Translate this page into:
Beta maritima mediated silver Nanoparticles: Characterization and evaluation of Antibacterial, Antifungal, and antioxidant activities
⁎Corresponding author. tahira.shafique23@gmail.com (Tahira Shafique)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract

Abstract
Silver nanoparticles (AgNPs) are known for their large surface area, which enhances their energy and antimicrobial activity. This study focused on producing silver nanoparticles using leaves from the Beta maritima (vulgaris) plant. Various techniques such as Fourier transform infrared (FT-IR) spectroscopy, ultraviolet–visible (UV–vis) spectroscopy, energy-dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM), and X-ray diffraction analysis (XRD) to characterize the synthesized AgNPs. The biologically synthesized AgNPs were then evaluated for their antimicrobial (antibacterial and antifungal) and antioxidant properties using different agents such as hydrogen peroxide (H2O2), 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2 azobis, 3-ethyl benzothiazoline-6-sulphonic acid (ABTS), and phosphomolybdate. The results showed that the AgNPs exhibited strong antifungal activity, particularly against fluconazole-resistant strains of Aspergillus niger and Aspergillus flavus, with a 61 % inhibition rate. Moreover, the AgNPs demonstrated significant antibacterial activity against various strains. At a concentration of 40 µg/mL, they exhibited 26 % inhibition against Staphylococcus aureus, 22 % against Micrococcus luteus, 18 % against E. coli, and 24 % against Klebsiella pneumonia, outperforming clarithromycin antibiotics. SEM analysis revealed spherical AgNPs, sized using nano measurer 1.2, EDX showed the presence of silver having composition at 80.38 %, and XRD confirmed a face-centered cubic crystalline structure. In addition to their characterization, the AgNPs also displayed strong antioxidant activity. At the same concentration, they showed percentages of 80.78 % (DPPH), 85.38 % (H2O2), 81.78 % (ABTS), and 80.66 % (phosphomolybdate), surpassing the antioxidant activity of the plant leaf extract alone. The synthesis of AgNPs using the extract of the Beta maritima (vulgaris) plant offers several advantages compared to other methods, including ease of handling, cost-effectiveness, wide availability, and eco-friendliness.
Keywords
Nanoparticles
Green synthesis
FTIR, SEM, XRD
Antioxidant: antimicrobial
1 Introduction
For an extended period, medicinal plants have been employed for nutritional and traditional medicinal purposes. Their efficacy is attributed to their strong biological properties (Erenler et al., 2023a). A nanoparticle is widely used in a new era of the scientific world. So, nanotechnology is a diverse field, i.e., an interdisciplinary field that covers all the major fields like physics, chemistry, biology, metaphysics, etc. Nanotechnology is a comprehensive concept that encompasses the manipulation and fabrication of materials at the nanoscale particles having a size of 1 nm to 100 nm (Kawasaki & Player, 2005). Extensive research has been conducted to explore the advantageous effects of silver nanoparticles in biotechnology and medicine, with a particular focus on their role as antibacterial agents (Zhang et al., 2023). Silver nanoparticles (AgNPs) find application in various fields, including medicinal uses, surface treatment, coatings, the chemical and food industries, and enhancing agricultural productivity. Furthermore, AgNPs have gained widespread utilization nanomedicine, drug delivery, biomedical devices, electronics, the energy sector, and environmental protection are prominent domains where nanotechnology finds extensive application. (Cui, 2023). The Green synthesis of nanoparticles can be conducted in different ways like viral DNA, prokaryotic and eukaryotic microorganisms, and diatom templates. The production of nanoparticles using plant and plant extracts is a significant area of research and development that drew the consideration of scientists for the reason that it has eco-friendly, nontoxic effects on the environment and human beings (Gecer & Erenler, 2023). Nanotechnology has captivated the interest of scientists owing to its environmentally friendly nature and clean characteristics, an environmentally friendly, and non-toxic mode of nanoparticle synthesis. Many nanomedicines are being used in the market to control, check, monitor, and repair human biological structures with the help of nanostructures and nanodevices (Emerich & Thanos, 2003).
Among the various types of metal nanoparticles, the silver nanoparticle stands out as an exceptionally noteworthy nanomaterial owing to its remarkable biological characteristics (Erenler et al., 2023a). Silver nanoparticles (AgNPs) are most extensively used in the business field and as a biocide (a chemical used to kill life, including poison, antibacterial, and fungicide. It has a very powerful antibacterial activity. AgNPs have a large surface area, which helps to increase the energy and improves the antimicrobial activity (Mansoor et al., 2021). AgNPs are being utilized in surgical instruments, surgical masks, and bone cement, among other medical devices. It has been reported that free radicals perform a great role in the pathogenesis of specific diseases and aging (Adom & Liu, 2005). Pathogenic bacteria are involved in causing various diseases in humans. Bacterial groups such as Staphylococcus aureus, Micrococcus luteus, Klebsiella pneumonia, and Escherichia coli cause serious disorders, e.g. typhoid fever, diphtheria, and food borne illnesses. The antimicrobial effectiveness of AgNPs is linked to various mechanisms, such as inducing oxidative stress, causing protein malfunction, disrupting the bacterial membrane, and inflicting DNA damage. These actions collectively result in the demise of bacteria (Timoszyk & Grochowalska, 2022).
AgNPs have astonishing protection against a variety of microorganisms and can resist commonly used antibiotics (Sharma et al., 2009). Synthesis of AgNP demonstrations hurdle against microbial activity in both gram-positive and negative bacteria [(Khan et al., 2021a; Mansoor et al., 2021)]. Synthesized AgNPs showed very potential and good results in antibacterial, antimicrobial, and anticancer activities (Khan et al., 2021b). AgNPs harmfully affect the cellular catabolism, transport of substrate system, DNA multiplication, and cell division of bacteria (Sellami et al., 2021). Nowadays, the best application is using AgNPs for wound healing and infections (Sher et al., 2020). AgNPs are extremely noxious and dangerous to microorganisms when they contact each other. The reduction in the size of silver to nano-sized increases its ability to control bacteria and fungi (Ijaz et al., 2017; Khan et al., 2020a).
Green synthesis of AgNPs applications has favorability and reliable potential, which are low-cost to be cultured (Khan et al., 2020b). The herbal source is composed of different phytochemicals like dehydrogenases, ascorbic acids, flavonoids citric acid, and extracellular electron shuttles, which exhibit the properties of reduction and stabilization AgNPs synthesizes. These compounds play an essential role in measuring metal-based nanoparticles (Khan, Shahid, Shahid et al., 2020). AgNP green plant syntheses have great importance due to easy availability, safe handling, non-hazardous, and having a wide multiplicity of metabolites. The synthesis of AgNPs indicates that plant phytochemicals actively engage in ion reduction and direct involvement in the production of AgNPs (Ahmed et al., 2017; Mansoor et al., 2021).
Beta maritima grows in wild environments, and which are subspecies of Beta vulgaris that grows in the coastal area of Eurasia. It is related to the family Amaranthaceae, which is found in Europe, Africa, Asia, and some of the Middle East. Moreover, a few previous studies proved that B. maritima exhibits anti-tumor effects, specifically against breast cancer cells. This medicinal plant, used for a very long time as both a traditional cure and food, is employed in folk remedies for various ailments, including leukemia, esophageal, glandular, prostate, and breast cancers (Abbasi et al., 2016; Sher et al., 2020). Considering the therapeutic worth and importance of B. maritima and green nanotechnology, the present study was designed to synthesize AgNPs using leaves of the B. maritima (vulgaris) plant and evaluate their antimicrobial and antioxidant activities.
2 Material and methods
2.1 Materials
Silver nitrate salt (AgNO3), Sigma-Aldrich (USA) provided the following materials: sulfuric acid (H2SO4), sodium phosphate, ascorbic acid, DPPH (1,1-Diphenyl-2-picrylhydrazyl), phosphomolybdate, hydrogen peroxide (H2O2), ABTS (2, 2 azobis, 3-ethyl benzothiazoline-6-sulphonic acid), potassium persulfate (K2S2O8), nutrient agar, and sabourad dextrose agar (SDA). Briefly, four clinically isolated bacterial strains, two gram-positive (Staphylococcus aureus ATCC 29213, Micrococcus luteus ATCC 4698) and two gram-negative (Klebsiella pneumonia ATCC 700603, and Escherichia coli ATCC BAA-2471) were used. Briefly, two fungus strains, the microbiology lab provided Aspergillus niger and Aspergillus flavus., in the department of Biotechnology, University of Science and Technology Bannu-KPK, Pakistan.
2.2 Plant collection and extract preparation
An experienced taxonomist from the University of Science and Technology, Bannu identified the plant Beta maritima (Vulgaris) that was collected in the Bannu region of KP, Pakistan. After being thoroughly washed and dried, the plant leaves were ground into a fine powder. The obtained powder of leaves is soaked in de-ionized water (1:3w/v) and heated up to 40°c in a conical flask for 20 min. The obtained mixture is After passing through Whatman filter sheets, the mixture is kept cold (4 °C) for later use.
2.3 Synthesis of AgNPs
A silver nitrate (AgNO3) solution of about 1 mM in water was prepared. To synthesize AgNPs 10 mL of plant leaves (1 g/100 ml water) extract is mixed-up with 90 mL of AgNO3 aqueous solution (1 mM) and stirring it constantly. The solution's color transitioned from clear yellow to brownish immediately; the UV–visible spectrum confirmed the spectrum analysis. After that, it was centrifuged for 20 min at 20,000 rpm to get supernatant for further use (Abbasi et al., 2016). washing post-centrifugation may be needed to eliminate remaining AgNPs and ensure the final product's purity, post-synthesis washing AgNPs from contaminated leaves after centrifugation with water containing peroxyacetic acid or chlorine, with or without an organic load, was found to only remove 3–7 % of the sorbed Ag from the leaves (Gunathilaka et al., 2023). The method is reproducible and same results are shown by (Adnan et al., 2022).
2.4 Characterization
The assessment of silver ion reduction in the solution was conducted using a Shimadzu UV spectrophotometer (UV-1800). To determine the existence of various functional groups, the purified AgNPs were analyzed using an FT-IR Shimadzu spectrometer (IR Prestige-21 Japan). Before conducting FT-IR analysis, the prepared materials and potassium bromide (KBr) powder were thoroughly dried. Pure KBr pellets were used for a blank study. Each sample was thinly pelletized with a 5 % of solid solution of KBr prepared for FT-IR spectra acquisition within the infrared radiation range of 400 to 4000 cm−1. The AgNPs' crystalline nature was ascertained through the use of a JDX-3532 X-ray diffractometer from JEOL JAPAN. The XRD patterns were obtained with A constant wavelength of radiation of λ-1.54A°. Concerning structural analysis, one square inch-sized samples were placed in a glass holding system and subjected to X-ray scanning. The scanning parameters included an acceleration voltage of 35 kV, a current of 20 mA, and a scanning degree range of 100-50°. From 20° to 80°, the diffracted intensities were measured.
To analyze the dimensions and form of the ready materials nanoparticles, A Japanese-made JEOL scanning electron microscope, model JSM-5910, was used. The SEM analysis was conducted with varying resolutions ranging from 10000X to 50000X, using a current voltage in the range of 5 kV-20 kV. The samples were appropriately placed on conductive tap-equipped aluminum stubs and examined using an SEM. All of the samples' micrographs were captured at the right voltage and resolution.
2.5 In Vitro-Antioxidant assay
2.5.1 DPPH scavenging activity
The DPPH assay quantifies the scavenging capacity of test samples against DPPH, a stable organic radical characterized by a deep purple hue. This assay measures the extent of DPPH radical neutralization, expressed as a percentage of radicals scavenged. A higher percentage indicates a more potent antioxidant activity of the analyzed compound (Erenler et al., 2023b). The stock solution of DPPH (3 mg/50 mL methanol) was prepared, and a UV spectrophotometer was used to record the absorbance value of the instrument which was found to be less than 0.9ƛ (Williams et al., 2004). Plant extract stock solution, ascorbic acid, and AgNPs were dissolved in 1 g/mL of de-ionized H2O. About 200 µL from varying concentrations of the samples at 10, 20, 30, and 40 µg/mL, mixed with 800 µL DPPH. The experimental procedure followed standard scientific protocol (Erenler et al., 2023a) and was conducted in duplicate. Absorbance was set at 517 nm as recommended for 24 hrs incubation in the dark. The % scavenging was calculated using the formula as follows: (i); (i).
The absorbance of the sample is As, whereas the absorbance of the control is Ac.
2.5.2 Hydrogen peroxide H2O2 scavenging Activity
For the activities of hydrogen peroxide (Pick & Mizel, 1981) method was followed. The experiment involved utilizing plant extract, ascorbic acid, and AgNPs at 10 µg/mL, 20 µg/mL, 30 µg/mL, and 40 µg/mL concentrations. Initially, a 2 mM solution of H2O2 was prepared and mixed with a 50 mL phosphate buffer with a pH of 7.4. Subsequently, 0.2 mL of the respective sample solution was added to 0.6 mL H2O2 and 0.4 mL phosphate buffer. After 15 min and a thorough shaking of the tubes, the absorbance was measured at 230 nm. The percentage was calculated using the formula (i).
2.5.3 ABTS free radical scavenging activity
ABTS (2, 2 azobis 3-ethyl benzothiazoline-6-sulphonic acids) was calculated according to the procedure of (Mathew & Abraham, 2006) with slight modifications. 2.45 mM solution of K2HSO4 was mixed with 7 mM ABTS solution and left in the dark overnight to get dark free radicals of ABTS. At 745 nm, the ABTS solution's absorbance was measured to be 0.936. A standard solution of AgNPs, ascorbic acid, and plant extract was in de-ionized H2O. The plant extract, ascorbic acid, and AgNPs were diluted in different values of concentrations as 10 µg/mL, 20 µg/mL, 30 µg/mL, and 40 µg/mL. From all running solutions, 0.2 mL was mixed with 0.8 mL of ABTS solution. After one minute of mixing both solutions, a clear reduction in absorbance was calculated, and after six minutes, duplicate solutions of each were measured. The formula (i) was used to calculate the percentage of age.
2.5.4 Phosphomolybdate free radicals scavenging activity
The technique of (Velavan et al., 2012) was used to perform the phosphomolybdate scavenging activity. A stock solution of AgNPs, ascorbic acid, and plant extract was prepared in de-ionized H2O. From each running solution (10 µg/mL, 20 µg/mL, 30 µg/mL, and 40 µg/mL), A 0.1 mL volume was mixed with 1 mL of a 28 mM sodium phosphate solution, 0.6 M sulfuric acid, and 4 mM phosphomolybdate. Through raping with a silver foil sheet, incubation was done, and after this was placed, the solution was boiled for 90 min at 95 °C. Methanol was used as absolute, and the absorbance measurement was performed on the sample mixture. as 0.621 at 765 nm at room temperature. The formula (i) was used to calculate the percentage of age.
2.6 Antimicrobial screening of AgNPs
2.6.1 Anti-fungal assessment of green synthesized AgNPs
About 1 mg/mL of AgNP solution was prepared in de-ionized water. Further, 4 different diluted concentrations of solution were prepared, i.e., 40 µg/mL, 60 µg/mL, 80 µg/mL, and 100 µg/mL. In a current study of anti-fungal activity, the technique of (Ruparelia et al., 2008) is followed. The fungicidal potential is measured against two strains of fungus, i.e., Aspergillus flavus and Aspergillus niger. First, 100 mL of de-ionized water with about 6.2 gm sabouraud dextrose agar (SDA) was poured, and the mixture was autoclaved at 121 °C for 15 min. After the autoclave, the solution was allowed to cool down to 40 to 50 °C. In 1 mL of DMSO, about 5 mg of fluconazole were dissolved. About 67 µL AgNPs and plant extract were present at a 40 µg/mL concentration, combined with 7 mL SDA and transferred into test tubes. Two control test tubes were taken, i.e., positive and negative, each of them 67 µL fluconazole, along with 4 mL of media, transferred to the positive control tube. For solidification, the tubes were kept in a slants position in a Laminar flow cabinet at room temperature. Afterward, the fungal strain was applied to each test tube and was placed inside the incubation for 9 days at 30 °C with water; after a few days, inhibition was identified and examined, which was expressed in millimeters (mm).
2.6.2 Anti-bacterial activity
Four different bacterial strands, two gram-negative and two gram-positive bacteria, namely Micrococcus luteus and Staphylococcus aureus, Escherichia coli, and Klebsiella pneumonia, respectively were selected (Duraipandiyan & Ignacimuthu, 2009). Nutrient agar prepared in water (2.8 g/100 mL). Each Petri dish is filled with 40 mL media and allowed to solidify. In the laminar flow cabinet, fresh bacterial cultures were taken with the help of a cotton swab and spread all over a nutrient agar plate aseptically. With the use of an aseptic cork borer, these five wells, measuring around 3–6 mm, were allowed. Now, 20 µL from AgNPs and plant concentration 40 µg/mL are poured into wells, respectively. Standard clarithromycin (5 mg/mL DMSO) is used as an anti-biotic and DMSO as a negative control loaded into wells respectively. After all this, Petri dishes were placed in incubation at 37°c for 24 hrs. After a 24-hour period, the outcomes were expressed by calculating the zone of inhibition, measured in millimeters, around each well.
2.7 Statistical analysis
One-way ANOVA was used for statistical analysis, and posthoc analysis followed subsequently. (d Steel & Torrie, 1986). For the determination to calculate the coefficient of correlation, Statistics (version 8.1 USA) was used. Graphs were plotted in the R-Studio 4.3.2 version.
3 Results
3.1 Ultraviolet spectroscopy
Using ultraviolet spectroscopy, the basic characterization of nanoparticles that are synthesized and also to observe their stability. The surface peak and size depend on the chemical environment and dielectric medium of Ag nanoparticles. The UV spectra of an aqueous solution, which are shown in Fig. 1 (range from 200 nm to 800 nm wavelength), and 1 mL of plant concentration, show a peak of 442 nm, which declares the presence of AgNPs.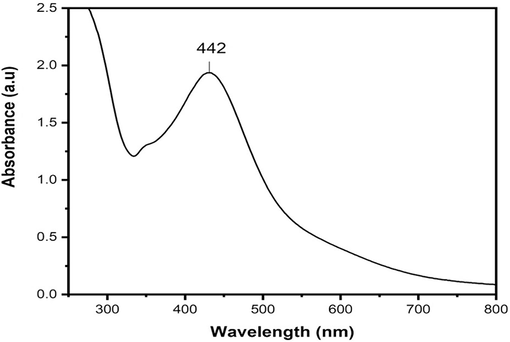
UV–visible spectrum by using extract of Beta maritima for synthesizing AgNPs.
3.2 Fourier Transformed infrared analysis of AgNPs
Fourier Transformed Infrared is a method for detecting functional groups that are partially or strongly bonded to the Nanoparticles (Table 1). Various peaks of distinct functional groups in the plant extract, and synthesized AgNPs exhibited absorption peaks at specific wave numbers: The following wavenumber ranges were observed in the infrared spectrum: 3400–3200 cm-1 (O–H stretch), 2935–2915 cm-1 (–CH (CH2) vibration), 2865–2845 cm-1 (–CH (CH2)), 1740–1725 cm-1 (C = O stretch), 1650–1600 cm-1 (C = O stretch), 1410–1310 cm-1 (O–H bend), 1100–1000 cm-1 (Phosphate ion), 995–850 cm-1 (P-O-C stretch), and 700–600 cm-1 (C-Br stretch). See Fig. 2.
Wave number cm−1
Absorption, cm−1 of plant extract
Absorption, cm-1 of AgNPs
Assignment
Class of Compounds
700–600
696.97
656.7
C-Br stretch
Aliphatic Bromo compounds
995–850
994.31
976.61
P-O-C stretch
Aromatic phosphates
1100–1000
1096.21
1100
Phosphate ion
Phosphate compound
1410–1310
1316.12
1368.9
O–H bend, Alcoholic group
Phenol or tertiary alcohol
1650–1600
1609.16
1600
C = O stretching vibration, Ketone group
Ketone compound
1740–1725
1727.8
1725
C = O stretch
Aldehyde compound
2865–2845
2840.38
2853.88
Symmetric stretching of –CH (CH2) vibration,
Lipids, protein
2935–2915
2920.72
2916.64
Asymmetric stretching of –CH (CH2) vibration
Saturated aliphatic compound-Lipids
3400–3200
3400
3346.89
O–H stretch
Poly Hydroxy compound
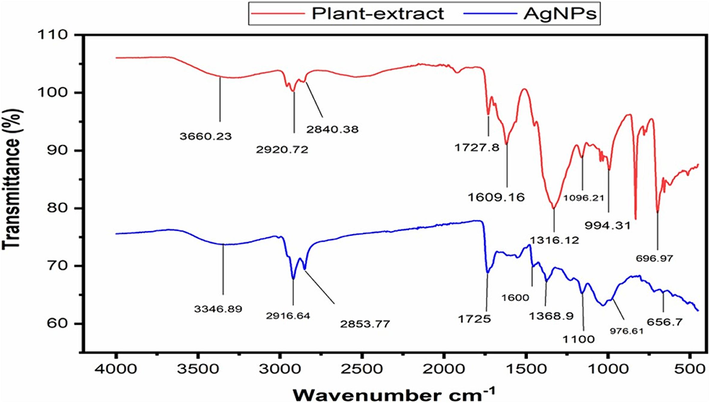
FT-IR results of plant extract and their AgNPs.
3.3 SEM activity
Scan Electron Microscopy (SEM) shows the morphology of AgNPs, as shown in Fig. 3a. The SEM analysis results presented spherical shaped AgNPs. To calculate the size, 15 particles were marked using nano measurer 1.2 software. The calculated dimensions was reported to be 144 nm.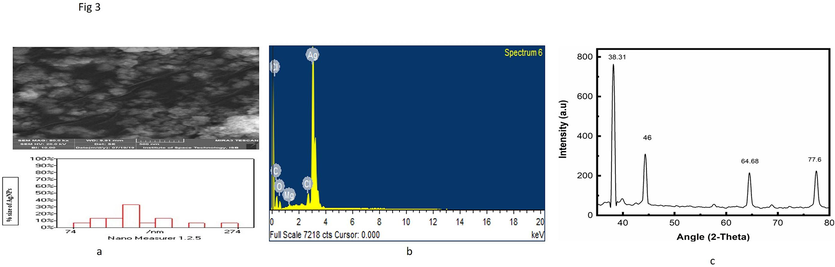
(a) SEM image of biosynthesized AgNPs. (b)The Nanomeasurer calculated size is 144 nm, EDX analysis of synthesized silver nanoparticles. (c) XRD analysis of synthesized silver nanoparticles by Beta maritime.
3.4 EDX analysis
The identified silver element in the solution was observed in the EDX analysis, as shown in Fig. 3b. The EDX spectrum illustrates the presence of significant metallic Ag signals. It ensures the elemental components of silver (80.38 %), oxygen (12.60 %), and carbon (5.45 %), correspondingly. Silver's strongest signal was recorded at around 3 keV. There were other peaks for Cl, C, O, and Mg detected. This graph indicates the presence of phytoconstituent's residual moieties of extract as an agent for surface stabilization of the AgNPs. (Table 2).
Elements
Weight in %
Atomic in %
C K
5.45
22.29
O K
12.60
38.67
Mg K
0.46
0.93
Cl K
1.10
1.53
Ag L
80.38
36.58
3.5 XRD analysis
XRD technique which is used for the detection of AgNPs particle crystallinity nature. The XRD of Beta maritima mediated AgNPs shows different peaks of AgNPs in given Fig. 3c from left to right, i.e., 38.31˚, 46.49˚, 64.68˚, and 77.60˚ at 2θ values. The XRD graph demonstrates that AgNPs are in a center cubic structure having an average calculated size carrying 71 nm. The size of Nanoparticles is calculated through an online XRD size calculator working on the principle of the “Brags equation”, which was launched online in 2017.
3.6 Antioxidant activities
3.6.1 Hydrogen peroxide activity
AgNPs showed the highest scavenging activity of 85.38 % as compared to plants at the highest concentration of 40 µg/mL, as shown in Fig. 4a. Ascorbic acid was taken as standard and showed maximum scavenging as compared to AgNPs.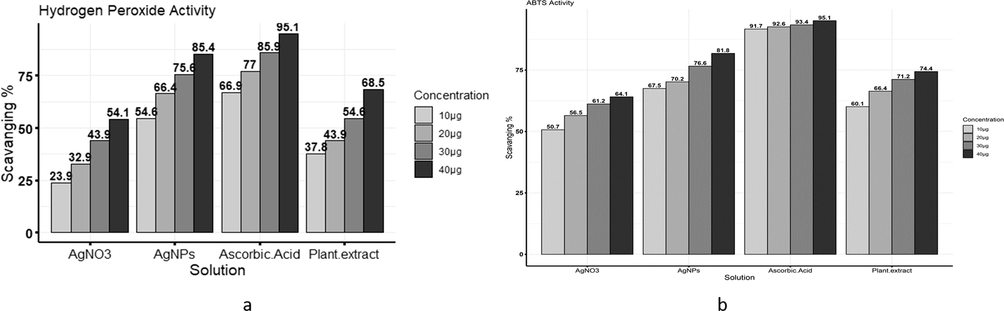
(a) Comparison among ascorbic acid, AgNO3, plant extract, and AgNPs scavenging activity against Hydrogen peroxide free radicals. (b) Comparison among ascorbic acid, AgNO3, plant extract, and AgNPs scavenging activity against ABTS free radicals.
3.6.2 ABTS activity
AgNPs showed the highest inhibition against ABTS than plant concentrations, and the highest inhibition shown by AgNPs at 40 µg/mL was 81.78 %. Ascorbic acid was taken as standard, as shown in Fig. 4b.
3.6.3 DPPH activity
AgNPs showed the highest inhibition against DPPH free radicals than plant concentrations, and the highest inhibition shown by AgNPs at 40 µg/mL is 80.78 %. Ascorbic acid was taken as standard, as shown in Fig. 5a.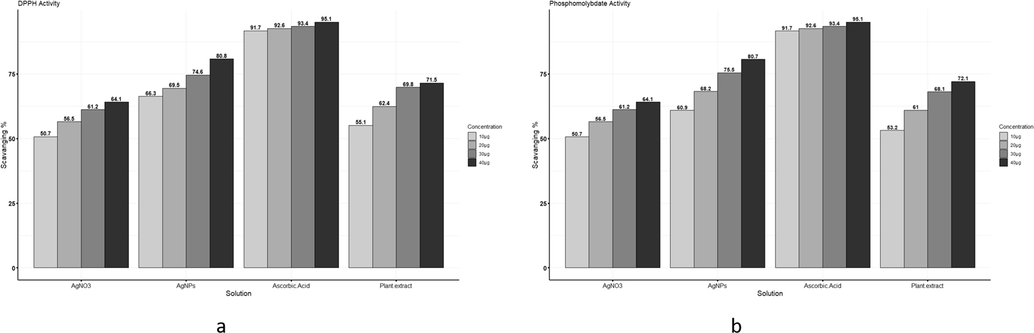
(a) Comparison among Ascorbic acid, AgNO3, plant extract, and AgNPs scavenging activity against DPPH free radicals. (b) Comparison among ascorbic acid, AgNO3, plant extract, and AgNPs scavenging activity against phosphomolybdate free radicals.
3.6.4 Phosphomolybdate activity
AgNPs showed the highest inhibition against phosphomolybdate than plant concentrations, and the highest inhibition shown by AgNPs at 40 µg/mL is 80.66 %. Ascorbic acid was taken as standard, as shown in Fig. 5b.
3.7 Antimicrobial assay
3.7.1 Antifungal activity
At a 40 µg/mL concentration, AgNPs exhibited the highest inhibition with a recorded antifungal activity of 77 % against Aspergillus niger, surpassing the effectiveness of the plant extract. The highest inhibition of Aspergillus flavous recorded at 40 µg/mL was 69 % as compared to plant extract (Fig. 6).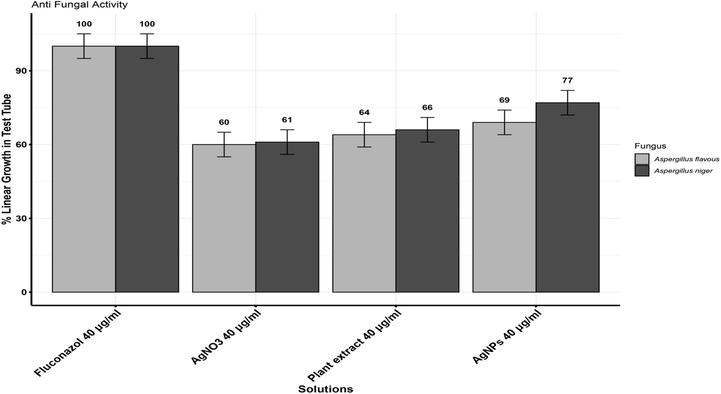
Effect of AgNPs and plant extract on the growth of Aspergillus niger and Aspergillus flavous.
3.7.2 Antibacterial activity
The antibacterial activity of plant extract and AgNPs with different concentrations is shown in Fig. 7. Clarithromycin was taken as standard. The zone of inhibition measuring 26 % and 40 µg/mL was the value observed as the highest against Staphylococcus aureus, 22 % against Micrococcus luteus, 18 % against E. coli, and 24 % against Klebsiella pneumonia. Fig. 8 shows a clear zone of inhibition.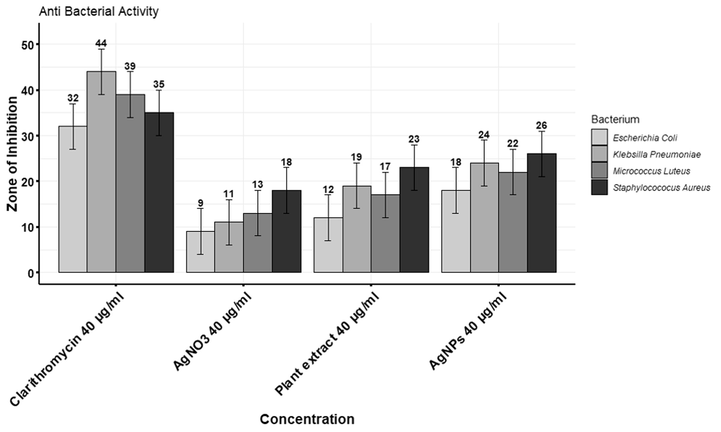
Comparison between effects of AgNPs and plant extract against the growth of four different bacterial strains.
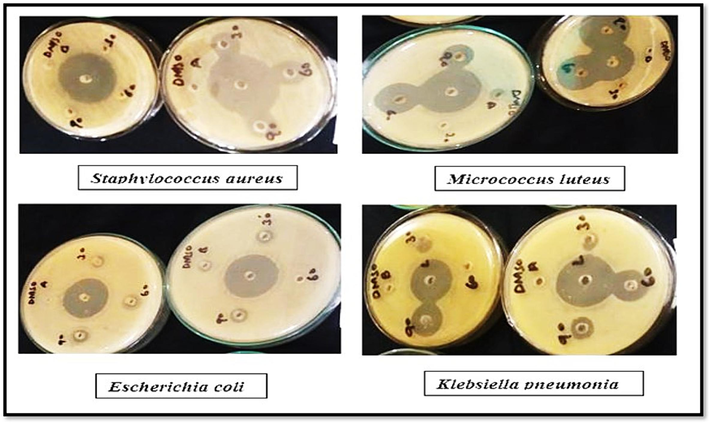
Zone of inhibition in Petri plates.
4 Discussion
In the last few decades, researchers have given primary focus to the green route for nanoparticle synthesis, which is still widely used and utilized in pharmaceutical markets. To investigate the biological potential of AgNPs for the treatment of various ailments, the plant Beta maritima was used in the current study's synthesis of AgNPs. The impact of the green synthesis process on the dimensions and uniformity of AgNPs has been demonstrated. Several organic extracts, including Cirsium japonicum, aloe vera, chamomile, and kiwi, have been employed as reducing agents in the AgNP synthesis (Devadharshini et al., 2023). The adjustment of the concentration of the extracts and the synthesis conditions allows for control over the size of the AgNPs (Fitriany et al., 2023). The extracts serve as reducing and stabilizing agents, leading to the production of diminutive and symmetrical AgNPs (Mallakpour & Amini, 2022).
UV–vis spectroscopy is employed for the primary or fundamental synthesized nanoparticles' characterization which monitors also and shows the stability of the nanoparticles. Dimension of the particles, chemical environment, and dielectric medium of the AgNPs are the factors on which surface plasmon resonance of the absorption peak is dependent. In the UV–Vis spectrophotometry analysis, the sample exhibited a definite peak value at 442 nm. The UV–vis absorption band of 420–450 nm was previously published, which is the evidence of surface plasmon resonance on AgNPs (Erenler et al., 2023b; Ramalingam et al., 2014). AgNP presence was demonstrated by peaks that ranged in size from 300 to 800 nm. XRD analysis further confirmed the UV–vis spectrophotometry results. The existence of different functional groups on the surface of the synthesized AgNPs was verified through FTIR analysis. For the synthesized silver nanoparticles, obtaining peaks were found, i.e., 605.49 cm-1 (CBr stretch) of the alkyl halide compound, 1529.10 cm-1 (N–H bend) with amide compound, 1695.55 cm- (C = O stretch), 2924.99 cm−1 (C–H stretch). In plant extract of (Beta maritima), obtained peaks were at 561.16 cm−1 (C-Br Stretch) Alkyle Halide compounds, at 688.30 cm−1 (=C–H bend) Alkenes Compounds, at 1526.96 cm−1 (N–H bend) having Amide compounds and at 1693.77 cm−1 found (C = O stretch) having Aldehyde compounds was found. The outcomes that were attained are reliable with the findings of the earlier report (Khane et al., 2022). SEM images of synthesized AgNPs depict the spherical-shaped AgNPs. The current findings demonstrated that AgNPs exhibited a spherical shape and high density, with most of them being dispersed while only a minority formed aggregates (Guzmán et al., 2009). AgNPs' tiny size allows them to enter the cells/microbes and execute their significant biological activities. Similar SEM results of green fabricated AgNPs have been published by (Gavade et al., 2015; Sher et al., 2020). EDX analysis was employed to determine the occurrence of the element Ag in AgNPs with the elemental constituents of silver, oxygen, and carbon maintained at proportions of 80.38 %, 12.60 %, and 5.45 %, respectively, which specifies that Ag ions are reduced to AgNPs. The EDX spectrum showcases prominent signals indicating the presence of metallic Ag, which aligns with the results of earlier research (Bhakya et al., 2016; Ranjan et al., 2014). The XRD spectrum analysis showed 38.31˚, 46.49˚, 64.68˚, and 77.60˚ at 2θ degrees found the nanoparticles, with an average size of 71 nm, had a centered cubic shape (Sahin Yaglioglu et al., 2022), which closely resembled the Joint Committee on Powder Diffraction Standards' stated reference value (JCPDS PDF no:89–3722) (Bhakya et al., 2016).
Different activities were done to check the therapeutic capacity of synthesized AgNPs. The H2O2 ABTS, DPPH, and phosphomolybdate scavenging activities were found to be highest as compared to a plant extract and pure AgNO3 salt. These outcomes demonstrated that AgNPs had a great potency of antioxidants against the extracts of plants (Adebayo et al., 2019; Bhakay et al., 2016; Kanipandian et al., 2014). Free radicals gain electrons from antioxidant compounds, such as AgNPs, which changes in absorbance can be quantitatively measured (Mensor et al., 2001). Free radicals are responsible for the development of several illnesses, including aging, Parkinson’s disease, neurological conditions, and moderate cognitive impairment. The results indicate that free radicals gain hydrogen or electrons from the AgNPs and become stable. These findings have been confirmed by (Carlson et al., 2008; Ramalingam et al., 2014), who showed that AgNPs interact with reactive oxygen species uniquely way within cells (ROS). This outcome was also confirmed by the findings of (Guntur et al., 2018), who observed a considerable increase in AgNP content indicated a strong scavenging ability. Our findings are supported by (Kumar et al., 2022; Reddy et al., 2013).
On the other hand, synthesized AgNPs were also tested for their anti-microbial potential. The highest inhibition value was recorded against gram-positive bacteria, whereas clarithromycin was used as a standard. The highest potency against gram-positive is because of the compositions of gram-positive and gram-negative cell walls, which are composed of thick structural layers of peptidoglycans. The higher activity of bacterial strains is due to the presence of silver cations, which act as a reservoir for the Ag+, which helps in penetrations in bacterial agents.
In the coming years, we may see the integration of green-synthesized silver nanoparticles across diverse fields, emphasizing sustainability and environmental impact. This includes advancements in biomedical applications, antimicrobial solutions, nano-bio interfaces, catalysis and energy, electronics and sensors, water purification, environmental remediation, and collaborative research with multidisciplinary approaches. In summary, upcoming research should prioritize understanding the intricate mechanisms behind metal nanoparticle formation, developing engineered nanoparticles with minimal toxicity, enhanced health benefits, and precise size and shape control. Moreover, there is a need to expand the utilization of plant-based metallic nanoparticles in related fields (Nadaf et al., 2022).
5 Conclusions
The current study concluded that the synthesis of AgNPs from Beta maritima (Vulgaris) plant extract can be beneficial over other methods because plant products are easier and safer to handle, cost-effective, widely distributed, easily available, and eco-friendly. The significance of nanomaterial research is closely correlated with nanoparticle dimensions. Since these particles are smaller in size, have been proven to play a more significant role. The present study, conferring with various recent literature, has demonstrated the medicinal value of AgNPs and describes them as a promising and good source of antioxidants and antimicrobials.
Consent to participate
All authors consent to participate in the manuscript publication
Consent for publication
All authors approved the manuscript to be published
CRediT authorship contribution statement
Abdur Rahman Khan: Writing – original draft, Methodology, Data curation. Mushtaq Ahmed: Visualization, Validation, Investigation, Formal analysis, Data curation. Hajra Khan: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis, Data curation. Nehal Abdel-Hamid Kamel Osman: Writing – review & editing, Visualization, Validation, Formal analysis, Data curation. Abdel-Rhman Z Gaafar: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis, Data curation. Tahira Shafique: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis, Data curation, Conceptualization.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2024R686), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Silver nanoparticles: synthesis methods, bio-applications and properties. Crit. Rev. Microbiol.. 2016;42(2):173-180.
- [Google Scholar]
- Antimicrobial and antioxidant activity of silver, gold and silver-gold alloy nanoparticles phytosynthesized using extract of Opuntia ficus-indica. Rev. Adv. Mater. Sci. 2019;58(1):313-326.
- [Google Scholar]
- Phyto-Extract-Mediated Synthesis of Silver Nanoparticles (AgNPs) and Their Biological Activities. Biomed Res. Int.. 2022;2022:1-10.
- [CrossRef] [Google Scholar]
- Rapid peroxyl radical scavenging capacity (PSC) assay for assessing both hydrophilic and lipophilic antioxidants. J. Agric. Food Chem.. 2005;53(17):6572-6580.
- [Google Scholar]
- Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif. Cells Nanomed. Biotechnol.. 2017;45(6):1192-1200.
- [Google Scholar]
- Incorporation of fenofibrate nanoparticles prepared by melt emulsification into polymeric films. J. Pharm. Innov.. 2016;11:53-63.
- [Google Scholar]
- Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci.. 2016;6:755-766.
- [Google Scholar]
- Physical education and academic achievement in elementary school: data from the early childhood longitudinal study. Am. J. Public Health. 2008;98(4):721-727.
- [Google Scholar]
- Silver Nanomaterials Applied in the Field of Diagnosis and Treatment. Theor. Nat. Sci.. 2023;4(1):101-109.
- [CrossRef] [Google Scholar]
- d Steel, R. G., & Torrie, J. H. (1986). Principles and procedures of statistics: a biometrical approach. McGraw-Hill New York, NY, USA.
- Green Synthesis of Silver Nanoparticles. Microbiology Research Journal International. 2023;33(5):1-9.
- [CrossRef] [Google Scholar]
- Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J. Ethnopharmacol.. 2009;123(3):494-498.
- [Google Scholar]
- Biosynthesis, Characterisation, and Antioxidant Activity of Silver Nanoparticles using Schinus molle L. Trends in Sciences. 2023;20(10):6105.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Stachys spectabilis: Identification, catalytic degradation, and antioxidant activity. Biochem. Biophys. Res. Commun.. 2023;659:91-95.
- [Google Scholar]
- Green Synthesis AgNPs menggunakan Bioreduktor Alami Ekstrak Buah Kiwi: Biosintesis, dan Karakterisasi. Justek : Jurnal Sains Dan Teknologi. 2023;6(1):162.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles by using carambola fruit extract and their antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol.. 2015;6(4):045015
- [Google Scholar]
- Biogenic synthesis of silver nanoparticles using Echium vulgare: Characterisation, quantitative analysis of bioactive compounds, antioxidant activity and catalytic degradation. J. Indian Chem. Soc.. 2023;100(5):101003
- [CrossRef] [Google Scholar]
- Persistence of Silver Nanoparticles Sorbed on Fresh-Cut Lettuce during Flume Washing and Centrifugal Drying. J. Food Prot.. 2023;86(6):100097
- [CrossRef] [Google Scholar]
- In vitro studies of the antimicrobial and free-radical scavenging potentials of silver nanoparticles biosynthesized from the extract of Desmostachya bipinnata. Anal. Chem. Insights. 2018;13 1177390118782877
- [Google Scholar]
- Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Biomol. Eng.. 2009;2(3):104-111.
- [Google Scholar]
- Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie. Trop. J. Pharm. Res.. 2017;16(4):743-753.
- [Google Scholar]
- Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater. Res. Bull.. 2014;49:494-502.
- [Google Scholar]
- Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomed. Nanotechnol. Biol. Med.. 2005;1(2):101-109.
- [Google Scholar]
- Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules. 2020;10(6):835.
- [Google Scholar]
- Green synthesis of MnO nanoparticles using abutilon indicum leaf extract for biological, photocatalytic, and adsorption activities. Biomolecules. 2020;10(5):785.
- [Google Scholar]
- Phytomolecules-coated NiO nanoparticles synthesis using abutilon indicum leaf extract: antioxidant, antibacterial, and anticancer activities. Int. J. Nanomed. 2021:1757-1773.
- [Google Scholar]
- Contact lenses coated with hybrid multifunctional ternary nanocoatings (Phytomolecule-coated ZnO nanoparticles: Gallic Acid: Tobramycin) for the treatment of bacterial and fungal keratitis. Acta Biomater.. 2021;128:262-276.
- [Google Scholar]
- Green synthesis of silver nanoparticles using aqueous Citrus limon zest extract: Characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials. 2022;12(12):2013.
- [Google Scholar]
- Kumar, G. C. A., Bodke, Y. D., Manjunatha, B., Satyanarayan, N. D., Nippu, B. N., & Muthipeedika, N. J. (2022). Novel synthesis of 3-(Phenyl)(ethylamino) methyl)-4-hydroxy-2H-chromen-2-one derivatives using biogenic ZnO nanoparticles and their applications. Chimica Techno Acta. 2022. Vol. 9.№ 1, 9(1).
- Green synthesis of Ag ultra-fine nano-catalyst supported on layered double oxide and chitosan: Accelerated reduction of 4-nitrophenol to 4-aminophenol. J. Clean. Prod.. 2022;381:135154
- [CrossRef] [Google Scholar]
- Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnol.. 2021;3:679358
- [Google Scholar]
- In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem. Toxicol.. 2006;44(2):198-206.
- [Google Scholar]
- Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res.. 2001;15(2):127-130.
- [Google Scholar]
- Green synthesis of gold and silver nanoparticles: Updates on research, patents, and future prospects. OpenNano. 2022;100076
- [Google Scholar]
- Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods. 1981;46(2):211-226.
- [Google Scholar]
- Biosynthesis of silver nanoparticles from deep sea bacterium Pseudomonas aeruginosa JQ989348 for antimicrobial, antibiofilm, and cytotoxic activity. J. Basic Microbiol.. 2014;54(9):928-936.
- [Google Scholar]
- Nanoscience and nanotechnologies in food industries: opportunities and research trends. J. Nanopart. Res.. 2014;16:1-23.
- [Google Scholar]
- 2, 2-Diphenyl-1-Picrylhydrazyl Free Radical Scavenging Assay and Bacterial Toxicity of Protein Capped Silver Nanoparticles for Antioxidant and Antibacterial Applications. Asian J. Chem.. 2013;25(16)
- [Google Scholar]
- Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater.. 2008;4(3):707-716.
- [Google Scholar]
- Biosynthesis of Silver Nanoparticles Using Astragalus flavesces Leaf: Identification, Antioxidant Activity, and Catalytic Degradation of Methylene Blue. J. Inorg. Organomet. Polym Mater.. 2022;32(10):3700-3707.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Olea europaea leaf extract for their enhanced antibacterial, antioxidant, cytotoxic and biocompatibility applications. Int. J. Mol. Sci.. 2021;22(22):12562.
- [Google Scholar]
- Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci.. 2009;145(1–2):83-96.
- [Google Scholar]
- Calligonum polygonoides reduced nanosilver: A new generation of nanoproduct for medical applications. Europ. J. Integr. Med.. 2020;33:101042
- [Google Scholar]
- Mechanism and antibacterial activity of gold nanoparticles (AuNPs) functionalized with natural compounds from plants. Pharmaceutics. 2022;14(12):2599.
- [CrossRef] [Google Scholar]
- Biological reduction of silver nanoparticles using Cassia auriculata flower extract and evaluation of their in vitro antioxidant activities. Nanosci Nanotechnol Int J. 2012;2(4):30-35.
- [Google Scholar]
- Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med.. 2004;36(7):838-849.
- [Google Scholar]
- Application of Silver Nanoparticles in Parasite Treatment. Pharmaceutics. 2023;15(7):1783.
- [CrossRef] [Google Scholar]







