Translate this page into:
Evaluation of date palm kernels’ biological activities and possible role in improving liver and kidney functions
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Global production of date fruit, Phoenix dactylifera, has exceeded 8 million tons, with thousands of tons of seeds ending up as waste. Here, a detailed functional study is presented on the biological activities of the date palm seed powder using chemical analysis carried out via in vitro and in vivo approaches to assess its medical application.
Material and method
Powdered fresh dates kernel from Al-Baha city, Saudi Arabia were used to treat rats, HepG2, and HCT-116 cell lines. Toxicity and biochemical analyses were carried out on the cells.
Result
Antibacterial tests on Al-Baha date palm kernel (AB-DPK) powder indicated no antibacterial activity on Staphylococcus aureus, Bacillus subtilis, Protius mirabilis and Escherichia coli. Cytotoxicity tests of cancer cells revealed a dose-dependent decrease in cell viability in both HCT-116 and HepG2 cell lines after 24 h with IC50 of 43.2 ± 4.9 µg/ml and 63.7 ± 5.4 µg/ml respectively. Rats that were treated with AB-DPK for 48 h also showed no difference in hepatic and renal functions. However, oxidative stress assessment in both the liver and kidneys showed improvement in antioxidant levels by stimulating the production of CAT, GPx, SOD and GSH. In addition, the peroxidation parameter was also reduced in both the liver and kidney assays.
Conclusion
The date seeds powder improved antioxidant activities in liver and kidney fractions of treated rats. Coupled with the possible anticancer effect on the colorectal and hepatocellular carcinoma cell lines, AB-DPK may prove a useful therapy for the treatment of some cancers, but this requires further investigation.
Keywords
Date palm kernel
Antioxidant
Bioactivity
Liver functions
Renal functions
Natural product
1 Introduction
Date palm kernel seed is borne by the date palm tree (Phoenix dactylifera), which is mainly grown in semi-arid and arid regions of Africa and the Arabian Peninsula. Indeed, the proceeds from the cultivation of date palm constitute the majority of income for those living in the Sahara (Mirghani, 2012). Date palm is also cultivated in some parts of Southern Europe, and South and Central America (Qadir et al., 2018). There are different varieties of the date fruit, such as the Deglet Noor and Medjool, which are popularly cultivated in the United States and some parts of Africa such as Morocco, Algeria, and the Middle East; and Ajwa date fruit, which is cultivated mainly in Al-Madinah (Zhang et al., 2013). Dates are widely consumed among the Arab population due to traditional and religious importance. For example, Ajwa dates are consumed due to their reference in the Koran, in which they are referred to as cures for many ailments. It is for this single reason that this variety is at least three times more expensive than any other variety.
Date fruits have fleshy pericarp of high nutritional value, containing a high level of fiber, vitamins, and minerals (such as magnesium, calcium, iron, zinc, iodine and potassium), fructose and glucose. They contain very little fat (0.5%) and protein (3%), with carbohydrates comprising about 80% of the total nutrients (Rambabu et al., 2020). There are numerous studies on the functional quality of date fruits in the literature. For instance, aqueous extract from date fruit has been reported to have anti-mutagenic and antioxidant properties due to free radical scavenging antioxidants such as anthocyanins, polyphenols, carotenoids, and tannins (Hussain et al., 2020).
Global production of date fruit was estimated to be around 8.5 million tons in 2016 (Altaheri et al., 2019). Considering that date seed accounts for 10% of the date fruit mass, this amounts to 850 thousand tons of date seed produced in 2016, most of which ended up as waste. If properly channeled, this cheap agricultural produce can be of immense economic use. A good example is the processing of date seed for its fiber content to be utilized in fiber-based foods. Asides from this, date seed has been employed as a low-cost solution for the removal of boron in seawater; achieving 47% decontamination efficiency (Al. Haddabi et al., 2016).
Al-Baha region of Saudi Arabia plays a prominent role in agriculture and other farming activities in the Kingdom and one of the crops widely grown in the area is the date palm fruit (Al-Zahrani et al., 2021). While many studies have investigated the biological functions of date fruit, studies on the biological properties of date seeds specifically are only just beginning to gain traction. Many of these studies have been focused on analyzing the seeds for bioactive compounds such as phenols, minerals and fatty acids (Habib et al., 2014; Golshan Tafti et al., 2017; Nadeem et al., 2019; Babiker et al., 2020). There are few studies, however, that have demonstrated the biological functions of the contents of date seeds. In those that do exist, extracts from date seeds have been shown to have anti-diabetic properties in streptozotocin- and alloxan-induced diabetic rats (Hasan and Mohieldein, 2016; Ayatollahi et al., 2019). The rats showed improved tolerance to glucose with reduced serum creatinine and urea levels and a better lipid profile. In another study, Ajwa date seeds were found to improve the lipid profile of hyperlipidemic rabbits (Mushtaq et al., 2017). Due to the relatively few studies on date seed, this study aims to carry out a detailed functional study on the biological activities of Al-Baha date palm kernel (AB-DPK) using chemical analysis through in vitro and in vivo approaches.
2 Materials and methods
2.1 Al-Baha date palm kernel (AB-DPK) collection
Fresh dates were purchased from a local market in Al-Baha city, Saudi Arabia and the seeds were extracted, washed several times with clean water, and air-dried at room temperature for 7 days. This was followed by oven-drying for 2 h at 370 K to ensure that the kernels dried completely. Next, the oven-dried AB-DPK were crushed to form a powder and used as described below.
2.2 Cell lines and culture medium
Human colorectal cancer cell line (HCT-116) and liver cancer cell line (HepG2) used in this study was obtained from Dr. Neamatallah’s lab, Faculty of Pharmacy, KAU. HCT-116 and HepG2 cancer cells were used in this study to evaluate the anticancer properties of AB-DPK. The cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 units/ml penicillin/streptomycin and 1% (v/v) L-glutamine at 37 °C in a humidified 5% CO2 incubator.
2.3 MTT assay
To evaluate the cytotoxic effect of AB-DPK extract compound on HCT-116 cell line, an MTT cell viability assay was carried out by culturing the cells in a 96-well plate at a density of (1 × 105 cells/well). After 24 h of incubation, the cells were then treated in triplicates with the compounds at 6 serial dilutions (250–3.9 µg/ml) for 72 h. Appropriate control wells containing unexposed cells were also incorporated. The medium was then removed and replaced with MTT solution (2 mg/ml). The plates were covered with aluminum foil and incubated at 37 °C for 4 h. The DPK powder was dissolved by adding 200 µL of 100% DMSO (not exceeding 0.5% of the total concentration). The plates were then incubated for 5 mins at 37 °C in a 5% CO2 incubator, and the colorimetric signals were measured at 570 nm with a SpectraMax M3 plate reader.
2.4 Gas chromatography-mass spectrophotometry (GC–MS) analysis
The extracts of AB-DPK were analyzed using GC–MS in an Agilent 6890–5973 N USA gas chromatograph (GC) equipped with an HP1 TG-5MS polydimethylsiloxane capillary column (30 m × 250 µm × 0.25 µm) and interfaced with a Hewlett Packard 5973 mass selective detector. The gas chromatographic parameters employed were 110 °C fixed temperature for 2 min initially, which was increased to 200 °C and then 280 °C at the rate of 10 °C min−1 and 5 °C min−1 respectively. The inlet temperature was set at 250 °C and a 10:1 split ratio was used; MS quadrupole temperature was 150 °C; thermal aux temperature was set at 285 °C; MS temperature was 230 °C; MS scan range was m/z 40–450; ionization current was 60 µA; ionization energy was set at 70 eV; the carrier gas was helium with a flow rate of 1.0 mL min−1. Compounds identified in the GC–MS mass spectra were compared with Wiley 275.L and Wiley/n.L library data of the GC/MS system and literature data (Jennings and Shibamoto, 1980; Adams, 2007). The relative abundance of each compound was evaluated using the raw data peak areas in the spectra with no response factor correction from the FID.
2.5 Antimicrobial susceptibility testing
The antimicrobial activity of AB-DPK extract (100 µg) was assessed against Gram-positive (Staphylococcus aureus and Bacillus subtilis) and Gram-negative (Protius mirabilis and Escherichia coli) pathogenic bacteria. Overnight cultures were incubated for 24 h at 36 °C ± 1 °C and bacterial suspension or inoculum was diluted with sterile saline solution to make 108 CFU/ml (McFarland standard: OD = 0.5 at 600 nm). To evaluate the antimicrobial activity of the extracts, the agar well diffusion method was adopted according to Kilany (2017) using Muller Hinton agar media. The experiment was conducted in triplicate.
2.6 In vivo studies
To test acute cytotoxicity, oxidant/antioxidant and carcinogenic effects in the AB-DPK powder, 5 healthy, adult Sprague Dawley rats (200–250 g) were injected with a single dose regimen of 500 µg (in 200 µL) (Ghramh et al., 2020). Unexposed (N = 5) and acetone treated (200 µL, N = 5) rats were used as controls. The rats were left for 48 h and then euthanized, with the sera, livers, and kidneys (after perfusion with phosphate-buffered saline to get rid of red cells) were collected. The rats were kindly supplied by the Animal House Foundation at King Khalid University in the southern region of Saudi Arabia and treated following guidelines set by the Research Ethics Committee at King Khalid University. Each liver and kidney was homogenized (10% w/v) in ice-cold 0.1 M Tris-HCl buffer (pH 7.4) using an electrical homogenizer. The homogenate was centrifuged at 22,000g at 4 °C for 15 min (Ogunlana et al., 2018), and then the supernatant was collected and used for all subsequent parameters measurement. All data are expressed as per gram tissue unless otherwise indicated.
Liver function was tested by assaying the level of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein, and total bilirubin, while kidney function was tested by measuring serum creatinine and urea, and evaluated using the methods described by Ibrahim et al. (Ibrahim et al., 2021). To evaluate the oxidative stress, lipid peroxidation capacity was measured by quantifying thiobarbituric acid reactive substance (TBARS), while total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), hydrogen peroxide (H2O2) and reduced glutathione (GSH) in liver and kidney homogenate supernatants were estimated utilizing the same methods and chemicals described by Ibrahim et al. (Ibrahim et al., 2021). Arginase activity in serum was also estimated utilizing the methods and chemicals described by Ibrahim et al. (Ibrahim et al., 2021). Sera were filtered through a 10 KdDa cutoff filter (Amicon® Ultra-4) to remove urea found in the serum. α-L-Fucosidase activity in serum was estimated using a colorimetric assay kit (IAZYME) according to the supplied instructions.
2.7 Human and animal rights
No humans were used in this study. All rats used in this study were euthanized and killed under general anesthesia. All procedures involving animals’ research were carried out following the published standards in the 8th Edition of the Guide for the Care and Use of Laboratory Animals (https://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf).
2.8 Statistical analysis
Results were expressed as means ± SD of the number of experiments. A student’s unpaired t-test (GraphPad Prism-Version 7.0 for windows) was performed and a p-value ≤ 0.05 was considered statistically significant.
3 Results
3.1 GC/MS analysis of AB-DPK
To determine the biochemical content of AB-DPK, GC/MS was employed and the chromatogram is shown in Fig. 1.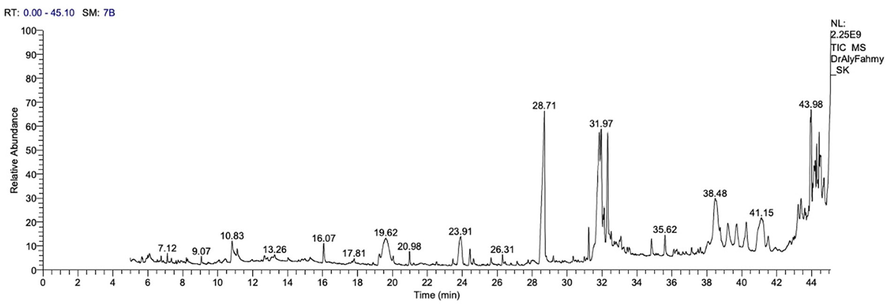
Chromatogram presenting results of GC/MS-analysis of AB-DPK.
The major constituents identified in the extract of AB-DPK were 1-Trilinolein, (Z,Z) 3Dioctadecenoyl Glycerol, Palmitic acid, N-carbonbenzyloxy-1-lyrosyl-1-valine, L-Arganile, 3,5-Heptadienol- 2ethyllidene-6-methyl, 1,2-Benzediol, 1-Dodecanamin N,N-Dimethyl, Benzene, 1,1-(3-(3-cyclopentylpropyl)-1,5 pentaedyl)pentane, Oleic acid, Cis – Vaccenic acid, Cholan-24-Oic-acid.3.7.12-trihydroxy-,(3α,5α,12α, Octadecanoic acid, 1H-purine-2-,6-dione,3,7- dihyro-1,3,7-trimethyl, Docosanoic acid,1,2,3-propanetriyl ester, Α-Sitasterol, 3-Acetoxy-12-Ursanol, Betulin, Α-Amyrin, Lupeol, Betuinaldehyde, Stigmasterol, LUP-20(29)-EN-3-YL acetate, Ursolic aldehyde and Ethyl isoallocholate.
3.2 Antimicrobial potential
To test the antimicrobial activities of AB-DPK, different bacteria (as shown in Fig. 2) were exposed to AB-DPK powder, with 30 µg of neomycin as control, for 24 h. As shown, none of the bacteria evidenced sensitivity to AB-DPK.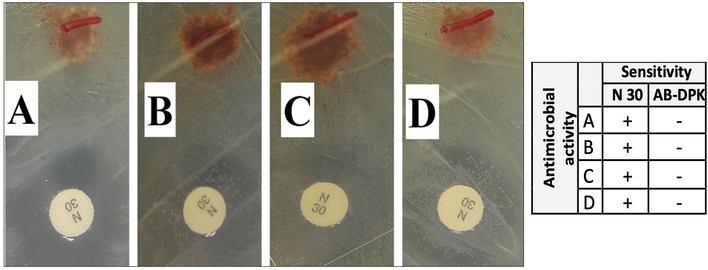
Antimicrobial activity of AB-DPK against Staphylococcus aureus (A), Bacillus subtilis (B), Protius mirabilis (C) and Escherichia coli (D). N = 30 µg of neomycin.
4 Toxicity
4.1 Cytotoxicity and IC50
To assess the anticancer potential of AB-DPK, colon cancer cell line (HCT-116) and liver cancer cell line (HepG2) were exposed to different concentrations of the AB-DPK extract for 24 h. MTT assay was then used to assess the cell viability post-exposure. A dose-dependent decrease in cell viability was observed in both HCT-116 and HepG2 cell lines after 24 h exposure (Fig. 3). The estimated IC50 was found to be 43.2 ± 4.9 µg/ml and 63.7 ± 5.4 µg/ml for HCT-116 and HepG2 cell lines respectively. This indicates that the HCT-116 cell lines were more sensitive to the AB-DPK extract for the exposure period observed here.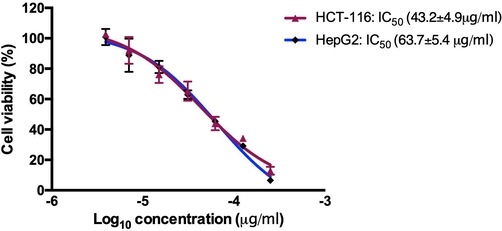
The effects of AB-DPK compound on the viability of HCT-116 and HepG2 cell lines.
4.2 Effect of AB-DPK on liver and kidney functions
Rat exposure to AB-DPK extract was found to be safe, without impairment to both the liver and kidney functions. Assessment of liver function parameters, such as ALT, AST and total bilirubin, as shown in Fig. 4, highlighted that AB-DPK extract did not influence the level of these liver function parameters in comparison to the control unexposed groups. Similarly, kidney function parameters, urea, creatinine and serum total proteins levels were also not affected due to the exposure of the rats to AB-DPK extract. This also indicates that the extract did not cause impairment of kidney function (Fig. 5).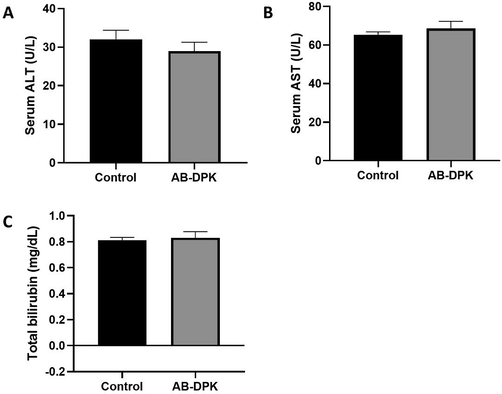
Liver function assessment after exposure to extract of AB-DPK. Levels of (A) ALT, (B) AST and (C) total bilirubin in control unexposed and AB-DPK exposed rats (n = 10) after 48 h exposure. Data is presented as mean ± SD.
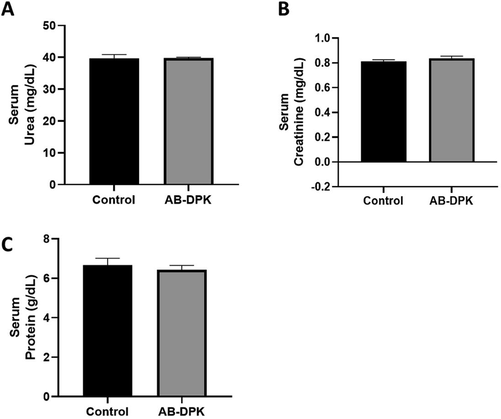
Kidney function assessment after exposure to extract of AB-DPK: Levels of (A) urea, (B) creatinine and (C) serum protein in control unexposed and AB-DPK exposed rats (n = 10) after 48 h exposure. Data is presented as mean ± SD.
4.3 Oxidant/antioxidant activity
The results from assessment of oxidative stress in the liver and kidney of rats exposed to the extract of AB-DPK are shown in Figs. 6 and 7. Seven oxidative stress markers (TBAC, CAT, GPx, GSH, H2O2, SOD, and TAC) were quantified in the livers and the kidneys of control unexposed and exposed rats. Findings showed significantly lower levels of TBARS in both the livers and kidneys of the exposed rats (p ≤ 0.05) when compared with the control unexposed rats. Similarly, GPx and GSH levels in the livers (p ≤ 0.0001 and p ≤ 0.01 respectively) and kidneys (p ≤ 0.0001) of rats exposed to AB-DPK extract were significantly higher than the levels in the control unexposed group. The CAT and TAC levels in the livers of rats exposed to the extract were significantly lower than those in the control unexposed rats (p ≤ 0.01 and p ≤ 0.001 respectively). Conversely, the CAT and TAC levels in the kidneys of rats exposed to the extract were significantly higher than those in the control unexposed rats (p ≤ 0.0001 and p ≤ 0.001 respectively). While H2O2 levels in the kidneys were significantly higher in the exposed rats when compared to the control unexposed rats (p ≤ 0.01), the SOD levels were similar across the two groups. On the contrary, the SOD levels in the livers of the extract exposed rats were significantly lower when compared with the unexposed control rats. Finally, the H2O2 levels were similar across the two groups.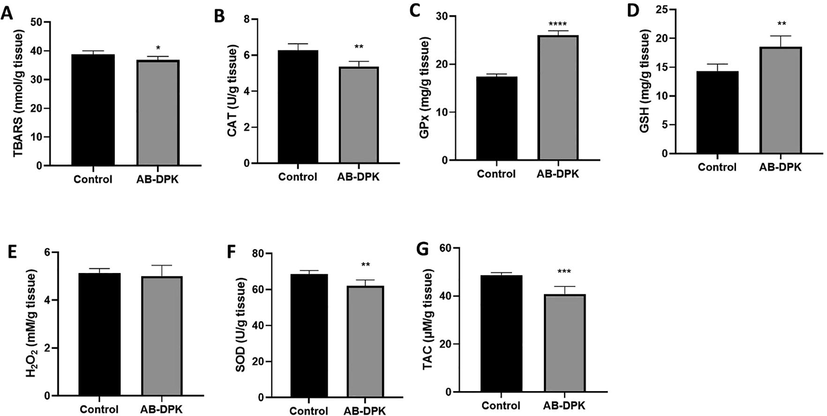
Oxidative stress assessment in RAT liver post-exposure to extract of AB-DPK. Levels of (A) TBARS, (B) CAT, (C) GPx, (D) GSH, (E) H2O2, (F) SOD and (G) TAC were quantified in homogenized livers of exposed and control rats (n = 10). Data is presented as mean ± SD, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
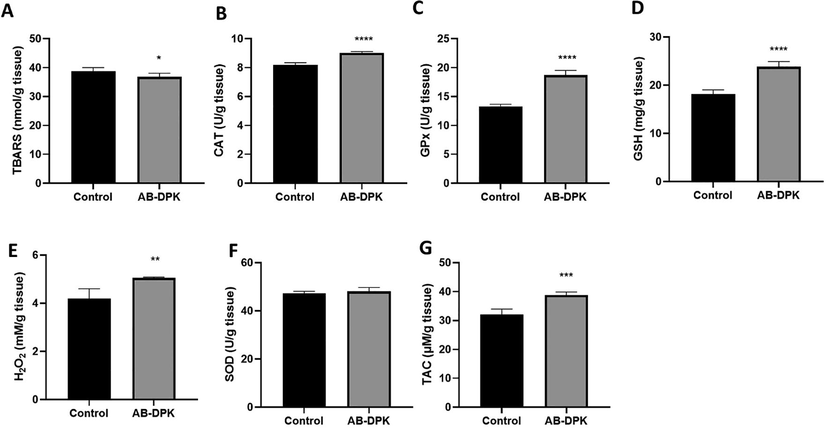
Oxidative stress assessment in RAT kidney post-exposure to extract of AB-DPK. Levels of (A) TBARS, (B) CAT, (C) GPx, (D) GSH, (E) H2O2, (F) SOD and (G) TAC were quantified in homogenized livers of exposed and control rats (n = 10). Data is presented as mean ± SD, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
4.4 Serum tumor markers
Anti-tumor activities of the extract of AB-DPK were evaluated by quantifying Arginase and α-L-Fucosidase levels, which are widely assayed tumor markers. Data here showed that AB-DPK extract does not affect the serum level of arginase. However, a significantly higher level of α-L-Fucosidase was observed in the treatment of healthy rats with AB-DPK extract, which showed no induction of any tumor formation, as indicated by normal levels of the tumor markers arginase and α-L-fucosidase in the AB-DPK treated rats (Fig. 8).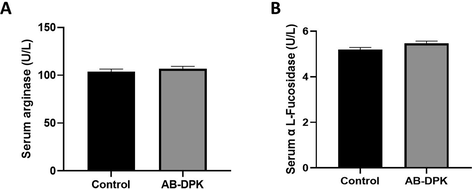
Anti-tumor effect of AB-DPK extracts. Levels of serum (A) Arginase and (B) α-L-Fucosidase in the control unexposed and AB-DPK extract exposed groups (n = 10) are shown here. Data is presented as mean ± SD, **p ≤ 0.01.
5 Discussion
There are reports in the literature on the anti-inflammatory and antioxidant effects of date palm seeds. In this study, a detailed functional study of date palm seed (AB-DPK) on liver and kidney functions was carried out. First, the extract of AB-DPK was analyzed with GC/MS. Compounds found in the analysis included antioxidants, such as 1-Trilinolein and (Z,Z) 3Dioctadecenoyl Glycerol, and anti-tumor, anti-inflammatory and antiviral agents such as betulin (Zhang et al., 2015; Pang et al., 2018). We also found ursolic aldehyde, which is readily oxidized to ursolic acid and modulates different targets of apoptosis, inflammation and angiogenesis to prevent chronic diseases, such as cancer, even before disease development (Kashyap et al., 2016).
Anti-bacterial activity assessment of the AB-DPK was carried out on different bacteria strains including Staphylococcus aureus, Bacillus subtilis, Protius mirabilis and Escherichia coli. Findings showed no antibacterial activities of AB-DPK on these bacteria. This is in contrast with other studies that have shown extract from date seeds possesses antibacterial properties. ALrajhi et al. (2019) demonstrated more potent antibacterial activity of ethyl acetate extract of date palm seed on P. aeruginosa, E. coli, and P. mirabilis in comparison to gentamycin. Metoui et al. (2019) also showed the extract of date palm seed to have antibacterial activities against bacteria strains like E. coli, S. aureus, S. epidermis, and S. Typhinurium. Although both ethyl acetate and methanolic extract, according to these studies, were active on E. coli, the contradiction to this finding in our study may still be explained by the fact that our extract may not contain more potent antibacterial effects as those of these studies. Although AB-DPK does not show antibacterial activities here, exposure to HCT-116 and HepG2 cancer cells revealed dose-dependent cytotoxicity on these cells. This anticancer activity is in agreement with a study by Khattak et al. (2020), where they showed a dose-dependent cytotoxic effect of different date fruit extract on breast cancer cell line, MDA-MB 231. In addition, in vivo work has demonstrated that aqueous extract of Ajwa date fruits reverses the development of liver cancer in rats induced with hepatocellular carcinoma (Khan et al., 2017).
One of the criteria for acceptance of pharmacoactive drugs onto the market for use is safety. Biochemical parameters that are widely used to assess safety are liver and kidney function tests, mainly due to the function of both organs in metabolism and excretion of drugs respectively (Newsome et al., 2018). Intracellular enzymes, such as the AST and ALT, as well as total bilirubin levels, are sensitive and significant indicators of hepatic injury. Increased activities of AST and ALT indicate leakage of the cell membrane and thus loss of the membrane integrity, while high levels of bilirubin are an indication of hepatic damage or liver injury due to impaired biliary function of the liver (Boone et al., 2005). Serum AST, ALT and total bilirubin levels in rats exposed to AB-DPK were unaffected in this study, which indicates that AB-DPK extract did not induce a toxic or detrimental effect on liver function. Not only is Phoenix dactylifera seed extract AB-DPK, as found here, not toxic to liver function, a previous study has also demonstrated that both methanolic and aqueous extracts for Phoenix dactylifera confer protective effects on oxidative and hepatic injury induced by paracetamol. This is due to the blocking of paracetamol-induced oxidative stress, causing hepatocellular injury and elevated serum ALT and AST (Salem et al., 2018). In contrast to this finding, another study has shown that aqueous extract of Ajwa date fruit reduces serum ALT and AST (Khan et al., 2017). However, this was in rats that were induced with hepatocellular carcinoma with diethylnitrosamine, which may be an attempt of the extract to skew biochemical parameters that help with tumorigenic effects.
Induced oxidative stress in the liver and the kidney is often as a result of increased oxidants such as peroxides and superoxides with reduced expression of antioxidants such as SOD, GPx and CAT, which scavenges of metabolizes the oxidative agents (Mates, 2000). As such, the assessment of intracellular antioxidant activities is not an isolated event but is a holistic assessment of intracellular antioxidants and oxidants ratios. In this study, the levels of these hepatic antioxidants were also assayed. While the levels of hepatic SOD and CAT were significantly reduced (p < 0.01) by AB-DPK treatment, GPx and GSH levels were significantly increased (p < 0.0001). In addition, the levels of oxidant H2O2 in the AB-DPK-treated rats were unaffected. GPx is a peroxidase that detoxifies the cells by regulating lipid peroxidation by free radicals, such as those generated by the decomposition of H2O2 (Mates, 2000). GSH on the other hand serves to assist both SOD, which deactivates superoxide radials and GPx to convert H2O2 to water (Zhan et al., 2004). Taken together, this evidence suggests that there is redox balance in the rat liver due to the antioxidant (SOD, CAT, GPx) to oxidant (H2O2) levels observed, which was maintained, as in the control.
Similar to that observed for the liver function, increased levels of serum creatinine, urea and total protein are also indicators of compromised renal function. Results from this study showed that AB-DPK extract did not influence the levels of these biochemical parameters in exposed rats; thus, not compromising renal function. Assessment of possible renal oxidative stress parameters in AB-DPK-treated cells showed significantly increased levels of H2O2, which may indicate oxidative stress at first glance. However from our study, examining the significantly increased levels of GPx, CAT, and GSH, coupled with normal levels of SOD, indicates a redox balance in the rats treated with AB-DPK (Gurudath et al., 2012; Puertas et al., 2012). Usually, a lower level of SOD, CAT, GSH and GPx, and an abnormally high level of H2O2 signal oxidative stress that will result in tissue damage, as observed in conditions such as cancer or neurodegenerative diseases (Gurudath et al., 2012; Puertas et al., 2012). This is because the antioxidant enzymes and molecules are unable to cope with the rising level of oxidants (e.g. peroxides and superoxides), resulting in tissue damage.
TBARS is used as a measure of lipid peroxidation, and increases in TBARS levels have been reported in neurodegenerative diseases and cancer with high active oxidant activities (Kolanjiappan et al., 2002; Puertas et al., 2012; Jablonska et al., 2015). In diseased conditions, high TBARS levels of this nature are accompanied by low GPx levels, similar to the pattern of SOD and CAT production concerning their substrate, H2O2, under the same health conditions. From this information, it is possible to suggest that AB-DPK seems also to have a protective antioxidant role by maintaining lower levels of TBARS than in untreated rats. However, further investigation is needed to prove this, even though this suggestion is supported by the findings of Salem et al. (2018). L-arginase and α-fucosidase are routinely used as tumor markers of hepatocellular and colorectal carcinoma based on the significantly higher levels of these enzymes in cancer patients compared with healthy matched individuals (Leu and Wang, 1992; Ayude et al., 2000; Kolanjiappan et al., 2002). Assessment of serum L-arginase and α-fucosidase upon AB-DPK extract exposure showed that the extract did not influence the levels of these enzymes in the rat serum, suggesting no tumorigenic tendencies of the extract. Although extensive studies on possible tumor-inducing effects beyond the assessment of serum L-arginase and α-fucosidase will need to be carried out, this finding is a first step in testing the safety of the extract concerning cancer development.
6 Conclusion
It has been shown here that while AB-DPK has no negative effect on the hepatic and renal functions, the extract seems to enhance both hepatic and renal redox balance by stimulating the production of CAT, GPx, SOD, and GSH that can handle any increases in peroxide or superoxide levels. In addition, AB-DPK may also confer protective effects on induced oxidative stress. Coupled with the possible anticancer effects on the colorectal and hepatocellular carcinoma cell lines, AB-DPK may prove as a useful therapeutic for the treatment of some cancers, although this requires further investigation intensively.
Acknowledgements
The author would like to thank Dr. Hossein Bayahia, Dr. Ali Moosa Mahzari, Dr. Azeez Oriyomi Yusuf, and Prof. Essam H. Ibrahim for their invaluable help in reviewing this manuscript before submission. The author also would like to thank the Deanship of Scientific Research at Al Baha University in Saudi Arabia for their support.
Funding
This research was funded by the Deanship of Scientific Research at Al Baha University in Saudi Arabia (Grant Number 5/1441).
Ethics approval and consent to participate
Ethical approval of the study protocol was given by the Research Ethics Committee at King Khalid University in Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification of essential oil components by gas chromatography/mass spectrometry. Vol vol. 456. IL: Allured publishing corporation Carol Stream; 2007.
- Biological yields through agricultural extension activities and services: A case study from Al-Baha region–Kingdom of Saudi. Arabia. 2021;28(5):2789-2794.
- [Google Scholar]
- Boron removal from seawater using date palm (Phoenix dactylifera) seed ash. Desalin. Water Treat.. 2016;57(11):5130-5137.
- [Google Scholar]
- Antibacterial activity of date palm cake extracts (Phoenix dactylifera) Cogent Food Agric.. 2019;5(1):1625479.
- [Google Scholar]
- Date fruit classification for robotic harvesting in a natural environment using deep learning. IEEE Access. 2019;7:117115-117133.
- [Google Scholar]
- Antidiabetic activity of date seed methanolic extracts in alloxan-induced diabetic Rats. Pakistan Vet. J.. 2019;39(4)
- [Google Scholar]
- Value of the serum alpha-L-fucosidase activity in the diagnosis of colorectal cancer. Oncology. 2000;59(4):310-316.
- [Google Scholar]
- Bioactive compounds, minerals, fatty acids, color, and sensory profile of roasted date (Phoenix dactylifera L.) seed. J. Food Process. Preserv.. 2020;44(7):e14495.
- [Google Scholar]
- Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet. Clin. Pathol.. 2005;34(3):182-188.
- [Google Scholar]
- Silver Nanoparticle Production by Ruta graveolens and Testing Its Safety, Bioactivity, Immune Modulation, Anticancer, and Insecticidal Potentials. Bioinorg. Chem. Appl.. 2020;2020:1-11.
- [Google Scholar]
- Physicochemical properties and applications of date seed and its oil. Int. Food Res. J.. 2017;24(4)
- [Google Scholar]
- Estimation of superoxide dismutase and glutathione peroxidase in oral submucous fibrosis, oral leukoplakia and oral cancer–a comparative study. Asian Pac. J. Cancer Prev.. 2012;13(9):4409-4412.
- [Google Scholar]
- Polyphenolic compounds in date fruit seed (Phoenix dactylifera): characterisation and quantification by using UPLC-DAD-ESI-MS. J. Sci. Food Agric.. 2014;94(6):1084-1089.
- [Google Scholar]
- In Vivo Evaluation of Anti Diabetic, Hypolipidemic, Antioxidative Activities of Saudi Date Seed Extract on Streptozotocin Induced Diabetic Rats. J Clin Diagn Res 2016 10(3):FF06-12
- [Google Scholar]
- Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.)–A review. Food. Bioscience. 2020;34:100509.
- [Google Scholar]
- Lepidium sativum and its biogenic silver nanoparticles activate immune cells and induce apoptosis and cell cycle arrest in HT-29 colon cancer cells. J. Biomater. Tissue Eng.. 2021;11(2):195-209.
- [Google Scholar]
- Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC Cancer. 2015;15(1)
- [Google Scholar]
- Quantitative analyses of flavour and fragrance by capillary gas chromatography. New York: Academic Press; 1980.
- Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci.. 2016;146:201-213.
- [Google Scholar]
- Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern. Med.. 2017;17(1)
- [Google Scholar]
- Anticancer activities of selected Emirati Date (Phoenix dactylifera L.) varieties pits in human triple negative breast cancer MDA-MB-231 cells. Saudi. J. Biol. Sci.. 2020;27(12):3390-3396.
- [Google Scholar]
- Isolation, screening and molecular identification of novel bacterial strain removing methylene blue from water solutions. Appl. Water Sci.. 2017;7(7):4091-4098.
- [Google Scholar]
- Measurement of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin. Chim. Acta. 2002;326(1–2):143-149.
- [Google Scholar]
- Clinical significance of arginase in colorectal cancer. Cancer. 1992;70(4):733-736.
- [Google Scholar]
- Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153(1–3):83-104.
- [Google Scholar]
- Chemical composition, antioxidant and antibacterial activity of Tunisian Date Palm Seed. Polish J. Environ. Stud.. 2019;28(1):267-274.
- [Google Scholar]
- Processing of date palm kernel (DPK) for production of nutritious drink. Adv. Nat. Appl. Sci.. 2012;6(5):575-582.
- [Google Scholar]
- Effect of AJWA date seed on lipid profile of diet induced hyperlipidemic rabbits. Khyber Med. Univ. J.. 2017;9(3)
- [Google Scholar]
- Mineral, vitamin and phenolic contents and sugar profiles of some prominent date palm (Phoenix dactylifera) varieties of Pakistan. Pak. J. Bot.. 2019;51(1):171-178.
- [Google Scholar]
- Assessment of the ameliorative effect of ruzu herbal bitters on the biochemical and antioxidant abnormalities induced by high fat diet in wistar rats. Int. J. Pharm.. 2018;14(3):329-341.
- [Google Scholar]
- Betulinic acid-induced expression of nicotinamide adenine dinucleotide phosphate-diaphorase in the immune organs of mice: A possible role of nitric oxide in immunomodulation. Mol. Med. Rep.. 2018;17(2):3035-3041.
- [Google Scholar]
- Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp. Gerontol.. 2012;47(8):625-630.
- [Google Scholar]
- Chemical composition of Saudi Arabian Sukkari variety of date seed oil and extracts obtained by slow pyrolysis. Indian J. Pharm. Sci.. 2018;80(5):940-946.
- [Google Scholar]
- Nutritional quality and physico-chemical characteristics of selected date fruit varieties of the United Arab Emirates. Processes. 2020;8(3):256.
- [Google Scholar]
- Phoenix dactylifera protects against oxidative stress and hepatic injury induced by paracetamol intoxication in rats. Biomed. Pharmacother.. 2018;104:366-374.
- [Google Scholar]
- Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: effect of antioxidant-rich diet. J. Hypertens.. 2004;22(10):2025-2033.
- [Google Scholar]
- Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J. Agric. Food Chem.. 2013;61(24):5834-5840.
- [Google Scholar]
- Betulinic acid and its derivatives as potential antitumor agents. Med. Res. Rev.. 2015;35(6):1127-1155.
- [Google Scholar]







