dependent bacteria-killing effect of NP on the aforementioned bacteria was seen by the time-killing kinetics. Therefore, the consequences of our data noticeably demonstrate strong antibacterial activity of NP against the tested bacterial isolates.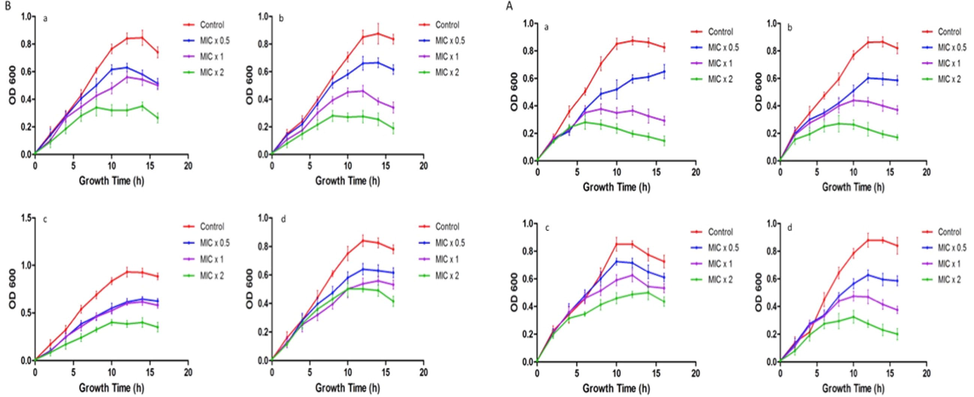
Effect of ZnO NP on bacterial growth kinetics. Representative bacterial strains of (4 A) a; Pseudomonas aeruginosa, b; Escherichia coli, c; Klebsiella pneumoniae, d; Salmonella (4B) a; Staphylococcus aureus, b; Staphylococcus saprophyticus, c; Streptococcus pyogenes, d; Enterococcus faecalis were treated with different concentrations (MIC × 0.5, MIC × 1 and MIC × 2) of ZnO NP. Growth cycle of untreated organisms served as growth control. Optical density at 610 nm was measured at regular time intervals of 2 h. Results are presented from three independent experiments using means ± SD.
4 Discussion
Nanoparticle research has seen a surge in attention in the last decade because of their outstanding catalytic and antibacterial properties (Trivedi et al., 2022; Oves et al., 2022). ZnO NP are widely employed in commercial applications. In this research, the antibacterial efficacy of ZnO NP was studied on the Gram-negative type and Gram-positive infectious bacterial isolates. The MBC and MIC consequences of ZnO NP established that Pseudomonas aeruginosa, Salmonella, Klebsiella pneumoniae, Staphylococcus saprophyticus, Staphylococcus aureus, Escherichia coli, Enterococcus faecalis and Streptococcus pyogenes growth was depleted with the elevated amount of nanoparticles MIC ranged from 15.625 to 125 μg/ml and MBC ranged from 62.5 to 250 μg/ml as shown in Fig. 1. We observed that Gram-positive (S. aureus, S. pyogenes, S. saprophyticus and E. faecalis) bacterial isolates are more vulnerable to ZnO NP when compared to Gram negative (K. pneumoniae, E. coli, Salmonella and P. aeruginosa) bacterial strains. Our findings are more in line with a study where it was found that as the amount of ZnO NP increases, the effect on Gram-positive bacterial strains becomes more prominent (Xie et al., 2011). Escherichia coli, Pseudomonas aeruginosa, Salmonella, Klebsiella pneumoniae, Staphylococcus saprophyticus, Staphylococcus aureus, Enterococcus faecalis and Streptococcus pyogenes strains were shown to be the most sensitive to ZnO NP based on the MIC and MBC results. This effect may be due to the Zinc ions well known effect at large concentration are known to have a deleterious impact on a variety of bacterial processes, including acid tolerance, transmembrane proton translocation and glycolysis, all of which can lengthen the bacterial lag phase (Applerot et al., 2009; Wahab et al., 2010; Jiang et al., 2009). Previous research have established that the liberation of H2O2 could be one of the plausible mechanisms for bactericidal action of ZnO NP (Yamamoto et al., 2004). Nevertheless, additional researches are needed to investigate the precise mechanism of the antibacterial efficacy of ZnO NP.
The emergence of drug resistance in S. saprophyticus and P. aeruginosa will lead to high morbidity and mortality. Therefore, it is necessary to develop alternative molecules against them. The turbidity of the bacterial culture was measured, and the bacterial colony were counted after treating all tested pathogens with ZnO NP (Fig. 2). The turbidity approach indicates that ZnO NP have a high toxicity and probable bacteriostatic impact on S. saprophyticus and lowest bacteriostatic effect against P. aeruginosa, based on the percentage of bacterial growth inhibition. Furthermore, Fig. 2 depicts that ZnO NP inhibits the growth of S. saprophyticus and results have validated that ZnO NP is better to inhibit bacterial cells. Results of MBC advocates that ZnO NP are much more efficacious against the S. saprophyticus and P. aeruginosa as per the results shown in Fig. 2.
The production of bacterial biofilms is a big problem in both the industrial and medicinal fields. Biofilm formation in implants such as catheters, alternatively food or dairy equipment pipes, industrial equipment or air ducts might trigger a cascade complication. Microbes use a variety of strategies to become resistant to antibiotics, including biofilm formation. Biofilm creation makes diseases more difficult to treat and is a primary contributor to disease-related infections, inadequate food safety and public health issues (Bjarnsholt, 2013; Ahmad et al., 2021). The hunt for novel anti-biofilm chemicals could lead to new ways for preventing infections and issues caused by biofilm formation. Many biogenic nanoparticles, such as gold and silver, have been shown to successfully check various stages of bacterial biofilms in the recent past (Rajput and Bankar, 2017; Namasivayam and Roy, 2013; Khan et al., 2022). We investigated the ability of the studied bacterial strains to generate biofilms as well as the efficay of ZnO NP on biofilm formation shown in Fig. 3. ZnO NP was discovered to efficiently suppress Escherichia coli, Pseudomonas aeruginosa, Salmonella, Klebsiella pneumoniae, Staphylococcus saprophyticus, Staphylococcus aureus, Enterococcus faecalis and Streptococcus pyogenes growth and biofilm formation. Overall, our findings imply that ZnO NP may reduce biofilm formation and development as it showed 2.3, 2.83 and 2.72-fold biofilm inhibition against S. pneumoniae Salmonella, and K. pneumonia respectively. As a result, such nanoparticles might be considered a potential active ingredient in various upcoming commercial formulations aimed at effectively preventing and controlling the ever-increasing threat of biofilms in the future.
The growth of strains Pseudomonas aeruginosa, Staphylococcus saprophyticus, Salmonella, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, and Streptococcus pyogenes were examined in the absence and presence of ZnO NP. Treated bacterial culture growth curves experience a depletion when compared to the untreated bacteria with time. The examination predicts that the ZnO NP have activity versus bacterial growth (Fig. 4). The growth curves in Fig. 4 indicates that ZnO NP has growth inhibiting property of bacterial strains concerning the concentrations.
5 Conclusions
The study results exhibited the dose dependent growth inhibition of ZnO NP on pathogenic bacterial isolates and diminished their pathogenicity. These findings support that ZnO NP might be the source of effective compound to be utilized for the treatment of the infections caused by these pathogenic microbes, which might draw these nanoparticles to the successive stage of drug development. Though, it is essential to assess the potential toxicity, pharmacokinetic properties, and their side effects. The study results suggest the potential of ZnO NP to be used as an antibacterial agent and might be recommended for biomedical and pharmaceutical applications.
Acknowledgements
The authors are grateful to Scientific Research Deanship at King Khalid University, Abha, Saudi Arabia for their financial support through the Large Research Group Project under grant number (RGP.02-87-43).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Inhibitory effect of Nepeta deflersiana on climax bacterial community isolated from the oral plaque of patients with periodontal disease. Molecules. 2021;26
- [Google Scholar]
- Evaluation of antibacterial properties of Matricaria aurea on clinical isolates of periodontitis patients with special reference to red complex bacteria. Saudi Pharm. J.. 2020;28:1203-1209.
- [Google Scholar]
- A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J.. 2018;30:283-291.
- [Google Scholar]
- Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Appl. Surf. Sci.. 2009;256:S3-S8.
- [Google Scholar]
- Synergistic effect of plant extracts on endodontic pathogens isolated from teeth with root canal treatment failure: an in vitro study. Antibiotics (Basel) 2021:10.
- [Google Scholar]
- Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett.. 2006;6:866-870.
- [Google Scholar]
- In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles. Sci. Total Environ.. 2009;407:3070-3072.
- [Google Scholar]
- Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut.. 2009;157:1619-1625.
- [Google Scholar]
- Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett.. 2008;279:71-76.
- [Google Scholar]
- Phytochemical screening, nutritional value, anti-diabetic, anti-cancer, and anti-bacterial assessment of aqueous extract from Abelmoschus esculentus Pods. Processes. 2022;10:183.
- [Google Scholar]
- Anti biofilm effect of medicinal plant extracts against clinical isolate of biofilm of Escherichia coli. Int. J. Pharm. Pharm. Sci.. 2013;5:486-549.
- [Google Scholar]
- Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci.. 2022;29:460-471.
- [Google Scholar]
- Bio-inspired gold nanoparticles synthesis and their anti-biofilm efficacy. J. Pharm. Invest.. 2017;47:521-530.
- [Google Scholar]
- Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr.. 2003;133:4077-4082.
- [Google Scholar]
- Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomed.. 2012;7:2767-2781.
- [Google Scholar]
- Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett.. 2018;13:141.
- [Google Scholar]
- Tayel, Ahmed A., Wael F. El‐Tras, Shaaban Moussa, Ashraf F. El‐Baz, Hoda Mahrous, Mohammed F. Salem, Leon Brimer, 2011. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens, J. Food Saf., 31: 211-18.
- Nanotechnological interventions of the microbiome as a next-generation antimicrobial therapy. Sci. Total Environ.. 2022;833:155085.
- [Google Scholar]
- Formation of ZnO micro-flowers prepared via solution process and their antibacterial activity. Nanoscale Res. Lett.. 2010;5:1675-1681.
- [Google Scholar]
- Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol.. 2011;77:2325-2331.
- [Google Scholar]
- Effect of lattice constant of zinc oxide on antibacterial characteristics. J. Mater. Sci. - Mater. Med.. 2004;15:847-851.
- [Google Scholar]
- TssB is essential for virulence and required for type VI secretion system in Ralstonia solanacearum. Microb. Pathog.. 2014;74:1-7.
- [Google Scholar]







