Translate this page into:
Histology and radiography studies of effects of Lepidium sativum seeds on bone healing in male albino rats
⁎Corresponding author. mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Lepidium sativum (garden cress) is a folk medicine that exhibited several therapeutic applications including anti-asthma, antihypertension, and bone healing. This study aimed to investigate the effects of L. sativum seeds on healing of fractured bone in male albino rats (Rattus rattus albinus). Twenty male albino rats were randomly divided into four groups (each group contains 5 rats); Non-Broken Control (NBC), Broken Control Non-Treated (BCNT), Broken Treated with 1 g/kg of seed powder (BT1g), and Broken Treated with 5 g/kg of seeds powder (BT5g). Right tibial closed fractures were created in the rats using a three-point bending technique. Serum Ca2+ level and Alkaline phosphatase tests were examined in all groups. Digital radiography was applied for three times, at the beginning, after 3 weeks, and 6 weeks. Histopathological study was done for right limb tibia bones. Results showed that the highest level of ALP was in the BT5g group after 3 weeks. However, after 6 weeks ALP level was the lowest in the BT5g group followed by the BT1g group. Serum Ca2+ level was higher in BT5g group after 6 weeks as compared to same group after 3 weeks. The X-ray photography shows that in treatment groups a clear improvement in bone healing that was revealed by an obvious callus and to a large extent a blurred fracture line. We also observed that after 6 weeks, the healing and recovery of broken bones were almost completed in L. sativum treated groups, especially in the BT5g group. Histology data further confirm the distinctive features of bone healing after feeding of seeds powder. Overall, our data suggested that seed of L. sativum has potential to speed up the callus formation, fracture line repairing, and new bone regeneration. This study highlights the importance of folk herbs in healing of fractured bones.
Keywords
Lepidium sativum seeds
Bone healing
ALP
Serum calcium
Histology
Radiography

1 Introduction
Fractures are one of the most recurrent injuries of the body‘s skeletal system. Bone fractures are a public health issue worldwide and pose a serious burden to the society and country (Watts et al., 2013; Ahamed et al., 2022a). Bone fractures can cause disability, diseased productivity, health loss, and a major burden to individuals, families, and healthcare systems (Nandra et al., 2016; Claes et al., 2012). Although treatment of bone fracture has upgraded substantially in recent decades, a large percentage of all fractures still show delayed healing and complications. Bone healing is a proliferation physiological process that enables the repair of a fractured bone. This process is characterized by secretion of a new bone organic matrix called osteoid and its subsequent mineralization, thus, the gap between two bony fragments is bridged (Davis et al., 2004; Ahamed et al., 2021a). Since several decades, numerous herbs have been widely used as traditional medicine for the treatment of several diseases. For instance, Stellate ganglion is being used for healing of fractured bones (Kizilay et al., 2020).
Lepidium sativum (garden cress) herb belongs to the Cruciferae family cultivated in Yemen, Saudi Arabia, Egypt, and Asia, Europe, and USA is an underutilized crop (Mahassni and Khudauardi, 2017). The L. sativum seeds are rich in protein, fiber, lipid, omega-3, iron, calcium, phosphorus, essential amino acids, and various phytochemicals. Several products of L. sativum seeds are being used either as a health drink or food integrated (Doke and Guha, 2014). It has been found that L. sativum seeds increase complete blood counting and hemoglobin level as well as increase the weight of spleen and total body weight (Ahsan et al., 1989; Ahamed et al., 2021b).
L. sativum seeds also recommended to treat inflammation, bronchitis, muscular pain, rheumatism (Mahassni and Khudauardi, 2017). Furthermore, L. sativum seeds had been found are significantly have anti-pyretic, analgesic and coagulant activities. Interestingly, L. sativum seeds have shown minimum side-effects (Attia et al., 2019). Hence, L. sativum seeds can be used as herbal medicines for bone healing and other diseases. This study aimed to investigate the effects of L. sativum seeds on the bone healing of an induced bone fracture in male albino rats (Rattus rattus albinus). This study determined the degree of callus formation and the degree of fracture line repairing potential of L. sativum seeds. Histopathological changes related to bone fracture and healing after L. sativum seeds feeding were also examined.
2 Materials and methods
2.1 Plant collection
Plant seeds were purchased from local market of Dhamar city, Yemen. The seeds were shade dried and grounded into fine powder (Ahamed et al., 2022b).
2.2 Preparation of L. sativum seeds powder dosages
Two dosages of dried powder of L. sativum seeds were prepared; a high concentration of 5 gm/kg BW and a low concentration of 1gm/kg BW. Seeds powder dosages were mixed with the daily meals of rats.
2.3 Experimental animals
Twenty male albino rats (Rattus rattus albunus) (weight 100–160 gm) were utilized in this study. Rats were supplied from the animal house, science faculty of Sanaà University, Yemen. They were housed in stainless steel cages containing husk as the bedding material under the condition of 25 °C with 12 h light-dark cycle. Animal experiments were carried out according to the “Guide for the Care and Use of Laboratory Animal” (Clark et al., 1996). The number of animals in this experiment has been carefully selected from the outset to avoid unnecessary increases and the animal handling was done as gently as possible to avoid pain. Also, an appropriate quantity of anesthesia was used at the time of dissection.
2.4 Animal feeding
Animals were fed on the diet formula supplied by the Department of Animal Production, Faculty of Veterinary, Thamar University, Yemen. The diet formula consisting of corn 30%, ssoybean 8%, wheat bran 7%, wheat 25%, and dried fish as a source of animal protein 10%, ssorghum stover 20%, and 1 teaspoonful of vegetable oil mixed with 3.5 kg of the above stock. The ingredients were supplemented with a suitable dose of multivitamins and minerals. The diet was prepared as pellets (Al-Amri, Veterinary Manufacturing Comp. Dhamar, Yemen). Rats received 100 gm/day of the dried pellets.
2.5 Experimental design
Twenty male albino rats were randomly divided into four groups (each group contains 5 rats); Non-Broken Control (NBC, Group 1), Broken Control Non-Treated (BCNT, Group 2), Broken Treated with 1 g/kg of seed powder (BT1g, Group 3), and Broken Treated with 5 g/kg of seeds powder (BT5g, Group 4). All rats received L. sativum of the standard pellets and tap water during the adaption period. Rats weight, experiment samples, and radiographs were studied at three time intervals; first day (initial period), after 3 weeks (mid period), and after 6 weeks (end period).
2.6 Bone broken induction
Rats were anesthetized with appropriate amount of chloroform (Dehkohneh et al., 2019). Right tibial closed fractures were created in the rats by using a three point bending technique as described earlier (Xu et al., 2020). Bone fractures were further stabilized with gypsum (Türkmen et al., 2017).
2.7 Blood samples collection
Samples were collected in the three defined experiment periods (first day, 3 weeks, and 6 weeks). Blood samples from each group were carefully collected from eye canthus of rats without anticoagulants using the heparinized micro-haematocrit capillary tubes. Serum was obtained after centrifugation of blood (3000 rpm for 10 min). Serum samples were immediately used for biochemical studies.
2.8 Biochemical assays
Serum Ca2+ levels were detected using commercial kit (SpinReact, Girona, Spain). Briefly, 10 µl serum was mixed with 1 ml of R solution (Imidazol Buffer pH 6, 5–100 mmol/L). At the optimal assay conditions (Cuvette 1 cm light path, Constant temperature 37 °C for 10 min and the adjust the instrument to zero with distilled water) the absorption of samples was recoded at 660 nm using Rayto Chemray-240 Spectrophotometer.
Alkaline phosphatase (ALP) assay was performed as per the protocol of Rosalki et al. (1993).
2.9 Radiography
Digital radiography of different group of rats was performed at Al-Mosali Hospital, Dhamar, Yemen. Radiographs were taken by XVISION-525 system (Korea) under 50 kV and 5 mA. The first set of radiograph was taken after first day of broken right tibia bone. The second set of radiograph was taken after three weeks of treatments. The third and final set of radiograph was recorded the rats right tibia bone after six weeks of treatment of L. sativum seeds. The digital radiographs was used to observe the bone fracture line, bone callus, and bone healing.
2.10 Histopathology
For histopathological study, rats were sacrificed and right limb tibia bones were removed from different groups for different trial periods of the treatment. The right limb tibia of each sample was labeled and fixed in 10% formalin. Tibias were decalcified with 0.5 M EDTA (pH8) and embedded in paraffin for standard histological procedures. Microtome sections were cut at 5 μm and stained with hematoxylin and eosin (H and E) (Takikawa et al., 2001). Images were captured at SINHER microscope at 40× magnification.
2.11 Statistical analysis
Quantitative data among groups were analysed by using one-way analysis of variance (ANOVA) followed by Dunnette‘s comparison tests. The significant difference between groups was accepted at p < 0.05.
3 Results
3.1 Body weight
As shown in Table 1, after 3 weeks from the beginning of this experiment, the rat's body weight was increased in all the groups and the percentage of increment was higher in the groups treated with L. sativum seeds than those of the control groups. Likewise, at the end of the experiment (after 6 weeks), the percentage of body weight gain was higher in the seed treatment groups as compared to the control groups. NBC = Non-Broken control. BCNT = Broken control-non treated. BT1g = Broken- treated with 1 g BT5g = Broke- treated with 5 g. N = Number of albino rats per group. A = P-value comparison significance between NBC and other groups. C = P- value comparison significance between the same group on the initial day and the end of 3 weeks. D = P- value comparison significance between the same group at the end of 3 weeks and the end of 6 weeks. + = an increase in body weight. % of change of the mean = (Final weight − Initial weight)/Initial weight. * % of change between the initial day and the end of the 3rd week. **% of change between initial day and the end of 6th week.
Period
GroupsInitial day
(Mean ± SD) gEnd of 3rd week
(Mean ± SD) g% of change in body weight mean
End of 6th week
(Mean ± SD) g% of change in body weight mean
NBC
N = 5154.00 ± 5.87
179.00 ± 19.78
+16.23*
202.40 ± 11.32
+31.42**
P value
C = 0.049
BCNT
N = 5151.80 ± 5.40
183.60 ± 25.21
+20.94*
222.00 ± 17.20
+46.24**
P value
C = 0.013
D = 0.003
BT1g
N = 5128.20 ± 18.36
195.40 ± 25.51
+52.41*
233.80 ± 22.92
+82.37**
P value
C = 0.001
A = 0.015; D = 0.003
BT5g
N = 5153.60 ± 6.65
207.40 ± 27.93
+35.02*
239.80 ± 27.30
+56.11**
P value
A = 0.026; C = 0.001
A = 0.004; D = 0.012
3.2 ALP levels
The comparison of the changes of ALP levels in rats serum between the experimental groups is shown in Table 2. The highest value of ALP level was at the end of the 3rd week in comparison with the rest of the periods in the all treatment groups (p < 0.001) in comparison with the NBC group. Moreover, at the end of the 3rd week, the highest level of ALP was in the BT5g group (524.80 ± 73.13) with a significance of 0.001, followed by the level in the BT1g group (412.80 ± 44.46) with the significance of 0.040, all in a comparison with the BCNT group. However, the ALP level in the BCNT group was the lowest among the treated groups in this period (361.40 ± 24.24). Generally, after six weeks of treatment and healing, the ALP level declined as is shown in Table 2. The highest decline in this level was in the BT5g group (307.40 ± 39.25) with a significance of 0.001 followed by the level in the BT1g group (322.00 ± 49.56) with a significance of 0.001, all compared to the levels after three weeks of the experiment, while ALP level in BCNT group remained high (366.40 ± 46.05) close to the level found at the end of the third week. NBC = Non-Broken control. BCNT = Broken control-non treated. BT1g = Broken- treated with 1 g BT5g = Broken- treated with 5 g. N = Number of albino rats per group. A = P-value comparison significance between NBC and other groups. B = P-value comparison significance between BCNT and other groups. C = P- value comparison significance between the same group on the initial day and the end of 3 weeks. D = P- value comparison significance between the same group at the end of 3 weeks and the end of 6 weeks.
Period
GroupsInitial day
(Mean ± SD)End of 3rd week
(Mean ± SD)End of 6th week
(Mean ± SD)
NBC
N = 5165.40 ± 28.66
194.80 ± 25.05
181.00 ± 34.08
BCNT
N = 5182.00 ± 13.60
361.40 ± 24.24
366.40 ± 46.05
P value
A = 0.001;
C = 0.001A = 0.001;
D = 0.001
BT1g
N = 5164.80 ± 27.06
412.80 ± 44.46
322.00 ± 49.56
P value
A = 0.001;
B = 0.040;
C = 0.001A = 0.001;
D = 0. 001
BT5g
N = 5171.00 ± 11.13
524.80 ± 73.13
307.40 ± 39.25
P value
A = 0.001;
B = 0.001;
C = 0.001A = 0.001;
B = 0.019;
D = 0.001
3.3 Serum Ca2+ level
Serum Ca2+ analysis did not reveal any significant difference at the end of the 3rd week of the treatment in all bone broken groups in comparison with the NBC group, as seen in Table 3, despite there was a slight increase found in L. sativum seeds treated groups. However, at the end of the 6th week, the serum Ca2+ level was increased more than the level at the end of the 3rd week in L. sativum seeds treated groups with significant values in BT5g group, 0.042 and 0.009 in comparison with NBC group and BCNT group, respectively (Table 3). NBC = Non-Broken control. BCNT = Broken control-non treated. BT1g = Broken- treated with 1 g BT5g = Broken- treated with 5 g. N = Number of albino rats per group. A = P-value comparison significance between NBC and other groups. B = P-value comparison significance between BCNT and other groups.
Period
GroupsInitial day
(Mean ± SD)End of 3rd week
(Mean ± SD)End of 6th week
(Mean ± SD)
NBC
N = 58.98 ± 0.79
8.88 ± 0.49
8.92 ± 0.41
BCNT
N = 58.40 ± 0.56
8.78 ± 0.64
8.66 ± 0.58
BT1g
N = 58.52 ± 0.96
9.24 ± 0.11
9.42 ± 0.35
BT5g
N = 58.76 ± 0.98
9.02 ± 0.56
9.76 ± 0.58
P value
A = 0.042;
B = 0.009
3.4 Radiography study
The radiographic examination using the X-Ray method was applied to show the differences in the bone healing features, most important were callus formation and fracture line, among the three studied periods in this study. On the initial day, X-ray photographs in all broken groups have shown the same appearance in which the callus was absent and the fracture line was visible and clear as shown in Fig. 1.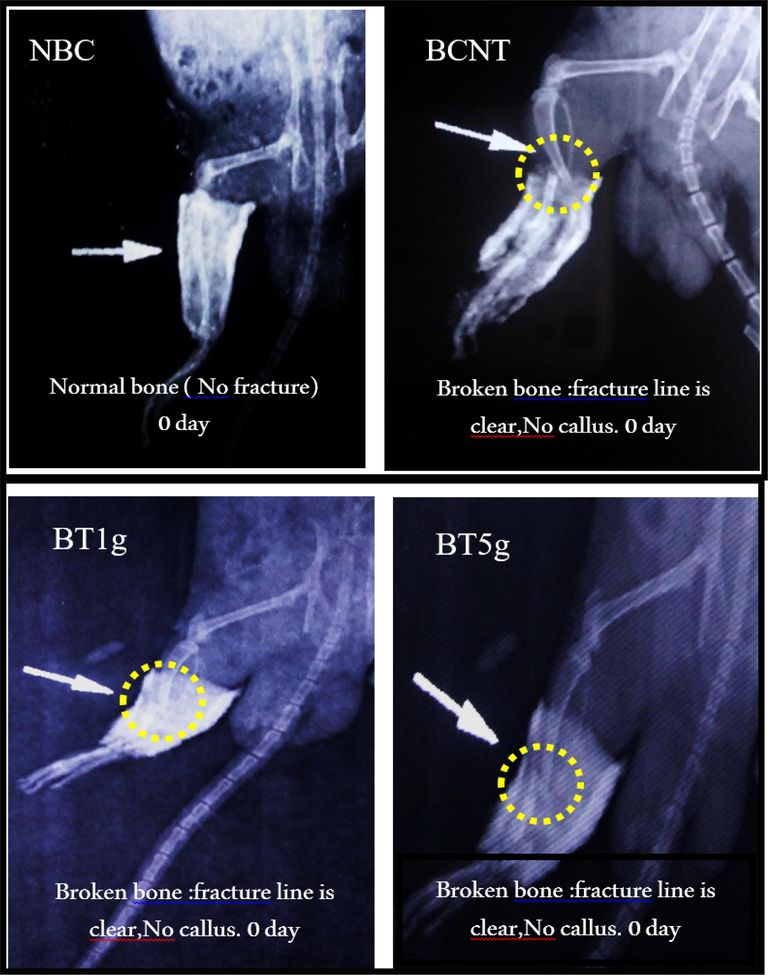
X-ray photos at initial day of tibial fracture induced in male albino rats. NBC = Non-Broken control; BCNT = Broken control-non treated; BT1g = Broken- treated with1g. BT5g = Broken- treated with 5 g; Yellow circle = the zone of induced fracture; White arrow indicate the fracture line.
After 3 weeks of treatment by L.sativum seeds, X-ray photographs in treatment groups showed a clear improvement in bone healing steps that revealed by an obvious callus and to a large extent a blurred fracture line, whereas in BCNT the callus was not clear and the fracture line remained visible. Furthermore, the progress of bone healing that appeared in the photographs was better in the BT5g group than in the BT1g group (Fig. 2). After six weeks of the treatment (Fig. 3), the features of healing and recovery of broken bones were less or more completed in L.sativum treated groups, especially in the BT5g group. In contrast, the broken bone in the BNCT group was still with a clear fracture line.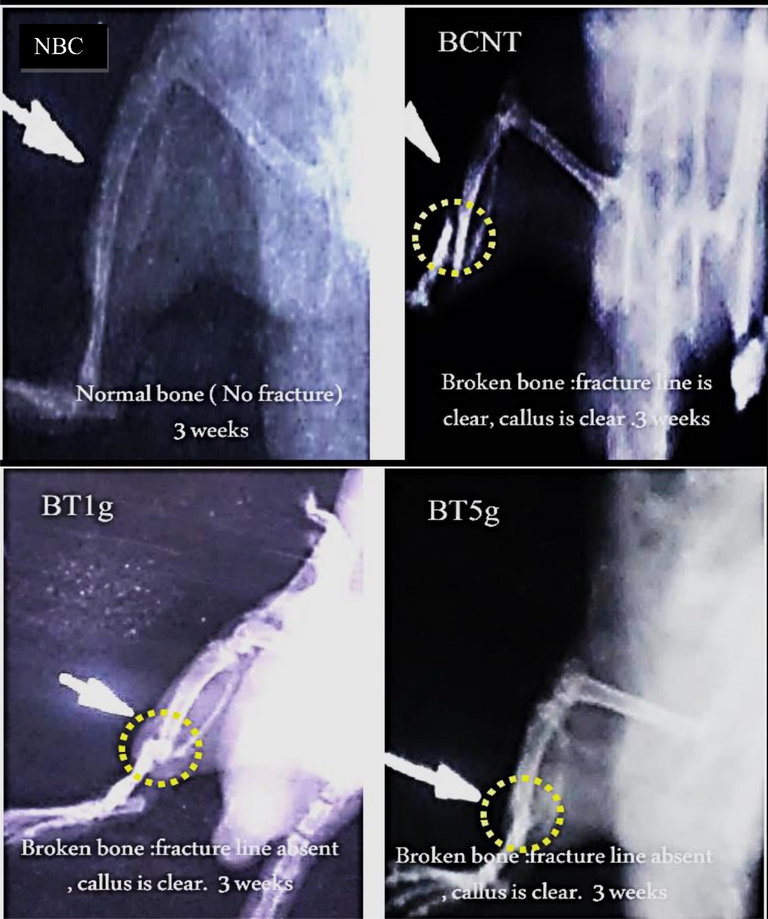
X-ray photos after three weeks of tibial fracture induced in male albino rats. NBC = Non-Broken control; BCNT = Broken control-non treated; BT1g = Broken- treated with1g. BT5g = Broken- treated with 5 g; Yellow circle = the zone of induced fracture; White arrow indicate the fracture line.
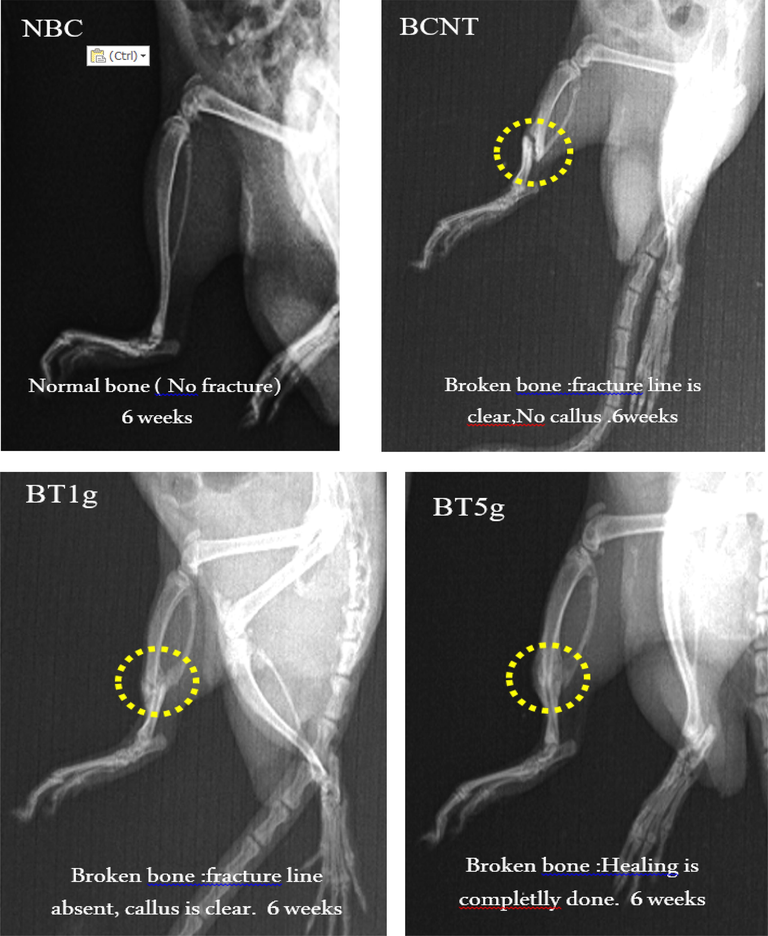
X-ray photo after 6 weeks of tibial fracture induced in male albino rats. NBC = Non-Broken control; BCNT = Broken control-non treated; BT1g = Broken- treated with1g. BT5g = Broken- treated with 5 g; Yellow circle = the zone of induced fracture; White arrow indicate the fracture line.
3.5 Histopathological study
The histopathological results of this study revealed the ameliorated effect of L. sativum seeds on bone healing in dose depending manner. In comparison to the BNTC group after 3 weeks of treatment, the distinctive features of bone healing like new bone formation, display of collagen fibers, repair of the fracture line, and bone organization, were fast and more clear in the BT5g group followed by BT1g group (Fig. 4).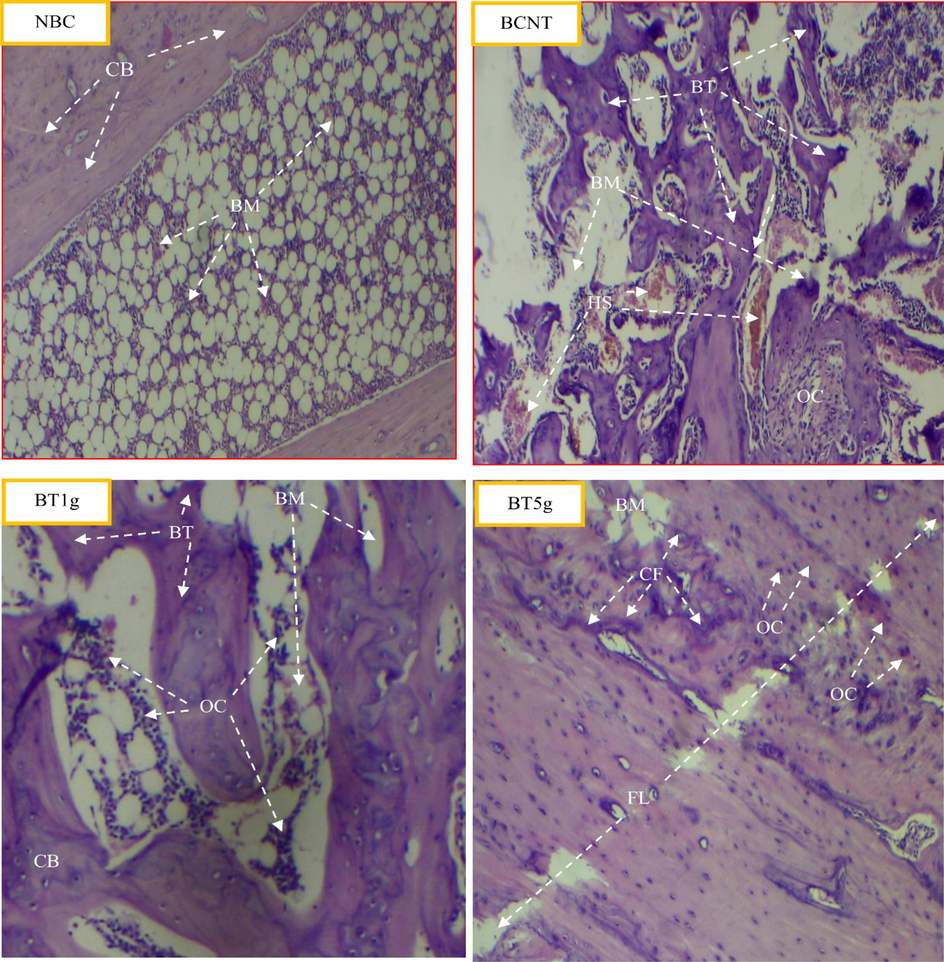
Light photomicrographs show the histological effect of L.sativum seeds on tibial fracture induced in male albino rats after three weeks compared to control groups. NBC = Non-Broken control; BCNT = Broken control-non treated; BT1g = Broken- treated with1g. BT5g = Broken- treated with 5 g. BM = bone marrow. BT = new bone formation. CB = compact bone. CF = collagen fibers. FL = fracture line. HS = Hamate stage. OC = osteocyte.
After 6 weeks of the treatment, the fractured bone in the BT5g group shown a high degree of bone healing in which the fracture line was close to being completely repaired and the processes of healing like new bone formation and display of collagen fibers were less, all indicate close healing termination. Progressing of the healing process was less in the BT1g group, whereas the healing in the BNTC group was slow (Fig. 5).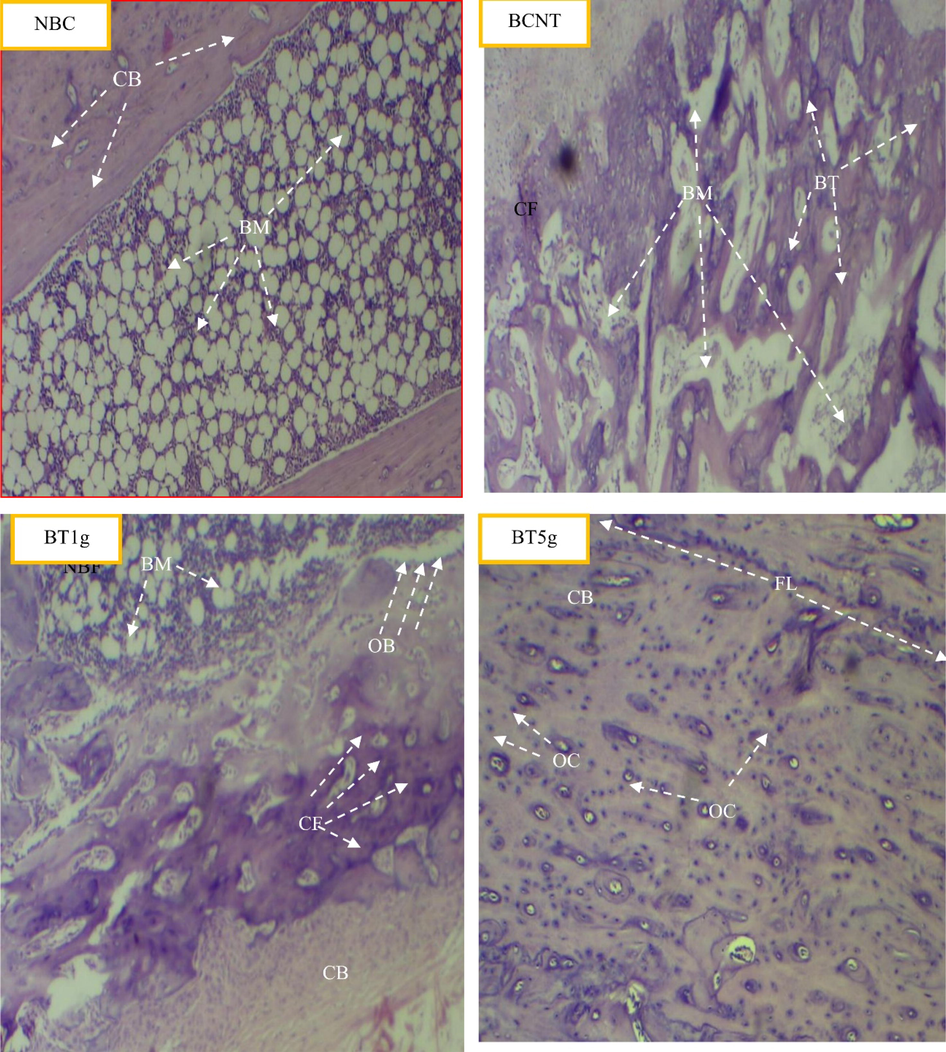
Light photomicrographs show the histological effect of L.sativum seeds on tibial fracture induced in male albino rats after six weeks compared to control groups. NBC = Non-Broken control; BCNT = Broken control-non treated; BT1g = Broken- treated with1g. BT5g = Broken- treated with 5 g. BM = bone marrow; BT = new bone formation; CB = compact bone; CF = collagen fibers; OB = Osteoblast; OC = osteocyte.
4 Discussion
The present study shows the effect of L. sativum seeds treatment increases the body weight. This result was in agreement with the earlier study where male swiss albino mice given extract of L. sativum seeds (0.5 ml and 1 ml daily) orally for twenty-one days (Mahassni and Khudauardi, 2017). Authors found a significant increase in body weight in the high dose group of L. sativum as compared to the control group. (Elshal et al., 2013). Furthermore, our result has shown a significant increase in serum calcium level after six weeks of the treatment in the BT5g group, which is in according with the study of Jadhav et al. (2016) that found association of high levels of calcium and phosphorus with the increase in the body weight in chicks. The increase of rat's body weight and serum calcium level can be attributable to L. sativum components because this herb contains high levels of protein as well as the seeds are a good source of essential minerals, such as potassium, phosphorus, calcium, and iron. Additionally, L. sativum seeds oil contains high levels of unsaturated fatty acids such as the essential fatty acid linoleic acid and linolenic acid (Agarwal and Sharma, 2013).
Our results have shown a significant increase of alkaline phosphatase level after three weeks of the treatment in the BT5g group that followed by the BT1g group. After six weeks, the ALP level decreased in the BT5g group followed by the BT1g group in comparison to the BCNT group. This result agrees with Sipani et al. (2020) that recorded an increase in ALP level in the 3rd week of bone fracture induction. Ajai et al. (2013) also observed an increase in ALP level from 4th to 6th weeks, while HangPham et al. (2017) found increament of ALP level after 5th week of L. sativum treatment.
As documented previously, ALP is an important component in hard tissue formation and is highly expressed in mineralized tissue cells (Golub and Boesze-Battaglia, 2007). It is also found in osteoblast plasma membrane before extracellular release, correlates with bone surveys, and with parameters of bone resorption (Ajai et al., 2013). Moreover, ALP elevation is correlated with the increase in the osteoblastic activity that occurs in bone growth, bone healing, and acromegaly. The ALP effect of proliferation and differentiation of osteoblasts, osteocytes, and macrophages is by the regulation of the different enzymes at bones healing duration in which osteoblasts release large quantities of ALP, which help in bone matrix formation and its mineralization. Besides, ALP is acting to increase the local concentration of inorganic phosphate, a mineralization promoter (Golub and Boesze-Battaglia, 2007; Ahamed et al., 2020).
In this study, serum Ca2+ level had no significant change in L. sativum treated groups, whereas at the end of the 6th week in the BT5g group. Serum Ca2+ was elevated when the bone fracture was nearly healed that may indicate the role of the plant as a source of calcium. This result is supported by other study that observed the lower concentration of serum Ca2+ in non L. sativum treated group comparing to L. sativum treated groups (Elshal et al., 2013). Besides, Paskalev et al. (2005) found a decrease in the serum Ca2+ during osteosynthesis and bone mineralizing. The L. sativum seeds are good source of linoleic acid, which has been shown to inhibit bone reabsorption and resistant the Ca2+ elimination. Another study also reported higher serum Ca2+ level upon complete healing of fractured bone (Claassen et al., 1995).
Radiographic examination of the callus formation and fracture line repairing in the fracture tibia bone in this study illustrated the speed up healing effect of L. sativum in the treated groups, especially in the BT5g group which showed the best result of healing at the end of this experiment. These results confirm the dose-dependent ameliorated the effect of this plant on bone heating. The callus is a soft external bridge that occurs in bone fracture healing which gradually hardens over time and restores the structural stiffness of the bone (Schwarzenberg, 2019). Similar to this work, Yadav and co-workers (2011) studied the effect of L. sativum seeds extract (400 mg/kg) on rats using X‐ray photography for eight weeks and found a significant increase in callus formation in the plant treated group compared to the control group. Another group studied the effect of L. sativum on fracture healing in the left femur of rabbits by X‐Ray photography after 6 and 12 weeks’ post-operation and found significant healing of fractures (Bin Abdullah Juma, 2007). Our results along with earlier studies it can be suggested that L. sativum seeds play an important role in promoting and accelerating callus formation in fractures and regarded as a beneficial recipe in conventional medicine for bone healing.
Histopathological analysis of the present study has also indicated L. sativum seeds promotes faster bone healing by collagen deposition at the fracture sites of bone. As reported previously, when a new bone is deposited, some of the osteoblasts are embedded in the mineralizing collagen matrix and differentiate to osteocytes, forming a dense network throughout the whole bone tissue (Kerschnitzki et al., 2011). Assisted role of L. sativum seeds in bone remoulding and regeneration may attribute to its compounds such as phytoestrogens which exist in higher quantities of this medicinal plant (Sirotkin and Harrath, 2014).
5 Conclusion
Radiography, histopathology, and biochemical (Serum Ca2+ and ALP) data demonstrated the ameliorated effect of L. sativum seeds in bone fracture healing through rapid callus formation, fracture line repair, and new bone reaeration. This work emphasizes the importance medicinal plant in bone related diseases.
Consent to participate or publication
Yes.
Availability of data and materials
Available opon reasonable request.
Declarations
This manuscript is an original investigation also it is not submitted elsewhere for publication.
Ethics approval
Animal experiments were carried out according to the “Guide for the Care and Use of Laboratory Animal (Clark et al., 1996)” prepared by the National Academy of Science and published by the National Institutes of Health.
Competing interests
There are no competing interests.
Acknowledgement
The authors extend their sincere appreciation to researchers supporting project number (RSP-2021/129), King Saud University, Riyadh, Saudi Arabia for funding this research.
References
- Appraisal of garden cress (Lepidium sativum L.) and product development as an all pervasive and nutrition worthy food stuff. Annals. Food Sci. Technol.. 2013;14:77-84.
- [Google Scholar]
- TiO2 nanoparticles potentiated the cytotoxicity, oxidative stress and apoptosis response of cadmium in two different human cells. Environ. Sci. Pollut. Res.. 2020;27(10):10425-10435.
- [Google Scholar]
- SnO2-doped ZnO/reduced graphene oxide nanocomposites: Synthesis, characterization, and improved anticancer activity via oxidative stress pathway. Int. J. Nanomed.. 2021;16:89-104.
- [Google Scholar]
- Enhanced anticancer performance of eco-friendly-prepared Mo-ZnO/RGO nanocomposites: Role of oxidative stress and apoptosis. ACS Omega. 2022;7(8):7103-7115.
- [Google Scholar]
- Facile green synthesis of ZnO-RGO nanocomposites with enhanced anticancer efficacy. Methods. 2022;199:28-36.
- [Google Scholar]
- A novel green preparation of Ag/RGO nanocomposites with highly effective anticancer performance. Polymers. 2021;13(19):3350.
- [Google Scholar]
- Studies on some herbal drugs used in fracture healing. Int. J. Crude Drug Res.. 1989;27(4):235-239.
- [Google Scholar]
- Evaluation of serum alkaline phosphatase as a biomarker of healing process progression of simple diaphyseal fractures in adult patients. Int. Res. J. Biol. Sci.. 2013;2:40-43.
- [Google Scholar]
- The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat. Prod. Res.. 2019;33(6):901-905.
- [Google Scholar]
- The effects of Lepidium sativum seeds on fracture-induced healing in rabbits. Medscape J. Med.. 2007;9:23.
- [Google Scholar]
- Supplemented gamma-linolenic acid and eicosapentaenoic acid influence bone status in young male rats: effects on free urinary collagen crosslinks, total urinary hydroxyproline, and bone calcium content. Bone. 1995;16(4):S385-S392.
- [Google Scholar]
- Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol.. 2012;8(3):133-143.
- [Google Scholar]
- Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996.
- Reliability of radiographs in defining union of internally fixed fractures. Injury. 2004;35(6):557-561.
- [Google Scholar]
- Effects of probiotic Lactobacillus paracasei TD3 on moderation of cholesterol biosynthesis pathway in rats. Iran. J. Basic Med. Sci.. 2019;22:1004.
- [Google Scholar]
- Garden cress (Lepidium sativum L.) seed-an important medicinal source: A. Cellulose. 2014;9:0.03.
- [Google Scholar]
- Synergistic antiosteoporotic effect of Lepidium sativum and alendronate in glucocorticoid-induced osteoporosis in Wistar rats. Afr. J. Tradit. Complement. Altern. Med.. 2013;10:267-273.
- [Google Scholar]
- The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop.. 2007;18:444-448.
- [Google Scholar]
- Investigating the effect of fibulin-1 on the differentiation of human nasal inferior turbinate-derived mesenchymal stem cells into osteoblasts. J. Biomed. Mater. Res. A. 2017;105(8):2291-2298.
- [Google Scholar]
- Effect of calcium, phosphorus premix with synergistic herbs supplementation in improving overall performance, carcass quality and tibial mineralization in broiler chickens. J. Adv. Vet.. 2016;3:268-273.
- [Google Scholar]
- The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. J. Struct. Biol.. 2011;173(2):303-311.
- [Google Scholar]
- Effects of Stellate Ganglion Block on Healing of Fractures Induced in Rats. Biomed Res. Int.. 2020;2020:1-7.
- [Google Scholar]
- A Pilot Study: The Effects of an aqueous extract of Lepidium sativum seeds on levels of immune cells and body and organs weights in mice. J. Ayurvedic Herb. Med.. 2017;3(1):27-32.
- [Google Scholar]
- Changes in some serum bone markers after experimental fracture and intramedullary osteosynthesis in dogs. Trakia J. Sci.. 2005;3:46-50.
- [Google Scholar]
- Multicenter evaluation of Iso-ALP test kit for measurement of bone alkaline phosphatase activity in serum and plasma. Clin. Chem.. 1993;39:648-652.
- [Google Scholar]
- Virtual Structural Analysis of Bone Fracture Healing from Low-Dose Clinical CT Scans. J. Biomech.. 2019;83:49-56.
- [Google Scholar]
- Serum alkaline phosphatase: A prospective biomarker for assessment of progress of fracture healing in diaphyseal fractures of long bones in adult patients. Int. J. Ortho.. 2020;6(2):248-251.
- [Google Scholar]
- Low-intensity pulsed ultrasound initiates bone healing in rat nonunion fracture model. J. Ultrasound Med.. 2001;20:197-205.
- [Google Scholar]
- The mechanical and physical properties of unfired earth bricks stabilized with gypsum and Elazığ Ferrochrome slag. Int. J. Sustain. Built Environ.. 2017;6(2):565-573.
- [Google Scholar]
- Watts, J.J., Abimanyi-Ochom, J., Sanders, K.M., 2013. Osteoporosis costing all Australian: a new burden of disease analysis-2012 to 2022. Report number: ISBN 978-0-9923698-1-1.
- Possible therapeutic effects of berberine on bone damage in high-fat diet-induced obese rats. Research Squire. 2020
- [Google Scholar]
- Fracture healing activity of ethanolic extract of Lepidium sativum L. seeds in internally fixed rats’ femoral osteotomy model. Int. J. Pharm. Pharmaceut. Sci.. 2011;3:193-197.
- [Google Scholar]






