Translate this page into:
Alleviation of Cd stress in maize by compost mixed biochar
⁎Corresponding authors at: Hainan Key Laboratory for Sustainable Utilization of Tropical Bioresource, College of Tropical Crops, Hainan University, Haikou 570228, China (S. Danish and S. Fahad). Department of Geology and Pedology, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska1, 61300 Brno, Czech Republic (R. Datta). sd96850@gmail.com (Subhan Danish), shah_fahad80@yahoo.com (Shah Fahad), rahulmedcure@gmail.com (Rahul Datta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cadmium (Cd) is a heavy metal that neutrally occurs in soil. It is carcinogenic in humans and caused a significant decline in the plant’s growth when up has taken beyond the threshold limit. The fertilizers, manure, sewage sludge, and aerial deposition are the main source of cadmium contamination in soil. Furthermore, poor soil organic matter is also one of the allied factors which facilitate the development of Cd toxicity in soil. The decomposition resistance nature of biochar makes it an effective amendment for cadmium remediation. Through crop production, Cd enters the food system. Individual studies on biochar and compost are found in the literature but the combined effect of biochar and compost are rarely documented especially in maize crops. The current pot study was conducted in Pesticide Quality Control Laboratory, Multan, Pakistan. However, the current study was novel and conducted by using compost mixed biochar (CB) against Cd toxicity in maize. Four application rates of CB i.e., 0, 0.50, 0.75 and 1.00% (1.00CB) were applied under 3 levels of Cd i.e., 0 (0Cd), 2.5 (2.5Cd) and 5 mg Cd kg−1 soil (5.0Cd). Overall, results indicated that 1.00%CB remained significantly best at higher 5.0Cd for improvement in soil organic matter, plant height, root length, number of leaves, leaves fresh and dry weight, plant fresh and dry weight, chlorophyll a, b, total and carotenoids. A significant decrease in soil pHs, leaves anthocyanin and lycopene also validated the efficacious functioning of 1.00%CB over control in 2.5 and 5.0Cd. In conclusion, the use of 1.00%CB is a better approach to decrease Cd harmful effects to improve gas exchange attributes, growth and chlorophyll contents in maize. Long-term research is required on co-composted biochar toward mitigation of cadmium toxicity under different geographical locations.
Keywords
Chlorophyll contents
Heavy metals
Zea mays L.
Organic amendments
Photosynthetic rate
Stomatal conductance
Transpiration rate
1 Introduction
Soil pollution is one of the important effects of disturbing the balance of nature (Soyingbe et al., 2020; Syed et al., 2021). The most important soil contaminants include heavy metals, acid rain and organic matter, which among these heavy metals are considered for their non-degradability, high toxicity, cumulative effects and carcinogenicity (Iram et al., 2012). Although heavy metals are naturally present in small concentrations in soil, their geographical distribution will pose problems both naturally and through human activities (Shafiq et al., 2020). Industrial wastewater is used to irrigate agricultural lands in many areas of the world, this leads to excessive accumulation of heavy elements in the soil, and by absorbing them with the plant, they eventually enter the human food cycle (Hassan and David, 2014). Heavy metals have the most adverse effects on fetuses and children, and this age group is more exposed to the dangers of heavy metals which leads to a decrease in their mental and physical growth and damage to their brain and organs (Azevedo et al., 2012).
Cadmium is a highly toxic metal, and its entry into the human food cycle has caused concern (Hassan et al., 2013). The toxic effects of this element on soil biological activities, plant metabolism, human and animal health have led to the study of cadmium behaviour in the environment by many researchers (Lamoreaux and Chaney, 1978). High levels of cadmium due to metabolic disorders increase free radicals' production and lead to oxidative stress in the plant (Hassan et al., 2016). Cadmium also causes leaf twisting, chlorosis, necrosis of leaves, reddening and browning of leaf margins, reduced leaf area, reduced total dry matter, browning of roots and reduced plant growth (Khan and Lee, 2013), and also harms physiological and metabolic processes of plants such as respiration, photosynthesis, plant water relations and gas exchanges of stomata (Hassan et al., 2016). It also interferes in the path of chlorophyll biosynthesis, Calvin cycle performance, photosynthetic light reactions and also absorption of certain nutrients such as potassium, iron and magnesium (Dong et al., 2006; Greger et al., 1991).
Biochar incorporation has been documented as the most favourable organic amendment to remove abiotic stresses i.e., lethal impacts of heavy metals in soils and crops (Danish and Zafar-ul-Hye, 2020; Jabborova et al., 2020). Specific characteristics of biochar i.e., large surface area, adsorption rates and porosity are dependent on the pyrolysis and feedstock (Ghodake et al., 2021), which efficiently increases the potential of char in the absorption of soil materials to cleans the soil from adverse (Hashmi et al., 2019; Ok et al., 2015). Additionally, the microporous structure, exchange capacity and active functional groups of biochar performed a critical role in lessening the bioavailability and mobilization of heavy metals (Jiang et al., 2012). Use of organic amendments (Izhar Shafi et al., 2020; Wahid et al., 2020), i.e., compost in agriculture, improves rhizobacterial proliferation, water and nutrients retention and soil aggregation. It also decreases soil pH when applied in soil (Schulz et al., 2014). Although foliar fertilizer application and micronutrients are demonstrated to be a better alternative to fast action in some cases (Rafiullah et al., 2020a, 2020b; Tariq et al., 2020), by maintaining the soil organic pool, compost in soil enhances the phyto availability of macro and micronutrients and ultimately improve improves soil health (Doan et al., 2014; Schulz et al., 2014).
Maize (Zea mays L.) has been dedicated third place after wheat and rice due to its ability to adapt to different climatic conditions (Hanif and Akhtar, 2020). In Pakistan, about 97% of corn production is allocated to Punjab and KPK (Tariq and Iqbal, 2010). Since maize is a very demanding plant in terms of nutrient uptake from the soil, cadmium-contaminated soils cannot meet the plant's needs and, by restricting plant growth, prevent the emergence of the full growth potential of maize (Drazkiewicz and Baszynski, 2005). The individual studies of compost and biochar on the cadmium mitigation in soil are available in literature but the combined effect of biochar and compost are rarely studied especially in Pakistan climatic conditions. Bass et al. (2016) found that combined use of compost and biochar improved water contents reduced bulk density and improved carbon stock as compared to individual application of biochar and compost in their field experiments. Individual studies on biochar and compost are already documented in the literature but limited information is available regarding the combined effect of biochar and compost are especially in maize. The current study is covering the knowledge gap regarding the compost mixed biochar application effects on Cd toxicity, especially in maize. It was hypothesized that the combined effect of biochar and compost will be more effective on cadmium effect mitigation as compared to individual effects in the current study. Therefore, a pot experiment was done to study the impacts of compost mixed biochar (CB) on the growth, pigment contents and gas exchange attributes of maize.
2 Materials and methods
2.1 The experimental site, design and soil characteristics
This study was conducted in a completely randomized design (CRD) at the Pesticide Quality Control Laboratory (30.154813, 71.442681), Multan, Pakistan. For soil texture analysis, hydrometer method (Gee and Bauder, 1986), available P (Olsen and Sommers, 1982), extractable K (Helmke and Sparks, 2018) and soil organic matter (Nelson and Sommers, 1982) were analyzed using standard protocols. Soil texture was loamy which contains pHs (8.05) organic matter (0.40%), ECe (2.32 dS m−1), commutable potassium (200 mg kg−1), available phosphorus (5.35 mg kg−1) and diethylenetriaminepentaacetic acid (DTPA) extractable Cd (0.40 mg kg−1) (Lindsay and Norvell, 1978). The climatic conditions were arid with low rainfall and high temperature (Fig. 1).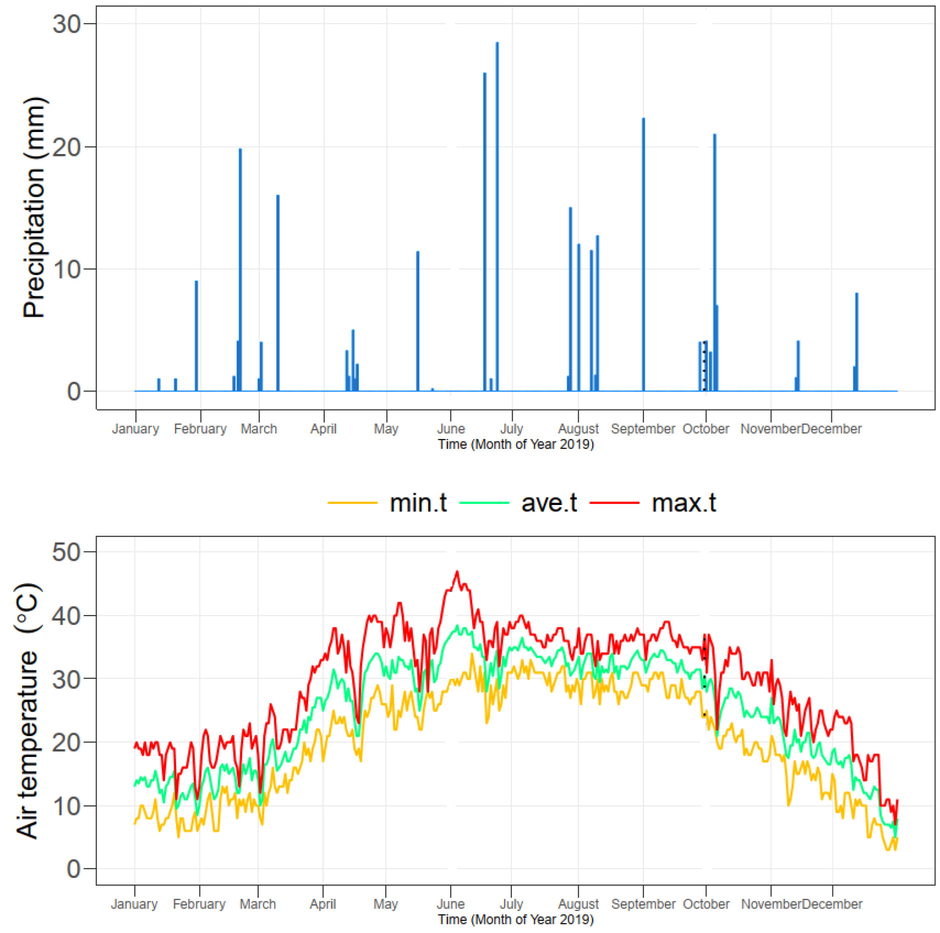
The daily minimum, maximum and average temperature and rainfall in Multan, Pakistan during the year 2019. The dotted lines are showing the duration of the cluster bean crop.
2.2 Cd toxicity
There were 3 levels of Cd i.e., control having 0.4 mg Cd kg−1 (0Cd), 2.5 mg Cd kg−1 soil (2.5Cd) and 5.0 mg Cd kg−1 (5.0Cd). All Cd levels were maintained by using analytical grade (Sigma) salt of CdCl2 (Zafar-ul-Hye et al., 2018).
2.3 Compost mixed biochar
Timber waste material was used for the manufacturing of biochar at 439 °C through pyrolysis for 2 h. After preparing biochar samples from the pyrolyzer, they were ground enough to transit via a 2 mm sieve. Compost was provided from Buraq Agro Chemicals, Industrial State Area, Multan. Both biochar and compost were mixed in a 1:1 ratio manually. After that, compost mixed biochar (CB) was applied in the soil as per the treatment plan i.e., 0, 0.5, 0.75 and 1.00%. The biochar and compost were charactered for physiochemical properties (Table 1).
Characteristics
Compost
Biochar
Characteristics
Irrigation Water
pHs
6.05
7.85
pHs
7.05
ECe (dS m−1)
5.36
3.00
ECe (dS m−1)
0.85
Total N (%)
1.46
1.21
TSS (ppm)
850
Available phosphorus (µg/g)
0.64
0.80
CO3−2
0
Extractable potassium (µg/g)
59
35
HCO3−1
2.5
Extractable Cd (µg/g)
0.35
0.45
Cl−1
0.5
Volatile matter (%)
–
12
Ca + Mg
2.0
Ash content (%)
–
20
Fixed carbon (%)
–
68
2.4 Irrigation
All the pots were irrigated regularly. Throughout the experiment, 65% of field capacity was preserved on a weighted base. The characteristics of irrigation water are provided in Table 1.
2.5 Fertilizer application
Initially, 12 kg of soil was added to each polyethylene bag. Based on the results of soil chemical analysis, 92 kg of N acre−1, 37 of K acre−1 and 58 kg of P acre−1 were used in the experiment (Zafar-ul-Hye et al., 2015). All triple superphosphate and potassium sulfate fertilizers were added evenly in one step to the individual pots before planting and were thoroughly mixed with soil. Nitrogen was added to the soil in two stages, two-thirds after thinning stage (in the 3–4 leaf stage) and the remaining one-third in the tassel emergence stage.
2.6 Sowing
The hybrid seed used in this experiment was YH 1898 hybrid. In stage 3–4 leaves, the plants were thinned, and the rest of the plants were watered evenly until the end of the growing season.
2.7 Harvesting
At the stage of ear silk formation, plant samples were taken to measure morphological, physiological traits, and nutrients contained in the plant's leaves and roots. Plant height and root length were measured using a meter and then reported in centimetres. However, the number of leaves, plant and root length and plant fresh weights were measured after harvesting. The samples' dry weight was measured by placing them in an oven at 65 °C for 48 h.
2.8 Chlorophyll contents
To measure chlorophyll (Chl.), at the stage of ear silk formation, to 0.2 g of freshly chopped leaves, 10 ml of 80% acetone and 0.5 g of calcium carbonate powder (to prevent the formation of Pheophytin) were added to separate the pigments and then extract all the chlorophyll from the leaf tissue and finally, the amount of light absorption of the solution was read using a spectrophotometer at 645 and 663 nm (Arnon, 1949). The chlorophyll a & b contents were obtained through the equations: where OD = Optical density (wavelength), V = Final volume made, fw = Fresh leaf weight (g).
2.9 Accessory pigments
The concentration of carotenoids, anthocyanin and lycopene were calculated through the following equations (Kirk and Allen, 1965; Rao et al., 1998; Sims and Gamon, 2002).
2.10 Gas exchange attributes
Gas exchange attributes were assessed at the tasseling stage by LCi- SD Ultra-Compact Photosynthesis System® (Danish and Zafar-ul-Hye, 2019; Danish et al., 2019).
2.11 Leaves N, P and K
Nitrogen was analyzed on the Kjeldahl distillation apparatus. The phosphorus was determined by the yellow colour method at 470 nm wavelength by using a spectrophotometer (Chapman and Pratt, 1961). For potassium determination, the digested sample aliquot was fed to the flame photometer (Nadeem et al., 2013).
2.12 Statistical analysis
Data analysis of variance was executed using a two-way analysis of variance (ANOVA) (Steel et al., 1997). The treatment means were compared using LSD's test at 5% probability level. Origin 2021Pro was used for making the graphs of data via pared comparison functions (OriginLab Corporation, 2021).
3 Results
Treatments impacts were significant on soil pH, EC and OM under different levels of soil Cd. Application of 0.5, 0.75 and 1.00%CB significantly decreased soil pH than control in 0, 2.5 and 5.0Cd soil. Treatment 1.00%CB significantly increased soil EC over control in 0 and 2.5Cd soils. For soil EC, 0.5%CB and 0.75%CB remained statistically similar in 0 and 2.5Cd soils. The increasing application rate of CB, i.e., 0.5, 0.75, and 1.00%, significantly increased soil OM in 0, 2.5 and 5.0Cd soil. Treatments 1.00%CB showed significantly better results for improvement in OM over 0.75 and 0.5%CB (Fig. 2).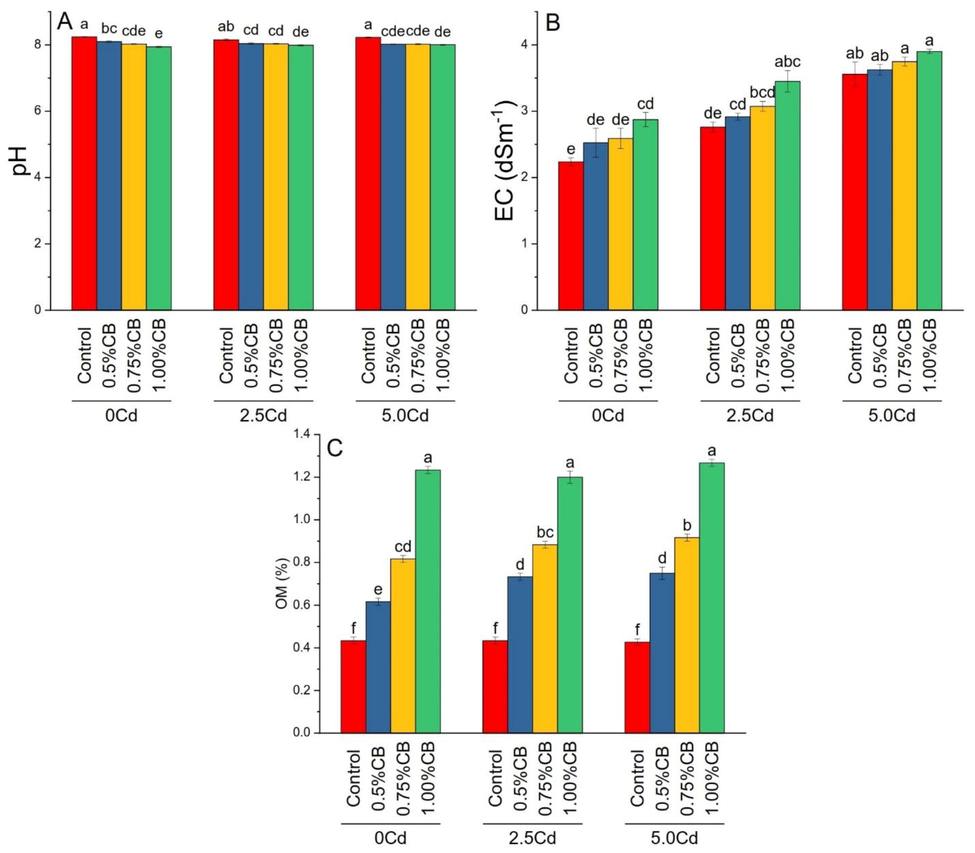
Variable rates of composted biochar impacts on soil pH (A), EC (B) and OM (C) under different levels of Cd. Means are average of 3 replicates compared with Tukey’s test. Columns with similar letters are not significantly different.
Different application rates of CB significantly affect maize plant height and root length cultivated under different Cd levels in the soil. Treatments 0.5, 0.75 and 1.00%CB significantly enhanced maize plant height than control in 0, 2.5 and 5.0Cd soil. A significant increase in maize plant height was noted in 1.00%BC over 0.5 and 0.75%CB in 0, 2.5 and 5.0Cd soils. Treatment 0.75 and 1.00%CB significantly enhanced root length over control in 0 and 2.5Cd soils (Fig. 3).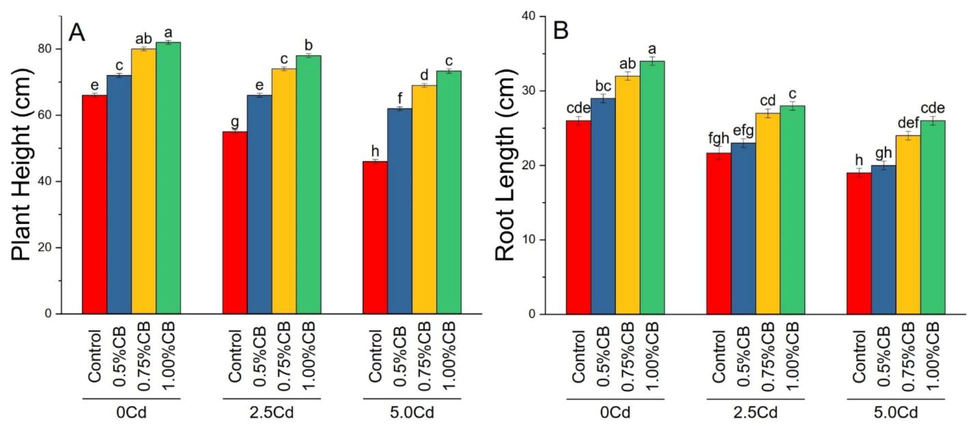
Variable rates of composted biochar impacts on maize plant height (A) and root length (B) under different levels of Cd. Means are average of 3 replicates compared with Tukey’s test. Columns with similar letters are not significantly different Number of leaves, fresh and dry leave weight.
Number of leaves, leaves fresh and dry weights differed significantly due to variable application rates of CB under different soil Cd levels. Application of 1.00%CB significantly improved maize number of leaves than control in 0, 2.5 and 5.0Cd soil. Treatment 0.5, 0.75 and 1.00%CB significantly increased leaves fresh weight over control in 0, 0.5 and5.0Cd soils. For leaves dry weight, 0.5%CB and 0.75%CB remained statistically similar with control in 0Cd soils. The increasing application rate of CB, i.e., 0.75 and 1.00% significantly increased leaves dry weight in 2.5 and 5.0Cd soil (Fig. 4).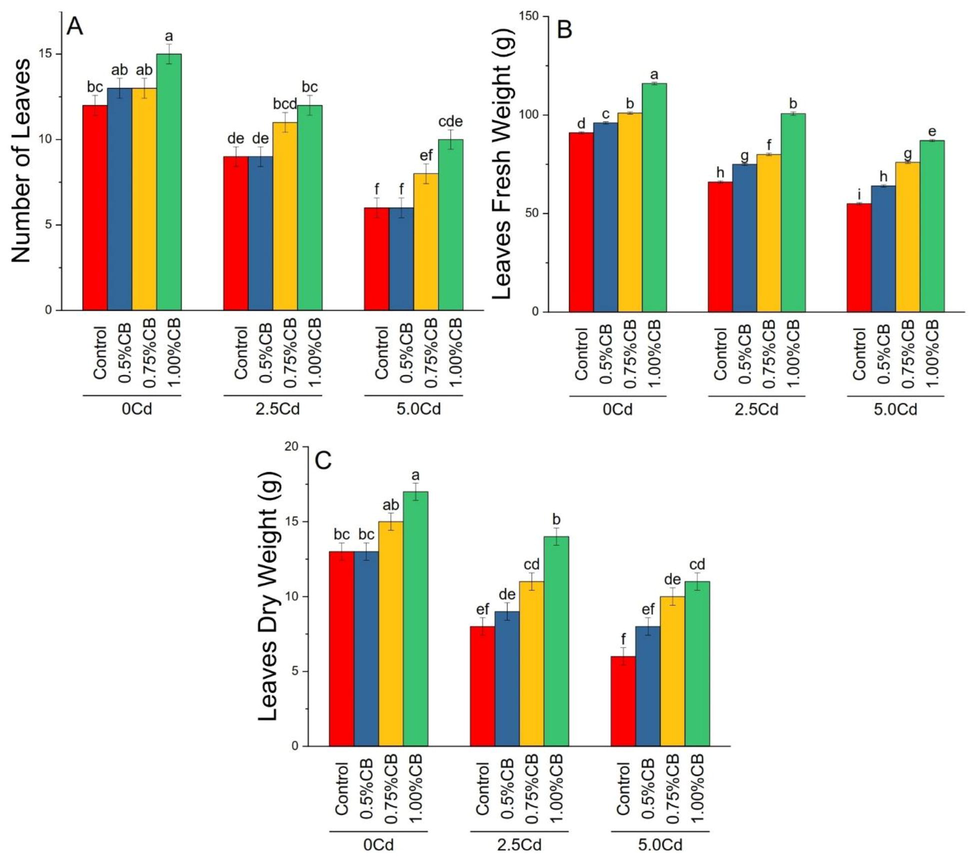
Variable rates of composted biochar impacts on maize number of leaves (A), leaves fresh weight (B) and leaves dry weight (D) under different levels of Cd. Means are average of 3 replicates compared with Tukey’s test. Columns with similar letters are not significantly different.
CB effect was significant for maize plant fresh and dry weights grown under different soil levels. Treatments 0.75 and 1.00%CB significantly enhanced maize plant fresh weight than control in 0, 2.5 and 5.0Cd soil. There was a considerable increase in maize plant fresh weight in 0.5%BC over control in 5.0Cd soils. Treatment 0.75 and 1.00%CB significantly enhanced plant dry weight over control in 5.0 and 2.5Cd soils (Fig. 5).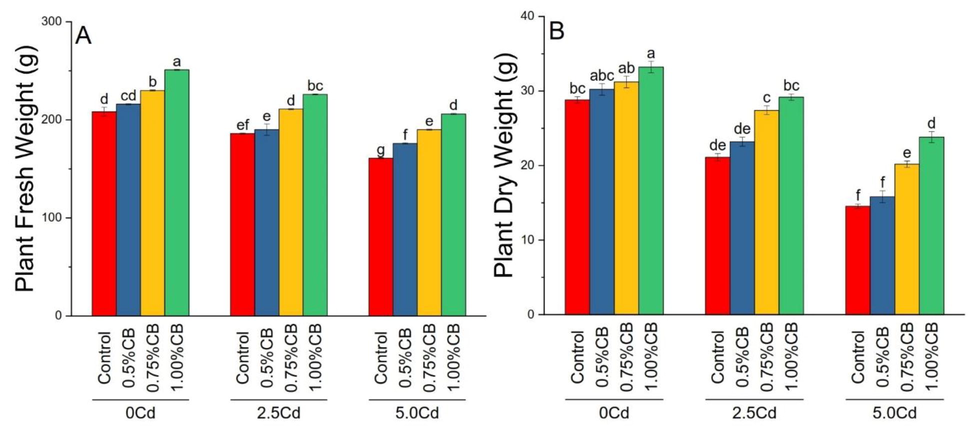
Variable rates of composted biochar impacts on maize plant fresh weight (A) and dry weight (B) under different levels of Cd. Means are average of 3 replicates compared with Tukey’s test. Columns with similar letters are not significantly different.
Different application rates of CB significantly affected chlorophyll contents (Fig. 6A-C), carotenoids (Fig. 6D), anthocyanin (Fig. 6E) and lycopene (Fig. 6F) of maize leaves under different levels of soil Cd. Treatments 0.75 and 1.00%CB significantly increased maize chlorophyll a and b than control in 0, 2.5 and 5.0Cd soil. For total chlorophyll, 0.75 and 1.00%CB cause a significant increase in 0 and 5.0Cd soils. However, only 1.00%BC significantly increased total chlorophyll in 2.5Cd soils. Treatment 1.00%CB significantly increased leaves carotenoids over control in 0Cd soils. Application of 0.75 and 1.00%BC significantly enhanced carotenoids in maize leaves cultivated in 2.5 and 5.0Cd soils. For anthocyanin, 1.00%CB gave a significant decrease over control in 0Cd soils. Application of 0.50, 0.75 and 1.00CB showed a significant decrease in anthocyanin over control at 2.5Cd soils. However, in 5.0Cd soils, a significant decrease in anthocyanin was noted where 0.75 and 1.00%CB were applied. Increasing application rate of CB i.e., 0.50, 0.75 and 1.00% significantly decreased lycopene in 0, 2.5 and 5.0Cd soil (Fig. 6).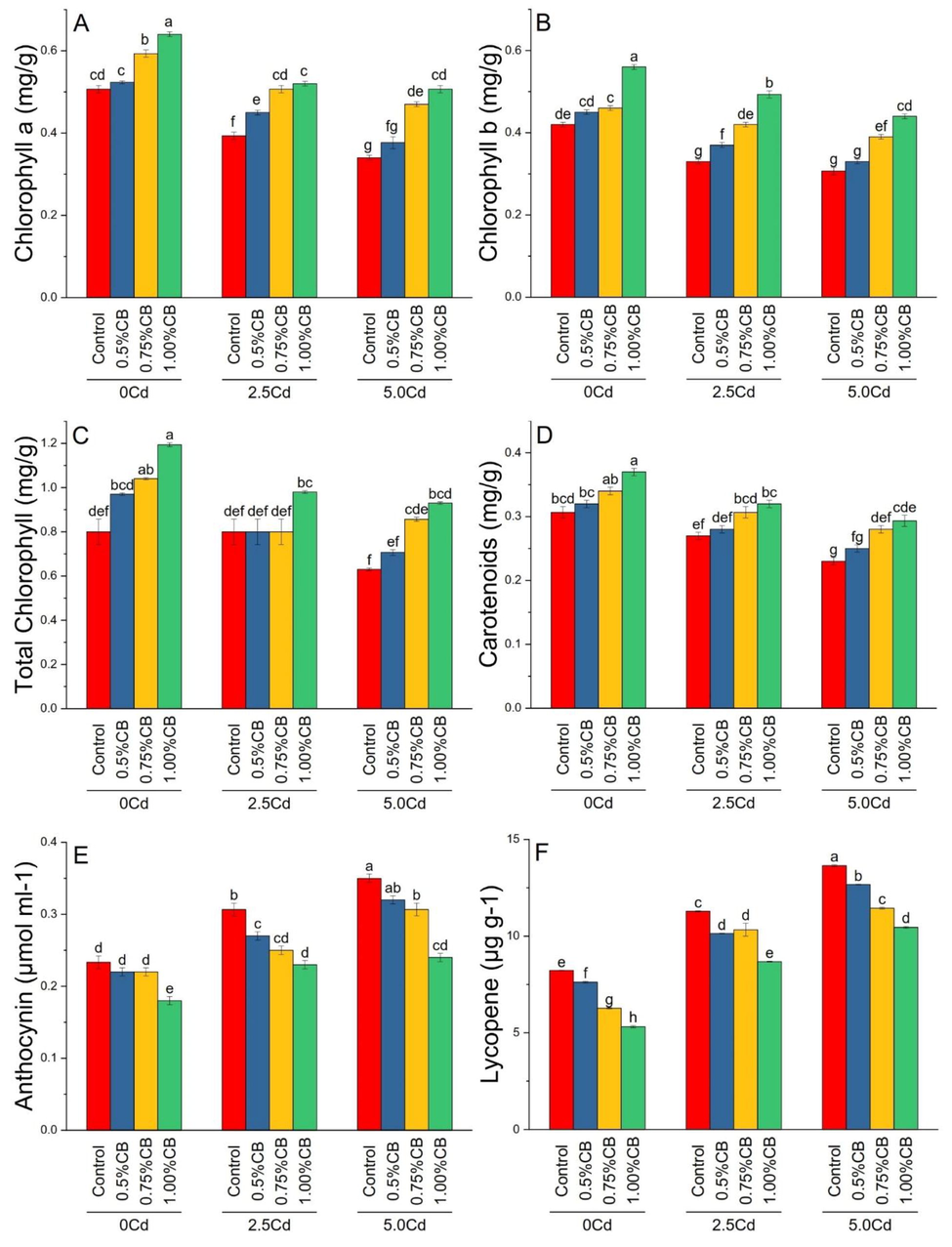
Variable rates of composted biochar impacts on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), carotenoids (D), anthocyanin (E) and lycopene (F) under different levels of Cd. Means are average of 3 replicates compared with Tukey’s test. Columns with similar letters are not significantly different.
Photosynthetic rate; Pn (A), transpiration rate; E (B) and stomatal conductance; gs (C) differed significantly due to variable application rates of CB under different levels of soil Cd. Application of 1.00%CB significantly improved maize photosynthetic rate than control in 0, 2.5 and 5.0Cd soil.. Treatment 0.75 and 1.00%CB significantly increased transpiration rate and stomatal conductance over control in 0, 0.5 and 5.0Cd soils. For transpiration rate and stomatal conductance, 0.5%CB and control remained statistically similar in 0, 2.5 and 5.0Cd soils (Fig. 7).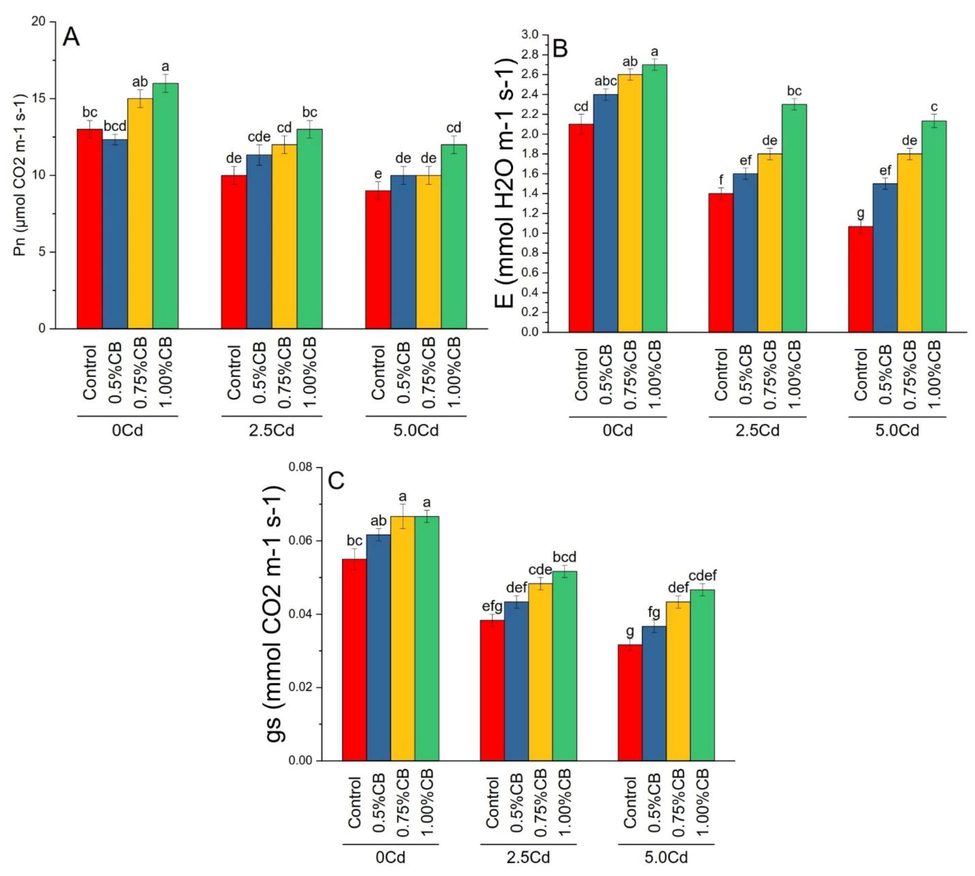
Variable rates of composted biochar impacts on maize photosynthetic rate; Pn (A), transpiration rate; E (B) and stomatal conductance; gs (C) under different levels of Cd. Means are average of 3 replicates compared with Tukey’s test. Columns with similar letters are not significantly different.
Application rates of CB significantly changed leaves nitrogen (A), phosphorus (B) and potassium (C) under different levels of soil Cd. Application of 1.00%CB significantly improved maize leaves nitrogen than control in 0, 2.5 and 5.0Cd soil. No significant change was noted between 0.5%CB and control for leaves nitrogen in 0, 2.5 and 5.0Cd soils. Treatment 0.75%CB significantly increased leaves nitrogen over control in 0 and5.0Cd soils. For leaves phosphorus, 1.00%CB caused a significant increase than control in 0, 2.5 and 5.0Cd soils. Treatment 0.75%CB significantly increased leaves phosphorus over control in 5.0Cd soils (Fig. 8). Pearson correlation showed that anthocyanin and lycopene were significant negative in correlation with leaves N, P, K concentrations and gas exchange attributes. Soil organic matter was significantly positive in correlation with plant height, root length, plant fresh and dry weight, chlorophyll a, chlorophyll b and total chlorophyll (Fig. 9).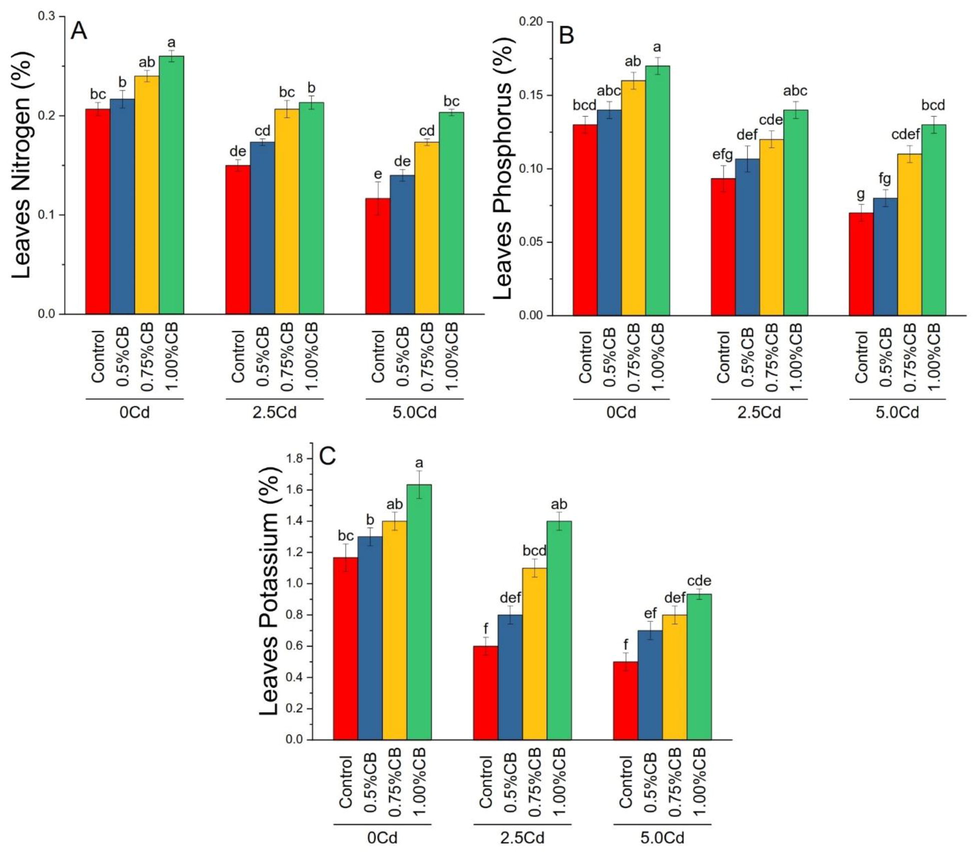
Variable rates of composted biochar impacts on maize leaves nitrogen (A), phosphorus (B) and potassium (C) under different levels of Cd. Means are average of 3 replicates compared with Tukey’s test. Columns with similar letters are not significantly different.
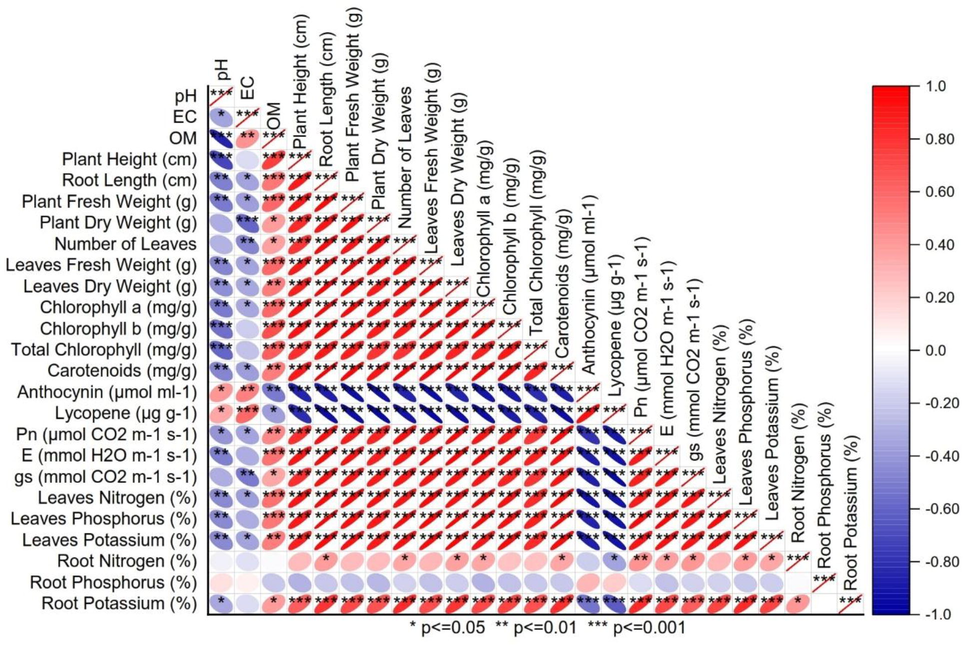
Pearson correlation for different maize attributes grown under various levels of Cd and CB.
4 Discussion
Maize is the cereal grain that comes at the third level after wheat and rice and is famous due to its nutritive values as it contains high quantities of macronutrients (Fat, fibre, protein and starch) and micronutrients (ß-carotene, Vitamin B) with essential mineral ions like Mg, Zn, P and Cu. In maize, a booster of antioxidants is present, which helps treat many diseases, but the quality of maize depends upon agricultural practices. Therefore, this study's main theme was to evaluate the biochar's role in the quality of maize under cadmium stress. As CB (biochar) has the ability for sorption of this metal ion on its surface and result in less toxicity of Cd on maize plants.
In the present study pH, EC and organic matter of the soil were significantly affected by applying biochar (Fig. 1). The soil pH decreased at 0, 2.5 and 5.0Cd with biochar percentages (0.5, 0.75 and 1.00%CB) than in control. Similarly, 1.00%CB significantly increased EC of soil than control at 0 and 2.5Cd soils. This effect is perhaps because of the absorption of Cd in soil by biochar and is by π-electrons that these are the part of functional groups (–OH,–COOH and C=N) and are aromatic in nature.
Similarly, Beesley et al. (2010), found the role of biochar in the sorption and immobilization of metals in soil. According to Bilgiç and Çaliskan (2001), π-electrons are involved in the immobility of toxic metals whenever biochar is used as an amendment. Similarly, Uchimiya et al. (2010), demonstrated that the semi sorption properties of biochar are due to d-electrons in coordination with π-electrons, which is the chief reason for changes in pH, EC organic components of soil as a result of biochar addition. Similar results on metal immobilization were noted by Park et al. (2011) using slush biochar as observed in this study that CB notably causes a reduction in soil pH by sorption of Cd.
The findings of the current study indicate a significant increase in plant growth attributes (Figs. 2, 3, 4) with the application of various CB percentages (0%, 0.5%, 0.75%, and 1.00%) as compared to control and Cd treatments (0, 2.5 and 5.0Cd soil). This increase in plant height and root length (Fig. 2) was mainly due to the verity that CB can improve physio-chemical properties of the soil in terms of WHC (water holding capacity), ions uptake (N, P, K) by plants (Uzoma et al., 2011). Similar sorts of results were observed in our experiment where CB caused increased plant growth by reducing Cd stress (Yeboah et al., 2009).
Cadmium causes negative effects on Zea mays fresh and dry weight, as indicated in our study (Fig. 4). But this reduction in plant biomass was less in CB than in the soil with various Cd levels. A similar trend was also noted for leaf attributes like leaf numbers, leaf fresh, and dry biomass (Fig. 3). This reduction was mainly due to less production of photosynthetic pigments with less ion uptake under Cd stress, but the increase under CB amendment was mainly the effect of biochar on ions uptake and Cd immobilization. Jiang et al. (2001) also observed a high decline in plant biomass and leaf area of garlic plants after applying Cd. The reduction in growth attributes like plant and leaves biomass was might be due to the toxic impact of Cd on physiological and biochemical attributes of maize (Figs. 5, 6).
Cadmium toxic effects are mainly observed on photosynthetic attributes as these parameters are served as physiological indicators for such type of stress (Xu et al., 2012). The decline in sub-stomatal CO2-concentration, transpiration, and photosynthetic rates were observed by increasing Cd levels from 0 to 5, but by applying BC, an improvement was seen (Fig. 6). Similar results for Cd-induced decline in physiological attributes of different plants have also been reported (Krantev et al., 2008; Wahid et al., 2008). These decreases are mainly due to Cd toxic effect on chlorophyll as Cd causes disintegration of chlorophyll molecules and demolition of Rubisco (Chaffei et al., 2004; Hajduch et al., 2001) with the closing of stomata. Enhancement in chlorophyll-contents was also mentioned with enhancing biochar percentages, as pragmatic earlier with Cd strain.
The photosynthetic attributes of maize planted in CB + Cd were not reduced in the current study, but a significant increase in chlorophyll and carotenoid was noted as compared to control (Fig. 5). Chlorophyll (a, b and total) and carotenoids were relatively more at 0.75% and 1.00% CB + Cd. The decline in leaf pigments concentration under Cd stress may be owing to chlorophyll less biosynthesis correspondingly, Cd causes a reduction in Mg and Fe supply vital for chlorophylls. This decline ultimately leads toward a reduction of transpiration rate, photosynthetic rate, and sub-stomatal CO2 concentrations synthesis (Liu et al., 2014).
To combat the stress of heavy metals, plants boost the manufacturing of antioxidants (Zaheer et al., 2020). A similar trend was also noted in the current study, whereby increasing Cd level from 0 to 5 the Lycopene and Anthocyanin concentrations were also increased (Fig. 5E and F). This increase mainly played a protective role against Cd toxicity (Xu et al., 2013). On the other hand, the increase in these pigments (Lycopene and Anthocyanin) was very less in CB soil, which demonstrated CB role for sorption of Cd in soil (Semane et al., 2010).
A higher concentration of cadmium retard plant growth by affecting biochemical, physiological attributes, and ion homeostasis. In the present study, nutrient percentages were significantly exaggerated by Cd and CB amendment (Fig. 7). Similarly, increasing Cd concentration retard maize growth due to less uptake of N, P, and K (Fig. 7A, B & C). However, biochar amendment in soil enhanced uptake of nutrients by maize plants at all Cd levels. Nitrogen, phosphorous, and potassium uptake by the maize plants were significantly increased in CB treatments at a high percentage (1.00%) irrespective of Cd. These findings suggested the role of biochar in increasing nutrient uptake by the sorption of Cd. Although, it might be the effect of biochar on cations exchange capacity of the soil and more bioavailability of N, P, and K for maize plants (Darby et al., 2016; Liang et al., 2006) and as a result of more Cd percentages at root that can interact with nutrient (N, P, and K) uptake (Danish and Zafar-ul-Hye, 2020; Gonçalves et al., 2011). The availability of nitrogen increased as biochar prepared from plant wastes contain various nitrogen sources like protein, amino acids, amines, and amino sugars. During biochar preparation by pyrolysis, these components became compressed in heterocyclic compounds of nitrogen (Koutcheiko et al., 2007). The porosity of the biochar surface is an important factor that affects the absorption of heavy metals. This structure with irregularly perforated plates at its surface increases the adsorption sites. Then, biochar can be a cheap and suitable adsorbent to reduce the bioavailability of heavy metals in soils that have been poisoned by contaminated water by improving soil properties and increasing the adsorption of cadmium.
5 Conclusions
The compost mixed biochar was found an effective amendment for alleviation of Cd stress on maize in the current study. Application of compost mixed biochar has the potential to enhance growth attributes and nutrient uptake in Cd toxic conditions. A significant improvement in chlorophyll contents and gas exchange attributes of maize validated the effectiveness of 1% compost mixed biochar for mitigation of Cd adverse effects. It is recommended to add 1% compost mixed biochar in soil for the cultivation of maize in Cd polluted soils. However, long-term and multiple location studies are suggested at natural Cd contaminated field conditions for a better and deep understanding of compost mixed biochar potential.
Acknowledgement
The authors extend their appreciation to the Researchers supporting project number (RSP-2021/316) King Saud University, Riyadh, Saudi Arabia.
Funding
The authors extend their appreciation to the Researchers supporting project number (RSP-2021/316) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol.. 1949;24:1-15.
- [CrossRef] [Google Scholar]
- What is new in the research on cadmium-induced stress in plants? Food Energy Secur.. 2012;1(2):133-140.
- [Google Scholar]
- Soil properties, greenhouse gas emissions and crop yield under compost, biochar and co-composted biochar in two tropical agronomic systems. Sci. Total Environ.. 2016;550:459-470.
- [CrossRef] [Google Scholar]
- Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut.. 2010;158:2282-2287.
- [CrossRef] [Google Scholar]
- Investigation of some Schiff bases as corrosion inhibitors for austenitic chromium-nickel steel in H2SO4. J. Appl. Electrochem.. 2001;31:79-83.
- [CrossRef] [Google Scholar]
- Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol.. 2004;45:1681-1693.
- [CrossRef] [Google Scholar]
- Methods of Analysis for Soils, Plants and Water. Berkeley, CA, USA: University of California, Division of Agricultural Sciences; 1961.
- Combined role of ACC deaminase producing bacteria and biochar on cereals productivity under drought. Phyton (B. Aires). 2020;89:217-227.
- [CrossRef] [Google Scholar]
- Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep.. 2019;9:5999.
- [CrossRef] [Google Scholar]
- Rhizobacteria with ACC-deaminase activity improve nutrient uptake, chlorophyll contents and early seedling growth of wheat under PEG-induced osmotic stress. Int. J. Agric. Biol.. 2019;21:1212-1220.
- [CrossRef] [Google Scholar]
- Short-term dynamics of carbon and nitrogen using compost, compost-biochar mixture and organo-mineral biochar. Environ. Sci. Pollut. Res.. 2016;23:11267-11278.
- [CrossRef] [Google Scholar]
- Influence of buffalo manure, compost, vermicompost and biochar amendments on bacterial and viral communities in soil and adjacent aquatic systems. Appl. Soil Ecol.. 2014;73:78-86.
- [CrossRef] [Google Scholar]
- Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum) Chemosphere. 2006;64:1659-1666.
- [CrossRef] [Google Scholar]
- Growth parameters and photosynthetic pigments in leaf segments of Zea mays exposed to cadmium, as related to protection mechanisms. J. Plant Physiol.. 2005;162(9):1013-1021.
- [Google Scholar]
- Gee, G.W., Bauder, J.W., 1986. Particle-size analysis, in: Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. Madison, pp. 383–411. https://doi.org/10.2136/sssabookser5.1.2ed.c15.
- Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod.. 2021;297
- [CrossRef] [Google Scholar]
- Phytoavailability of toxic heavy metals and productivity in wheat cultivated under residual effect of fertilization in soybean culture. Water Air Soil Pollut.. 2011;220:205-211.
- [CrossRef] [Google Scholar]
- Uptake and physiological effects of cadmium in sugar beet (Beta vulgaris) related to mineral provision. J. Exp. Bot.. 1991;42:729-737.
- [CrossRef] [Google Scholar]
- High-resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: drastic reductions/fragmentation of ribulose-1,5-bisphosphate carboxylase/oxygenase and induction of stress-related proteins. Electrophoresis. 2001;22:2824-2831.
- [CrossRef] [Google Scholar]
- Nutritional evaluation of maize plant fodder grown in spring and autumn season in Punjab, Pakistan. J. Bioresour. Manag.. 2020;7:74-93.
- [CrossRef] [Google Scholar]
- Pongamia pinnata L. leaves biochar increased growth and pigments syntheses in Pisum sativum L. exposed to nutritional stress. Agriculture. 2019;9:153.
- [CrossRef] [Google Scholar]
- Response of soil microbial biomass and enzymes activity to cadmium (Cd) toxicity under different soil textures and incubation times. Aust. J. Crop Sci.. 2013;7:674-680.
- [Google Scholar]
- Role of ACC-deaminase and/or nitrogen fixing rhizobacteria in growth promotion of wheat (Triticum aestivum L.) under cadmium pollution. Environ. Earth Sci.. 2016;75:267.
- [CrossRef] [Google Scholar]
- Effect of lead pollution on soil microbiological index under spinach (Spinacia oleracea L.) cultivation. J. Soils Sedim.. 2014;14:44-59.
- [Google Scholar]
- Helmke, P.A., Sparks, D.L., 2018. Lithium, sodium, potassium, rubidium, and cesium, in: D.L. Sparks, A.L. Page, P.A. Helmke, R.H. Loeppert, P. N. Soltanpour, M. A. Tabatabai, C. T. Johnston, M. E. Sumner (Eds.), Methods of Soil Analysis: Part 3 Chemical Methods, 5.3. John Wiley & Sons, Ltd, pp. 551–574. https://doi.org/10.2136/sssabookser5.3.c19.
- Distribution of heavy metals in peri-urban agricultural areas soils. J. Chem. Soc. Pakistan. 2012;34:861-869.
- [Google Scholar]
- Application of single superphosphate with humic acid improves the growth, yield and phosphorus uptake of wheat (Triticum aestivum L.) in calcareous soil. Agronomy. 2020;10:1224.
- [CrossRef] [Google Scholar]
- Co-inoculation of rhizobacteria and biochar application improves growth and nutrients in soybean and enriches soil nutrients and enzymes. Agronomy. 2020;10:1142.
- [CrossRef] [Google Scholar]
- Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mater.. 2012;229–230:145-150.
- [CrossRef] [Google Scholar]
- Hyperaccumulation of cadmium by roots, bulbs and shoots of garlic (Allium sativum L.) Bioresour. Technol.. 2001;76(1):9-13.
- [CrossRef] [Google Scholar]
- Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol.. 2013;13:86-100.
- [CrossRef] [Google Scholar]
- Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem. Biophys. Res. Commun.. 1965;21(6):523-530.
- [Google Scholar]
- Preparation and characterization of activated carbon derived from the thermo-chemical conversion of chicken manure. Bioresour. Technol.. 2007;98:2459-2464.
- [CrossRef] [Google Scholar]
- Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol.. 2008;165:920-931.
- [CrossRef] [Google Scholar]
- The effect of cadmium on net photosynthesis, transpiration, and dark respiration of excised silver maple leaves. Issue Physiol. Plant. Addit. Inf.. 1978;43:231-236.
- [CrossRef] [Google Scholar]
- Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J.. 2006;70(5):1719-1730.
- [Google Scholar]
- A DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J.. 1978;42:421-428.
- [Google Scholar]
- Effects of cadmium (Cd) on seedling growth traits and photosynthesis parameters in cotton (Gossypium hirsutum L.) Plant Omics. 2014;7:284-290.
- [Google Scholar]
- Qualitative and chemical analysis of rice kernel to time of application of phosphorus in combination with zinc under anaerobic conditions. Asian J. Agric. Biol.. 2013;1:67-75.
- [Google Scholar]
- Nelson, D.W., Sommers, L.E., 1982. Total Carbon, Organic Carbon, and Organic Matter, in: Page, A.L. (Ed.), Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, USA, pp. 539–579.
- SMART biochar technology-A shifting paradigm towards advanced materials and healthcare research. Environ. Technol. Innov.. 2015;4:206-209.
- [CrossRef] [Google Scholar]
- Olsen, S.R., Sommers, L.E., 1982. Phosphorus, in: Page, A.L. (Ed.), Method of Soil Analysis, Agron. No. 9, Part 2: Chemical and Microbiological Properties. American Society of Agronomy, Madison, WI, USA, pp. 403–430.
- OriginLab Corporation, 2021. OriginPro. OriginLab, Northampton, MA, USA.
- Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil. 2011;348:439-451.
- [CrossRef] [Google Scholar]
- Phosphorus nutrient management through synchronization of application methods and rates in wheat and maize crops. Plants. 2020;9(10):1389.
- [Google Scholar]
- Rafiullah, Tariq, M., Khan, F., Shah, A.H., Fahad, S., Wahid, F., Ali, J., Adnan, M., Ahmad, M., Irfan, M., Zafar-ul-Hye, M., Battaglia, M.L., Zarei, T., Datta, R., Saleem, I.A., Hafeez-u-Rehman, Danish, S., 2020b. Effect of micronutrients foliar supplementation on the production and eminence of plum. Qual. Assur. Saf. Crop. Foods 12, 32–40. https://doi.org/10.15586/qas.v12iSP1.793
- Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res. Int.. 1998;31:737-741.
- [CrossRef] [Google Scholar]
- No effect level of co-composted biochar on plant growth and soil properties in a greenhouse experiment. Agronomy. 2014;4:34-51.
- [CrossRef] [Google Scholar]
- Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J. Plant Physiol.. 2010;167:247-254.
- [CrossRef] [Google Scholar]
- Growth, protein expression and heavy metal uptake by tobacco under heavy metals contaminated soil. Pakistan J. Bot.. 2020;52:1569-1576.
- [Google Scholar]
- Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ.. 2002;81:337-354.
- [CrossRef] [Google Scholar]
- Ameliorative effect of exogenously applied oxalic acid on nickel (heavy metal) induced stress in Zea mays. Pakistan J. Bot.. 2020;52:413-418.
- [Google Scholar]
- Principles and Procedures of Statistics: A Biometrical Approach (3rd ed.). Singapore: McGraw Hill Book International Co.; 1997.
- Heavy metals induced modulations in growth, physiology, cellular viability, and biofilm formation of an identified bacterial isolate. ACS Omega. 2021;6(38):25076-25088.
- [Google Scholar]
- Effect of micronutrients foliar supplementation on the production and eminence of plum (Prunus domestica L.) Qual. Assur. Saf. Crop. Foods. 2020;12(SP1):32-40.
- [Google Scholar]
- Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem.. 2010;58(9):5538-5544.
- [Google Scholar]
- Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manage.. 2011;27:205-212.
- [CrossRef] [Google Scholar]
- Effect of cadmium on photosynthesis, nutrition and growth of mungbean. Agron. Sustain. Dev.. 2008;28:273-280.
- [CrossRef] [Google Scholar]
- Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture. 2020;10:334.
- [CrossRef] [Google Scholar]
- Subcellular distribution and toxicity of cadmium in Potamogeton crispus L. Chemosphere. 2012;89:114-120.
- [CrossRef] [Google Scholar]
- Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. Int.. 2013;20:358-368.
- [CrossRef] [Google Scholar]
- Improving soil productivity through biochar amendments to soils. Afr. J. Environ. Sci. Technol.. 2009;3:34-41.
- [Google Scholar]
- Bacteria in combination with fertilizers promote root and shoot growth of maize in saline-sodic soil. Braz. J. Microbiol.. 2015;46:97-102.
- [Google Scholar]
- Mitigation of cadmium toxicity induced stress in wheat by ACC-deaminase containing PGPR isolated from cadmium polluted wheat rhizosphere. Pakistan J. Bot.. 2018;50:1727-1734.
- [Google Scholar]
- Iron-lysine mediated alleviation of chromium toxicity in Spinach (Spinacia oleracea L.) plants in relation to morpho-physiological traits and iron uptake when irrigated with tannery wastewater. Sustainaibilty. 2020;12:6690.
- [CrossRef] [Google Scholar]







