Translate this page into:
Antibiofilm efficacy of novel biogenic silver nanoparticles from Terminalia catappa against food-borne Listeria monocytogenes ATCC 15,313 and mechanisms investigation in-vivo and in-vitro
⁎Corresponding authors. mthlakshmi27@gmail.com (Lakshmanan Muthulakshmi), jesuaroa@srmist.edu.in (Jesu Arockiaraj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Listeria monocytogenes (LM) is a potential foodborne pathogen, known to form biofilms, which ultimately leads to serious problems in the food industry. This study demonstrates a comprehensive approach for the synthesis of silver nanoparticles using the phytochemicals present in the leaf aqueous extract of Terminalia catappa for the inhibition of L. monocytogens biofilms. The phytochemical analysis by UV–vis spectrum of the extract revealed the presence of polyphenolic compounds such as ellagic acid, gallic acid, chebulinic acid, and chebulgic acid. The shape, structure and size of the synthesized AgNPs were determined as 23–100 nm using the scanning electron microscopic images, and particle size distribution curve analysis. The in vitro studies using the AgNPs against L. monocytogenes revealed the inhibition of biofilm formation, reduction in the Virulence factors such as protease production at sub-inhibitory concentrations of AgNPs. In vivo experiments performed with the Caenorhabditis elegans model showed that AgNPs prolonged the lifespan of infected worms by about 90%. In addition, the non-hazardous nature and in vivo anti-adherence potential of AgNPs were also established with LM - C. elegans infection model.
Keywords
Terminalia catappa
Listeria monocytogenes
Caenorhabditis elegans
Silver nanoparticles
Antibiofilm
1 Introduction
World Health Organization (WHO) has placed Listeria monocytogenes on the top in the list of most serious food-borne pathogens due to its high level of pathogenicity. L. monocytogenes is an intracellular rod-shaped, Gram-positive foodborne pathogen that can survive in varying temperatures and environments (Lecuit 2020, Dos Santos et al., 2021). It is a ubiquitous pathogen with the ability to shift from commensal to pathogenic virulence stage causing a high level of mortality or morbidity. The high rate of fatality is due to its ability to survive in the cytoplasm, spread to other cells using cytoskeleton, cross the blood–brain barrier, intestinal and placental lining (Pizarro-Cerda and Cossart 2018, Lecuit 2020).
To withstand the heat and other stress forces in the food processing atmospheres, L. monocytogens can form develop biofilms which in turn can lead to the contamination of food, a severe public health hazard to consumers, and serious economic consequences for the companies. As per the regulatory guidelines, L. monocytogenes count should be less than 100 colony-forming units for every gram of food. Even though these criteria are met during the food processing and packing, a lack of temperature control at the supply chain, distribution, or consumer levels will lead to an increase in the L. monocytogenes population. Further, the ability of the L. monocytogenes to survive and multiply at 4 °C in the presence of food-grade preservatives causes a serious problem in the food industry (Colagiorgi et al., 2017). As L. monocytogenes is of serious concern in human health, there is great commercial as well as scientific interest in discovering novel biocompatible, edible preservatives or materials that could extend the shelf life of the various food products (Mazaheri et al., 2021).
Several biological and non-biological classes of compounds such as antibiotics, antimicrobial enzymes, cationic compounds, antimicrobial peptides, natural products, metals, and metal oxides are been explored for controlling bacterial biofilms (Jiao et al., 2019). Among them, metal-based nanoparticles are gaining much interest among researchers due to their ease in synthesis, good shelf life, show broad range of activities. Nanoparticles are proposed as next-generation novel promising antimicrobial agents because of their ability to inhibit and eradicate the biofilms formation, kill the planktonic bacteria (Cao et al., 2020, Mohanta et al., 2020, Subhaswaraj et al., 2020).
Terminalia catappa is widely grown all around the world for its fruits and the leaves are used in herbal medicines for treating various ailements such as liver, intestine infections, and related diseases. T. catappa leaves are large in size and contain large amounts of flavonoids, tannins (terflavin A and B, tergallagin, chebulagic acid, graniin, punicalin, punicalagin, and tercatain), saponines, and phytosterols. Recent scientific literature showed that the T. catappa derived flavonoids and tannins have bacterial antiquorum sensing activity (Taganna et al., 2011, Anand et al., 2015, Dwevedi et al., 2016) and could play a role in preventing biofilm formation. As T. catappa has good medicinal properties as well as the presence of more flavonoids which play vital role in the NPs synthesis we selected this plant.
Due to the absorption of toxic substances onto nanoparticles, during synthesis or purification of Nanoparticles synthesized either by physical or chemical methods do not have any utility in the food industry (Abbasi et al., 2016, Ali et al., 2016, Olga et al., 2022). Green synthesis is best for the synthesis of nanoparticles as it decreases and eliminates the need for harmful chemicals synthesis, availability of biodegradable bioactive metabolites and simple filtration or centrifugation will give the nanoparticles of the required quality and purity in a simple, eco-friendly, and economic feasible way for use in the food industry (Mousavi et al., 2018).
A nanoparticle that can destroy the biofilm and then kill the released planktonic bacteria also will be of great commercial value. Our literature survey about various metal properties concerning their antimicrobial activity, biocompatibility and toxicity nature led us to narrow down to silver nanoparticle as it is renowned for its broad-spectrum antimicrobial properties against a wide range of pathogens and is approved by the FDA as a topical antimicrobial agent (Naganthran et al., 2022). Silver nanoparticles (AgNPs) have been shown to eliminate, inhibit human and animal pathogens belonging to bacteria, fungi, viruses, and parasites groups (Naganthran et al., 2022). The anionic form of silver has been used for ages to cure several bacterial diseases caused by pathogens such as Staphylococcus aureus, Klebsiella sp. and Pseudomonas sp (Huang et al., 2021). Sliver, being a lewis acid, interacts with the phosphorous, sulfur sulfhydryl, amino, imidazole, electron-donating groups, or carbonyl groups present on the biomolecules such as proteins, DNA, etc (Tang and Zheng 2018). Based upon this, we are speculating that the AgNPs might interact with the extracellular DNA or protein, which are a core structural component of the biofilm structure. Strangely, our literature survey showed that AgNPs have not been investigated for the antibiofilm activities against L. monocytogenes. Given this, to decided to investigate whether the AgNP synthesis in a biogenic way could inhibit the biofilm formation in the foodborne pathogens especially, L. monocytogenes.
2 Materials and methods
2.1 Material
T. catappa plant leaves were collected from the Kalasalingam University campus, Krishnankoil, Tamil Nadu, India (latitude & longitude coordinates: 9.5747° N, 77.6798° E). The test organism L. monocytogens ATCC 15,313 was procured from the American Type Culture Collection (ATCC) and maintained in Tryptone soy Broth (TSB) (HiMedia, India) media at − 80 °C. For every experiment, 10 µl of the bacterial stock culture was inoculated into TSB and incubated at 37 °C for 24 h to prepare the seed culture. For the biofilm assays, Brain Heart Infusion (BHI) Broth was used for the stimulation of biofilm formation. Caenorhabditis elegans was obtained from the Caenorhabditis Genetics Center (CGC), University of Minnesota, USA, and maintained in NGM (Nematode growth medium).
2.2 Preparation of leaf extract and phytochemical analysis
Fresh and healthy leaves of T. cattappa were collected and washed with tap water for 1 h to remove the dust and other particles adhering to the surfaces of the leaves. Then with deionized water before cutting them into small pieces and pulverization into powder for the preparation of the aqueous extract after cutting. About 300 g of T. catappa leaf powder was extracted with distilled water for 72 h using a Soxhlet apparatus, at 80 °C. The extract was collected and filtered through Whatman filter paper (No. 1) and the filtrate was centrifuged at 3500 rpm for 10 min. The presence of bioactive components was confirmed by phytochemical analysis. The amount of crude extract yield was calculated using the following formula:
2.3 Biosynthesis of AgNPs
T. catappa aq. extract was mixed with AgNO3 (5 mM) in a 1: 4 ratio in an Erlenmeyer flask which was incubated with continuous shaking for 24 h at room temperature. The obtained AgNPs were collected and washed thrice with distilled water by centrifugation at 10,000 RPM for 15 min before confirming their identity by characterization studies.
2.4 Characterization of AgNPs
UV–visible spectra measurements were performed at the wavelength range of 200 nm – 600 nm at room temperature with a UV–Vis spectrophotometer (UV-mini, Shimadzu, Japan). X-ray diffraction (XRD) was used to determine the nature and average size of the synthesized particles was performed at 2 ⊖ in the range between 20° − 80° using a Bruker D8 X-Ray-Diffractometer. The morphology and size of AgNPs were analyzed by SEM (ZEISS 018-Japan). Samples were mounted on the SEM stage by attaching them with silver tape, and the silver sputter loaded under the pressure of 1.3⋅10-3 mm bar.
2.5 Antibiofilm assays
2.5.1 Growth inhibition effects of AgNPs
To analyze the effect of quercetin-based AgNPs against mid-log phase culture of L. monocytogenes was incubated in the presence and absence of AgNPs from 5 to 500 µg scale levels. Rifampicin was used as a positive control. Briefly, L. monocytogenes was inoculated into 10 ml of TSBS medium and then incubated for 12–16 h at 37 °C to prepare the seed inoculum. After incubation, 1% (1 X 106 CFU/mL)of the inoculum was incubated with and without quercetin-based AgNPs in TSBS medium and incubated at 37 °C. The observed absorption spectral changes at 600 nm were monitored at every 1 h and viable count (as CFU/mL) at every 24 h respectively as reported elsewhere.
2.5.2 Biofilm biomass analysis
The biofilm inhibitory effect of AgNPs was evaluated by microtiter plate assay methods as reported elsewhere (Mohanta et al., 2020, Xu et al., 2021). Briefly, L. monocytogenes was resuspended in a 96-well plate and incubated at the RT temperature for 18 h in the presence and absence of AgNPs (3 and 6 μg/mL). After incubation, the planktonic cells were removed and the wells rinsed gently with distilled water. The surface-adhered biofilm cells were stained with 0.4 % crystal violet for 10 min. Excess stain was removed by washing the wells with distilled water and allowing them to dry. To quantify adherent cells, 1 ml of 20 % glacial acetic acid was added to solubilize the bound crystal violet, and the absorbance was measured at 570 nm in a UV/visible spectrophotometer.
2.5.3 Light microscopic and biofilm biomass analyses
For the biofilm analysis, the bacterial cells were allowed to attach on glass slides (1 cm × 1 cm) in triplicates with and without AgNPs for 24 h at 37 °C (Reis-Teixeira et al., 2017, Xu et al., 2021). After the incubation, the slides were washed to remove the planktonic cells, and stained with 0.4% crystal violet. Excess dye was removed by washing and the slides were air-dried. Finally, the glass slides were examined under the magnification of 400 X in a light microscope (Nikon Eclipse 80i, USA). For confocal laser scanning microscopy analysis, the glass slides were stained with 0.1% acridine orange for 2 min in the dark, later they were washed with sterile distilled water to remove the excess dye. After air drying, the stained biofilm on the glass slides was examined immediately under CLSM with the COMSTAT software package.
2.5.4 Protease quantification assay
The whole proteolytic ability of foodborne pathogen was analyzed using azocasein (Sigma, USA) as a substrate. Briefly, 75 μl of AgNPs-treated (3 and 6 μg ml−1) and untreated cell-free culture supernatant were added to 125 μl of 0.3% azocasein. The mixtures were incubated at 37 °C for 30 min and the reactions stopped by adding 600 μl of 10% trichloroacetic acid. Then, 700 μl of 1 M NaOH was added to the mixtures, and absorbance was measured at 440 nm.
2.5.5 Quantification of EPS
EPS quantification was done using a well-reported protocol (Masuko et al., 2005). Briefly, 24-well plates containing 1 ml of LB medium along with L. monocytogenes were incubated at the optimum temperature for 18 h in the presence and absence of AgNPs. After incubation, the wells were washed with 0.9% NaCl and equal volumes of 5% phenol were added. Five volumes of concentrated H2SO4 were added to the mixture and the mixture was incubated for 1 h in dark. After incubation, the mixtures were centrifuged (at 16,770 g for 10 min) and the supernatant was transferred to fresh before the absorbance was measured at 490 nm.
2.6 In vivo toxicity assay
2.6.1 Toxicity and survival assay
A bioassay on C. elegans survival was performed to determine the toxicity and impact of AgNPs against the biofilm of L. monocytogenes under in vivo conditions. The Bristol N2 C. elegans, obtained from Caenorhabditis Genetics Center (CGC), Minnesota, USA was used to study the toxicity AgNPs. To evaluate the toxicity, age-synchronized L4- worms were transferred to an M9 medium containing 6.25, 12.5, and 25 µg/ml of AgNPs along with E. coli OP50. The assay plate was incubated at 20 °C and observed for survival for 7 days. To assess the impact of AgNPs on LM biofilm in vivo, the survival rates of worms during L. monocytogenes infection in the presence and absence of AgNPs were compared. For the killing assay, approximately 10 worms were transferred to the medium containing AgNPs at its sub-MICs along with an inoculum of ∼ 3.9 X 109 cells/ml of L. monocytogenes. Finally, survival of the worms was recorded every 2 h, and worms that did not respond to a touch stimulus with a platinum loop were scored as dead 23.
2.6.2 CFU Assay
To quantify the bacterial burden inside the worms exposed to LM, a CFU assay was performed as described earlier 24. Briefly, worms infected with LM in the presence and absence of AgNPs at 6.25, 12.5, and 25 µg/mL were washed thoroughly with M9 buffer containing 1 mM sodium azide to prevent intestinal expulsion of bacteria from worms. A countable number (∼10) of worms were transferred to a micro-centrifuge tube and the final volume was brought to 250 µl with M9 buffer. To the washed worms, 100 µg of silicon carbide particles (1.0 mm; Hi-media, India) was added, and the tubes vortexed vigorously for a minute to disrupt the worm and to release the colonized bacteria into the M9 suspension. Finally, the resulting suspension was serially diluted and plated on Hichrome Listeria isolation agar (Hi-media, India) to determine the CFU.
3 Results
3.1 Phytochemical components present in the T. Cattapa plant leaf extract
During the initial analysis, different plants were evaluated for their growth dynamics, utility and availability around the globe for their potential uses in nanoparticles synthesis both at the lab scale as well as commercial scale. Among various plants, T. catappa has drawn our attention as this plant grows all around the globe and is reported to be widely used as traditional medicine for the treatment of dermatosis and hepatitis. Pharmacological studies on the T. catappa leaves and fruits have demonstrated by the researchers and they possess compounds that have the anticancer, antioxidant, retroviral, anti-inflammatory, antidiabetic and hepatoprotective activities. The phytochemical analysis of crude aqueous leaf extract of T. catappa revealed the presence of tannins, saponins, flavonoids, terpenoids, alkaloids, reducing sugars, and glycosides at high levels. Our analysis showed that the main constituents in the aqueous leaf extract of T. catappa was polyphenols which may be responsible for the synthesis of silver nanoparticles. To elucidate the compounds profile in the aqueous extract of T. catappa leaf, further confirmation studies were done by UV analysis with the range of 200 nm − 600 nm. The absorption maxima (λmax) of the leaf extract were noted to be at the wavelengths of 304 and 366 nm, corresponding to ellagic acid, gallic acid, chebulinic acid and chebulgic acid (Engida et al., 2015, Masek et al., 2019). These observed phytochemical results are in good agreement with an earlier report (Anand et al., 2015, Zhang et al., 2019).
3.2 Morphological analysis of AgNPs
The biogenic synthesis of AgNPs were visually confirmed by the color change (green to brown) of the reaction solution as shown in Fig. 1. UV–visible spectrum sharp peak at 423 nm corresponds to the transverse plasmon observed for AgNPs. Our data is in complete agreement with the results were obtained by various other researchers (Flieger et al., 2021, Jaast and Grewal 2021, Nawabjohn et al., 2022). We believe that the secondary metabolites polyphenols that exist in the leaf aqueous extract is responsible for the reduction of silver nitrate into silver ions as reported by others (Liu et al., 2018, Tyagi et al., 2021). AgNPs crystalline nature was observed by the peaks at 2⊖ = 38.28° (1 1 1), 44 (2 0 0), 64.36°(2 2 0) and 77.12° (3 1 1), respectively in the X-ray diffraction analysis in the range 20° to 80° for the face-centered cubic (FCC) silver with a lattice parameter of a = 4.08 (JCPDS card number 04–0783) Fig. 2. The synthesis of AgNPs was noted within 24 h of incubation using the secondary metabolites of T.catappa leaf and they were generated as polydispersed in shape along with porous surface Fig. 3A. The particle size distribution was found to be in the range of 26–100 nm to be curved as illustrated in Fig. 3B. AgNPs were found to be highly stable and polydispersed in nature, as revealed by TEM and HR-TEM analysis (Fig. 3C-G). SEM analysis proved that the AgNPs are formed and have a uniform size in the range of 26 nm with a polydispersed structure.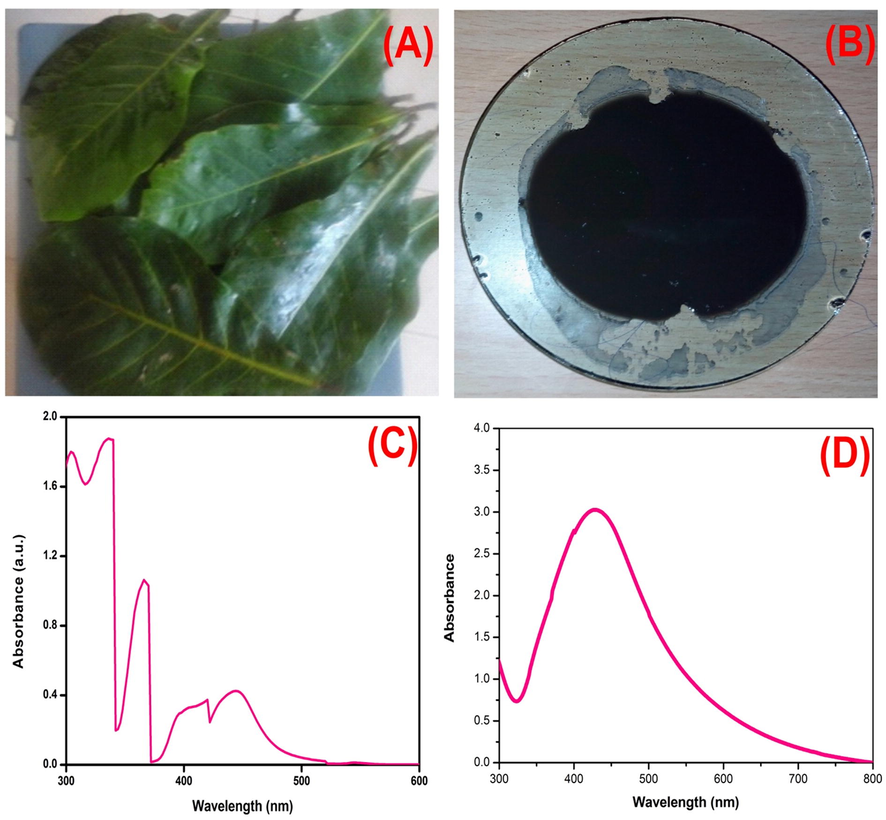
UV /VIS Spectrometric analysis (a) T.Cattappa leaf (b) synthesized AgNPs from T.Cattappa leaf (c) UV–Vis Spectra of T.Cattappa leaf extract (d) UV–Vis Spectrum of AgNPs.
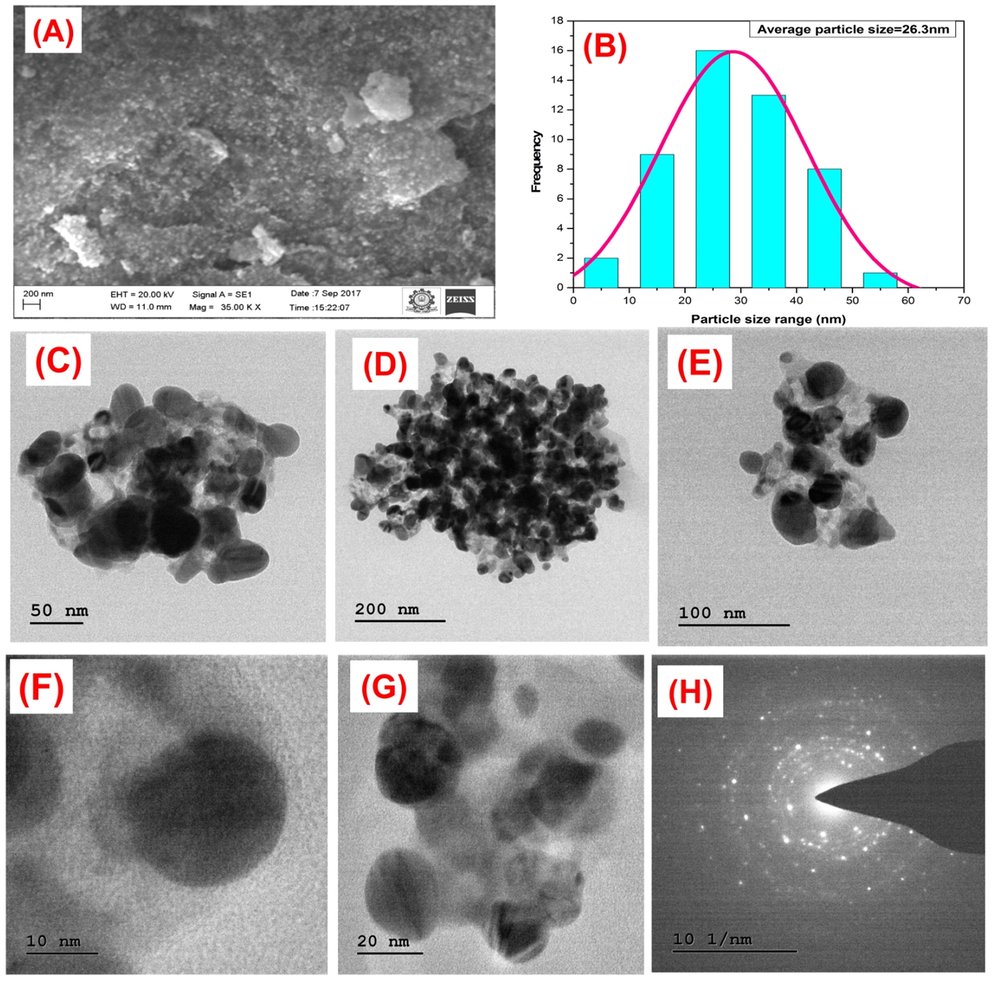
a) Depicting SEM microphotographs of synthesized AgNPs b) Particle size distribution curve. TEM micrograph of AgNPs. (c-g) different magnifications of AgNPs, (h) SEAD pattern of silver nanoparticles.
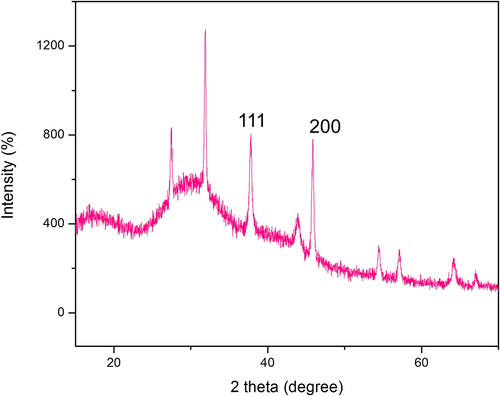
X-ray diffraction analysis of AgNPs from T.Cattappa plant leaf extract.
3.3 Biofilm inhibitory potential of AgNPs
Crystal Violet based biofilm quantification and confocal laser scanning.
microscopy was used to study the in vitro efficiency of AgNPs towards L. monocytogenes biofilm. At concentrations of 50 μg/ml and 100 μg/ml MIC concentration of AgNPs, 33% and 45.5% of biofilm development, was observed respectively (Fig. 4). The confocal microscopy biofilm imaging results (Fig. 5.) revealed that the AgNPs were significantly effective in destroying pre-formed biofilms, and entrenched biofilm cells. Based upon the Z-stack analysis of CLSM, it is very clear that at 100 μg/ml of L. monocytogenes could not form clusters and multi-layered microcolonies. The effects of AgNPs were examined on planktonic cells of L. monocytogenes and MIC was determined. The complete inhibition of bacterial growth was observed at 220 µg/ mL of AgNPs (data not shown). These results were proposed that the AgNPs have a tangible antibacterial potential against L. monocytogenes at very high concentrations. The L. monocytogenes ability to survive in the cell and escape membrane vacuoles depends upon the production of metalloprotease (Kannan et al., 2020). Studies have shown that the L. monocytogenes protease mutants fail to show signs of successful infection or spread from cell to cell (Yeung et al., 2005). So we investigated the ability of the AgNPs to inhibit protease production. Given the information that protease production is important for the L. monocytogenes pathogenesis and the propagation of the biofilms, we next investigated whether AgNPs can inhibit protease production. The inhibitory effect of AgNPs on protease production in L. monocytogenes was measured by the proteolytic assay. At 50 and 100 µg/ mL AgNPs could be able to inhibit 73% and 84% of the protease production respectively in 24 h of treatment. In nutshell, in vitro experiments indicate that the AgNPs have the potential to inhibit the L. monocytogenes in the biofilm and prevent the virulence factor production.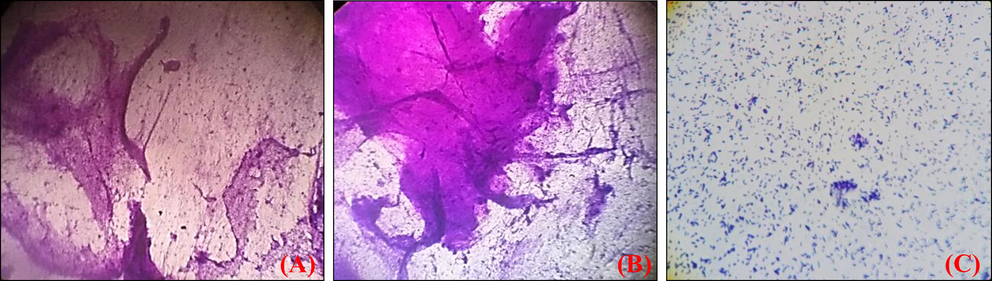
Crystal Violet images of biofilm development (a) Untreated control (b) Listeria monocytogens biofilm with ¼ MIC (c) Biofilm with ½ MIC.
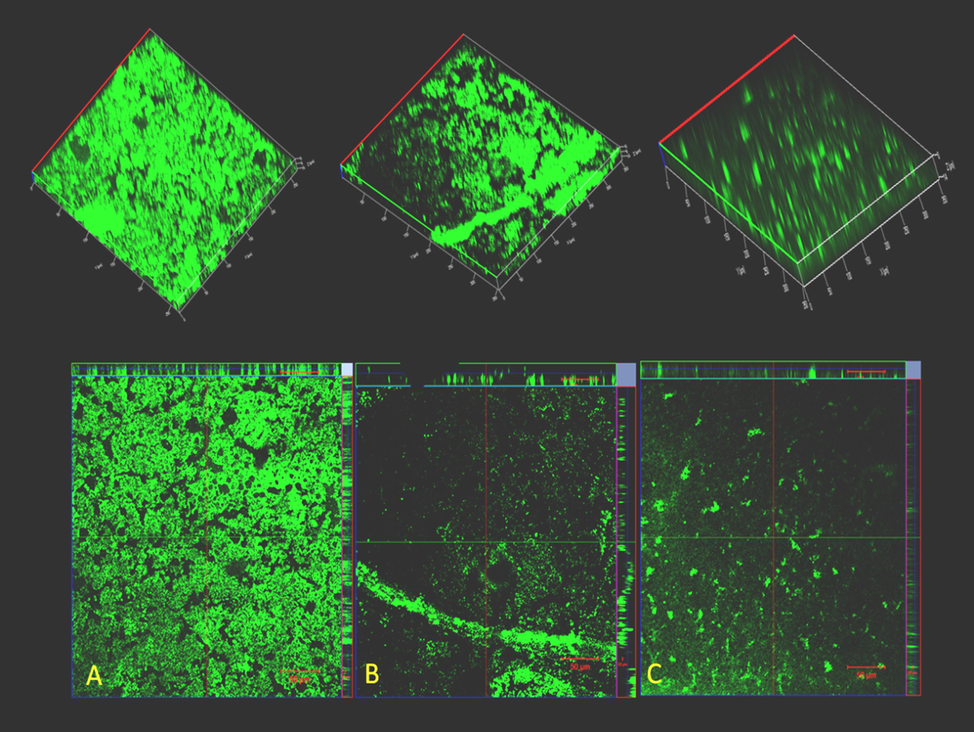
CLSM ortho and 3D images of biofilms of L. monocytogenes development (a) Untreated control (b) Listeria monocytogens biofilm with ¼ MIC (c) Biofilm with ½ MIC.
3.4 In-vivo impact of AgNPs on C. Elegans
Given the encouraging in vitro results, we next focussed our attention on exploring the potential of the AgNPs under in vivo conditions using free-living tropical nematode, Caenorhabditis elegans as a model system. We in this study choose C. elegans for the reason that the intestinal cells are quite similar to that of humans and L. monocytogenes can colonize in the intestinal tract (Yang et al., 2019). Moreover, 60–80% of genes, signal transduction pathways, specific epigenetic markers, human disease genes, and disease pathways are highly conserved in C. elegans and humans (Pimentel-Acosta et al., 2020). Our in vivo results showed that L. monocytogenes infected C. elegans when treated with the AgNPs showed a survival rate (up to > 50%) over the untreated group even after 100 h of infection (Fig. 6.). Further, the control group showed evidence of bacterial colonization in the intestinal region, while those treated with different doses of AgNPs showed a dose–response pattern. The CFU assay was performed to corroborate the results of the survival assay. As can be seen from (Fig. 7.) bacterial colonization was very low in the AgNPs-treated worms compared with the untreated group (control). The CFU count in the C. elegans infected with L. monocytogenes alone (without any treatment) was found to be a log value of 9.6 ± 0.3, whereas the worms treated with AgNPs showed a log value of 2.8 ± 0.5. Together with the previous investigation on survival, the present study endorses the fact that AgNPs can be used to constrain the biofilm formation, destroy the preformed biofilm of the food-borne pathogen L. monocytogenes.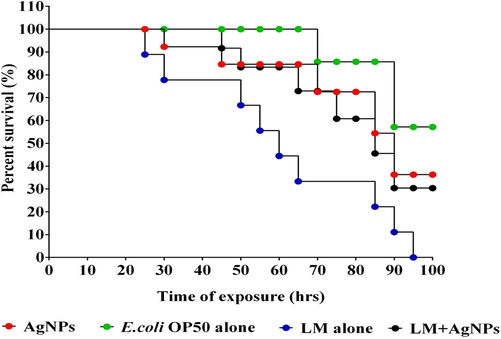
Kaplan–Meier survival curves of C. elegans fed with Listeria monocytogens and Protected with AgNPs.
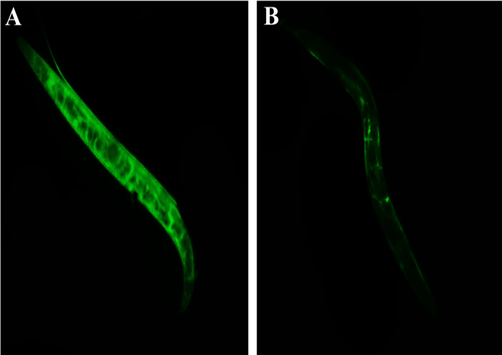
Intestinal colonization potential of L. monocytogenes (A) untreated (B) AgNPs treated.
4 Discussion
Among all the food-borne pathogens L. monocytogenes is of great concern as it can survive in widespread environments, surfaces and propagates at low temperature, form biofilms in the production lines as well as pack food which is hard to eliminate. The treatment of L. monocytogenes infection is a major challenge for clinicians also around the globe due to its pathogenic supremacy, resistant antibiotics treatment, chain washing, pasteurization, to form biofilms and regulate the virulence factors which influence the progression of the disease.
As predicted, AgNPs synthesis occurred with the T. catappa leaves in the 24 h. TEM studies showed that the surface morphology of AgNPs is diverse. Previous studies have shown that the AgNPs synthesized by the chemical route or photochemical process were distinct, spherical, and well separated, whereas those prepared by the phytochemical approach showed poly-dispersed with roughly spherical shape. The polydispersed nature along with the enhanced surface area of the nanoparticles might be due to the rich chemical diversity of the leaf extract. The crystalline nature of AgNPs, indicated by the XRD results obtained, agrees with the earlier report of other researchers (Raj et al., 2018, Hamedi and Shojaosadati 2019, Gul et al., 2021). Shah et al. (2019) recent reports indicate that the AgNPs synthesized using the Piper betle leaf extract showed an average size of 20–70 nm and polydispersed structure. Similarly, AgNPs synthesized using Ricinus communis leaf extract showed almost spherical morphology with a mean size of 29–38 nm (Gul et al., 2021). On the contrary, AgNPs synthesized using the leaf extracts of Semecarpus anacardium (62.72 nm), Glochidion lanceolarium (93.23 nm), and Bridelia retusa (74.56 nm) showed a higher average particle size (Mohanta et al., 2020). Based upon the results, it can be deduced that the size of AgNPs depends upon the method followed, a plant extract used in the biogenic synthesis. It is reported that in addition to the phytochemicals, proteins present in the extract might play a significant role in the synthesis of the AgNPS also. The amino acids like glutamine, aspartic residues tyrosine residues were reported to function as size and shape controlling agents (Zahoor et al., 2021). The images obtained showed the presence of agglomerated AgNPs in small aggregates with clear borders. The aggregation with the clear boundaries indicated that the presence of the coating and stabilizing agents in nanoparticles formed (Tang and Zheng 2018, Hamedi and Shojaosadati 2019). Corroborating the SEM pictures with extra unassigned peaks in XRD spectra confirms the presence of bio-organic phase that occurs on the surface of nanoparticles (Raj et al., 2018, Geissel et al., 2022). It is interesting to see whether there is any type of correlation between the synthesis method, shape, and size of AgNPs to that of biofilm activity among different pathogens.
It is very important to separate the antimicrobial activity from that of the antibiofilm activity. There is some ambiguity in the literature, where the researcher proposed antimicrobial activity as antibiofilm activity. To rule out these issues, we investigated the antibacterial activity of AgNPs against the L. monocytogenes also. Based upon the antimicrobial assay, 220 µg/mL was found to be the effective concentration for the AgNPs against mid-log planktonic L. monocytogenes, and this concentration was fixed as MIC values. Our size distribution analysis results show that the AgNPs have a mean size of 26 nm, hence exhibiting lower antimicrobial activity in comparison to the antibiofilm activity. It is reported that the size of AgNPs decides the antimicrobial activity and larger size nanoparticles tend to show lesser activity in the local environments (Osonga et al., 2020). Recent Agnihotri et al prepared highly monodispersed different sized AgNPs using a precise size control method and demonstrated that as the size increases the antibacterial activity and toxicity decrease (Agnihotri et al., 2014). It is reported that the AgNP's activity and toxicity depend upon the size, concentration, exposure time, efficiency of stabilizer/reducing agent used, and target organism sensitivity (Zahoor et al., 2021). As the activity depends upon the shape, it would be interesting to see how the size, shape, specific surface area, and surface chemistry impact the antibiofilm activity in the L. monocytogenes.
In this work, we decide to investigate the potential of the AgNPs at 50 and 100 μg/ml, to separate the antibiofilm from that of antibacterial activity. Crystal violet-based standard high throughput antibiofilm assay showed that at the tested concentrations i.e., 50 μg/ml and 100 μg/ml, 33% and 45.5% inhibitions were observed at 24 h. Our results show that there was no bacterial cell clumping in the presence of AgNPs as opposed to the control group Fig. 5. Recent studies demonstrated that the silver-based nanoparticles reduce the biofilm formation by inhibiting the biofilm-related genes, i.e fim H in E.coli, las I and rh II in P. aeruginosa (Xu et al., 2021). Even though tempting to speculate that AgNPs might also inhibit the biofilm-related genes in L. monocytogenes, we refrain as nanoparticles are shown to interact with various cellular mechanisms like enzymatic degradation of homeostasis related proteins or gene products or generation of reactive oxygen species (Mousavi et al., 2018) Given this, the molecular mechanism of AgNPs inhibiting biofilm in L. monocytogenes needs to be explored as the understanding of physical parameters' influence on the antibiofilm activity will help in further broadening the AgNP use in food industry applications. Not only that, it is well known that the synthesis route followed will decide various nanoparticle properties.
L. monocytogenes high level of pathogenicity is due to unique features like the ability to produce a high amount of proteases, multidrug surface transporters proteins, murein, deacetylated N-acetyl glucosamine residues, intrinsically resistance to cephalosporins, which gives them an ability to survive in diverse conditions (Pizarro-Cerda and Cossart 2018, Kannan et al., 2020, Dos Santos et al., 2021). To assess the toxicity of the AgNPs, C. elegans was used as model organisms as they are widely used to analyze the virulence and survival of L. monocytogenes (Yang et al., 2019). The Kaplan-Meier survival curves indicate a better survival rate (>30%) up to 100 h of infection. The colony-forming units account for the significantly decreased bacterial colonization (up to 2.8 ± 0.5) in AgNPs-treated C. elegans infected with L. monocytogenes compared with the control, which shows the highest log value of 9.6 ± 0.3 for bacterial colonization.
In this present study, we have demonstrated that the AgNPs can be synthesized in a biogenic way using T. catappa leaves. The synthesized AgNPs have a polydispersed structure with a mean size in the range of 26 nm and can significantly inhibit the L. monocytogenes biofilm formation not only that AgNPs can protect the C. elegans from the L. monocytogenes infection.
5 Conclusion
In this present study was concluded that, we produced AgNPs in an eco-friendly by the green approach using the T. catappa leaves to inhibit the biofilm production of food-borne pathogen L. monocytogenes without any toxicity issues. This present study highlights the effect of silver nanoparticles were significantly inhibit the biofilm formation of L. monocytogenes.
CRediT authorship contribution statement
Lakshmanan Muthulakshmi: Conceptualization, Methodology, Writing – original draft. Kannan Suganya: Conceptualization, Methodology, Writing – original draft. Maruthamuthu Murugan: Conceptualization, Methodology, Writing – original draft. Jamespandi Annaraj: Conceptualization, Methodology, Writing – original draft. Veeramuthu Duraipandiyan: Data curation, Formal analysis, Validation, Writing – review & editing, Funding acquisition. Dunia A. Al Farraj: Data curation, Formal analysis, Validation, Writing – review & editing, Funding acquisition. Mohamed S. Elshikh: Data curation, Formal analysis, Validation, Writing – review & editing, Funding acquisition. Annie Juliet: Data curation, Formal analysis, Validation, Writing – review & editing, Funding acquisition. Mukesh Pasupuleti: Conceptualization, Methodology, Resources, Formal analysis, Validation. Jesu Arockiaraj: Conceptualization, Methodology, Resources, Formal analysis, Validation.
Acknowledgement
Lakshmanan Muthulakshmi acknowledges the Department of Science and Technology-Science and Engineering Research Board (PDF/2017/001574), Government of India, for the award of a National Postdoctoral Fellowship. The authors extend their appreciation to the Researchers Supporting Project Number (RSP-2021/190), King Saud University, Riyadh, Saudi Arabia.
Ethics approval
Not Applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit Rev Microbiol. 2016:1-8.
- [Google Scholar]
- Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Advances.. 2014;4(8):3974-3983.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl.. 2016;9:49-67.
- [CrossRef] [Google Scholar]
- An updated review of Terminalia catappa. Pharmacogn Rev.. 2015;9(18):93-98.
- [CrossRef] [Google Scholar]
- Non-antibiotic antimicrobial agents to combat biofilm-forming bacteria. J Glob Antimicrob Resist.. 2020;21:445-451.
- [Google Scholar]
- Listeria monocytogenes Biofilms in the Wonderland of Food Industry. Pathogens.. 2017;6(3):41.
- [Google Scholar]
- Listeria monocytogenes: health risk and a challenge for food processing establishments. Arch Microbiol.. 2021;203(10):5907-5919.
- [CrossRef] [Google Scholar]
- Exploration of Phytochemicals Found in Terminalia sp. and their Antiretroviral Activities. Pharmacogn Rev.. 2016;10(20):73-83.
- [CrossRef] [Google Scholar]
- Analysis of major antioxidants from extracts of Myrmecodia pendans by UV/visible spectrophotometer, liquid chromatography/tandem mass spectrometry, and high-performance liquid chromatography/UV techniques. Journal of Food and Drug Analysis.. 2015;23(2):303-309.
- [CrossRef] [Google Scholar]
- Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules.. 2021;26(16):4986.
- [Google Scholar]
- Antibiofilm activity of nanosilver coatings against Staphylococcus aureus. J Colloid Interface Sci.. 2022;608:3141-3150.
- [Google Scholar]
- Green Synthesis, Characterization, Enzyme Inhibition, Antimicrobial Potential, and Cytotoxic Activity of Plant Mediated Silver Nanoparticle Using Ricinus communis Leaf and Root Extracts. J Biomolecules.. 2021;11(2):206.
- [Google Scholar]
- Rapid and green synthesis of silver nanoparticles using Diospyros lotus extract: Evaluation of their biological and catalytic activities. Polyhedron.. 2019;171:172-180.
- [CrossRef] [Google Scholar]
- Evaluation of Antibacterial Effects of Matrix-Induced Silver Ions against Antibiotic-Resistant ESKAPE Pathogens. Pharmaceuticals (Basel). 2021;14(11):1094.
- [Google Scholar]
- Green synthesis of silver nanoparticles, characterization and evaluation of their photocatalytic dye degradation activity. Current Research in Green and Sustainable Chemistry.. 2021;4:100195
- [CrossRef] [Google Scholar]
- Y. Jiao F.R. Tay L.-N. Niu et al. Advancing antimicrobial strategies for managing oral biofilm infections Int J Oral Sci. 11 3 2019 28 28 10.1038/s41368-019-0062-1.
- Listeria monocytogens - Amended understanding of its pathogenesis with a complete picture of its membrane vesicles, quorum sensing, biofilm and invasion. Microb Pathog.. 2020;149:104575
- [CrossRef] [Google Scholar]
- Listeria monocytogenes, a model in infection biology. Cell Microbiol.. 2020;22(4):e13186.
- [Google Scholar]
- Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. Journal of Food and Drug Analysis.. 2018;26(2):649-656.
- [CrossRef] [Google Scholar]
- Polyphenolic profile and antioxidant activity of Juglans regia L. leaves and husk extracts. Forests.. 2019;10(11):988.
- [Google Scholar]
- Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem.. 2005;339(1):69-72.
- [Google Scholar]
- Listeria monocytogenes Biofilms in the Food Industry: Is the Current Hygiene Program Sufficient to Combat the Persistence of the Pathogen? Microorganisms.. 2021;9(1):181.
- [Google Scholar]
- Anti-biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front Microbiol.. 2020;11
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biotechnol.. 2018;46(sup3):S855-S872.
- [Google Scholar]
- Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials.. 2022;15(2):427.
- [Google Scholar]
- Green Synthesis and Characterisation of Silver Nanoparticles Using Cassia tora Seed Extract and Investigation of Antibacterial Potential. Applied Biochemistry and Biotechnology.. 2022;194(1):464-478.
- [Google Scholar]
- Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules.. 2020;25(11):2682.
- [Google Scholar]
- Molecular Effects of Silver Nanoparticles on Monogenean Parasites: Lessons from Caenorhabditis elegans. Int J Mol Sci.. 2020;21(16):5889.
- [Google Scholar]
- Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochemical and Biophysical Research Communications.. 2018;503(4):2814-2819.
- [CrossRef] [Google Scholar]
- Growth, viability and architecture of biofilms of Listeria monocytogenes formed on abiotic surfaces. Braz J Microbiol.. 2017;48(3):587-591.
- [CrossRef] [Google Scholar]
- Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling.. 2019;35(1):34-49.
- [Google Scholar]
- Novel Nanotherapeutics as Next-generation Anti-infective Agents: Current Trends and Future Prospectives. Curr Drug Discov Technol.. 2020;17(4):457-468.
- [CrossRef] [Google Scholar]
- Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. J Ethnopharmacol.. 2011;134(3):865-871.
- [Google Scholar]
- Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv Healthc Mater.. 2018;7(13):e1701503.
- [Google Scholar]
- Tyagi, P. K., S. Tyagi, D. Gola, et al., 2021. Ascorbic Acid and Polyphenols Mediated Green Synthesis of Silver Nanoparticles from <i>Tagetes erecta</i> L. Aqueous Leaf Extract and Studied Their Antioxidant Properties. Journal of Nanomaterials. 2021 6515419. https://doi.org/10.1155/2021/6515419.
- Enhanced Antibacterial and Anti-Biofilm Activities of Antimicrobial Peptides Modified Silver Nanoparticles. Int J Nanomedicine.. 2021;Volume 16:4831-4846.
- [Google Scholar]
- Simple Evaluation of Listeria monocytogenes Pathogenesis Using Caenorhabditis elegans Animal Model. Food Sci Anim Resour.. 2019;39(1):84-92.
- [Google Scholar]
- The metalloprotease of Listeria monocytogenes controls cell wall translocation of the broad-range phospholipase C. J Bacteriol.. 2005;187(8):2601-2608.
- [CrossRef] [Google Scholar]
- A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water.. 2021;13(16):2216.
- [Google Scholar]
- The Genus Terminalia (Combretaceae): An Ethnopharmacological, Phytochemical and Pharmacological Review. Nat Prod Bioprospect.. 2019;9(6):357-392.
- [Google Scholar]







