Evaluation of hydrophilicity and moisture adsorption characteristics of inorganic mineral pigments to sustain ancient paintings
⁎Corresponding authors. lifj@lzu.edu.cn (Feng-jie Li), yhwlzu@yeah.net (Hong-wei Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Inorganic mineral pigments were widely used in ancient painted artworks, and their adsorption characteristic of water vapor in the environment is one of the reasons affecting the deterioration process of paintings under environmental humidity. This paper investigates the characteristics and mechanism of the water vapor adsorption process of inorganic mineral pigments in order to guide the conservation of ancient painting artworks.
Material and methods
The contact angle and isothermal hygroscopic curves of common inorganic mineral pigments were measured, as well as the nanoscale pore characteristics of mineral pigments and the effect of gelatin on the hygroscopic process of mineral pigments. The hydrophilic and hygroscopic properties of different mineral pigments were analyzed and compared.
Results
Earthen red and Kaolinite have been the strongest hygroscopic minerals, and their adsorption processes include adsorption on the particle surface and also capillary condensation. The hygroscopicity of mineral pigments containing iron oxide and carbonate was moderate, with moisture adsorption occurring mainly on the particle surface, whereas the other mineral pigments had little hygroscopicity. Furthermore, the gelatin used to mix mineral pigments has the ability to invade the nanoscale pores of the pigments and modify the pigments' water vapor adsorption process.
Conclusion
The results of the study can provide a theoretical basis for revealing the deterioration process of ancient painting artworks under the influence of the climatic environment, and can be useful in proposing conservation measures for painted artworks.
Keywords
Environmental humidity
Mineral pigment
Vapor adsorption
Contact angle
Isothermal hygroscopic curve
Nitrogen adsorption method
1 Introduction
Inorganic mineral pigments are a common type of pigment used in ancient painted artworks (Fig. 1) (Li, 2005; Ariadne et al., 2019; Zhou and Li, 2019; Li, 2020). Common inorganic mineral pigments include Cinnabar, Earthen red, Calcite, Realgar, Red lead, Yellow lead, and, Azurite (Yin et al., 2019; Li et al., 2021). The use of mineral pigments often requires the addition of a cementing material, which not only binds the powdered mineral pigment particles but also makes the painting process smoother. The most commonly used cementing material is usually cowhide glue, which is similar in composition to modern gelatin, being a natural macromolecular polymer derived from the collagenous parts of animal bones, rawhide, tendons, and other connective tissues. Today, gelatin is used directly when painting or restoring paintings using mineral pigments, and it is added to mineral pigments at a rate of approximately 2–10% (Li, 2017).

- Ancient painted artworks using mineral pigments: (a)wall painting (b)rock painting (c)sculpture (Li,2020).
The presence of water vapor in the environment is one of the most important factors in the deterioration of ancient artworks (Wilson-Yang and Burns, 2011). Previous research had focused on the chemical reactions between mineral pigments and water molecules, and had concluded that most mineral pigments are stable and do not react with water molecules to form new substances within a short period of time, which is one of the reasons why so many ancient paintings have been preserved to this day (Yin, 1990; Jie and Zhang, 2019; Li, 2017). However, the pigment layer still has physical moisture adsorption capacity. Under the influence of environmental humidity fluctuations, the surface of ancient paintings still undergoes a series of deterioration processes, such as cracks caused by differences in deformation of different materials, salt diseases caused by the interaction of water and salt (Germinario and Oguchi, 2022), and microbial diseases caused by the involvement of moisture, etc. (Li et al., 2018; Shen et al., 2020; He et al., 2022).
The physical adsorption of water vapor by materials is mainly due to two processes (Ning and William, 2012; Li et al., 2018; Li, 2020): 1) The adsorption of water molecules is caused by van der Waals forces and electrostatic forces on the surface of the particles, this process is related to the surface characteristics of the material; 2) the adsorption process of capillary condensation under the influence of the pore characteristics within the material. Since capillary condensation in porous materials theoretically only occurs in pores in the range of 0.1–200 nm, only the microporosity characteristics in this range can influence water vapor adsorption (Coasne and Pellenq, 2004; Ning and William, 2012). The pigment layer on the surface of painted artworks is a mixture of mineral pigments and cementing material, both of which are hygroscopic. Therefore, the process of water vapor adsorption of paint layer in artworks can arise from three sources: 1) The adsorption on the surface of the pigment particles, caused by Van der Waals forces and electrostatic forces, which are directly related to the hydrophilicity of the material; 2) The capillary condensation dominated by the micropore distribution characteristics (pores within 0.1–200 nm) of the mineral pigment, and 3) Hygroscopicity of cementing materials (such as gelatin). In addition, whether the addition of gelatin to mineral pigments affects the water vapor adsorption characteristics of the mineral pigments themselves is also a question that needs to be investigated.
Therefore, the objectives of this study include: (1) analyzing water vapor adsorption characteristics and mechanism of different mineral pigments by measuring the hydrophilicity, isothermal hygroscopic curve, specific surface area, and pore characteristics; (2) exploring the effect of gelatin addition on the water vapor adsorption characteristics of mineral pigments; (3) providing a theoretical basis for the conservation of ancient paintings and artworks.
2 Materials and methods
2.1 Test material
We discuss the water vapor adsorption characteristics of several most common inorganic mineral pigments in this study. Table 1 summarizes their name and color, as well as their mineral percentage and particle size. And the gelatin used in this study is analytically pure.
| Mineral pigments | Color | Average particle size (μm) |
Main components |
|---|---|---|---|
| Earth Red | brownish red | 9.871 | Kaolinite 40%, Ilmenite 18%, Needle iron ore 24%, Quartz 18% |
| Ochre powder | brownish red | 12.341 | Hematite 72%, Quartz 18%, Calcium carbonate 10% |
| Kaolinite | White | 15.623 | Kaolin > 85%, impurities mainly quartz |
| Calcite | White | 49.549 | Calcite (CaCO3) > 90%, impurities mainly quartz |
| Cinnabar | Bright red | 15.672 | Cinnabar (HgS) > 90%, impurities mainly quartz, calcite |
| Realgar | Yellow | 18.451 | Andrographis (AsS) > 90%, impurities mainly quartz |
| Orpiment | Yellow | 17.645 | Estradiol (As2 S3) > 90%, impurities mainly quartz |
| Red lead | Shallow orange | 19.763 | Pb3 O4 > 90%, impurities mainly quartz |
| Yellow lead | Deep orange | 16.875 | PbO > 90%, impurities mainly quartz |
| Azurite | Blue | 22.352 | Lime (Cu3 [CO]2 (OH)2) > 90%, impurities mainly quartz |
| Green stone | Green | 16.342 | Green stone (Cu2 [ CO3] (OH) 2) > 90%, impurities mainly quartz |
2.2 Powdered mineral pigments hydrophilic
Contact angle of a material directly reflects its hydrophilicity, whereas the contact angle of powdered material cannot be measured directly and is usually deduced using the capillary rise method. The theoretical basis for this method is the Washburn equation (Kirdponpattara et al., 2013; Valencia and Pileggi, 2018):
Vv (cm3), Vs (cm3), and V (cm3) are the pore volume, solid particle volume, and total volume respectively, where Vs (cm3) can be expressed as.
ms (g), ρs (g/cm3), Gs (dimensionless) are the dry mass and dry density of the particles as well as the specific gravity, and ρw (g/cm3) is the density of water. Combining equation (2) with (3), we obtain.
Therefore, the same pore ratio (e = 0.7) and the sample filling volume (V = 1.256 cm3, Filling height = 10 cm; capillary tube inner diameter = 0.4 cm) in the capillary tube is first set for the different mineral pigment samples in the capillary tube, and then the required sample mass (ms) can be deduced from the specific gravity of the different mineral pigments (Gs) (Table 2). The test device and specific test procedures are shown in Fig. 2. For each sample, 8 sets of moisture rise timings and their related rise heights were measured, with the room temperature kept at 23 °C altogether.
| Mineral pigments | Specific gravity | Filling mass (g) |
|---|---|---|
| Cinnabar | 2.86 | 1.2430 |
| Red lead | 2.85 | 1.2386 |
| Yellow lead | 2.85 | 1.2386 |
| Azurite | 2.75 | 1.1952 |
| Green stone | 2.75 | 1.1952 |
| Calcite | 2.75 | 1.1952 |
| Ochre powder | 2.75 | 1.1952 |
| Kaolinite | 2.60 | 1.1300 |
| Realgar | 2.60 | 1.1300 |
| Orpiment | 2.60 | 1.1300 |
| Earth Red | 2.60 | 1.1300 |
*Sample pore ratio e was set at 0.7 and a sample filled to a height of 10 cm in the capillary (i.e. a filling volume of 1.256 cm3).
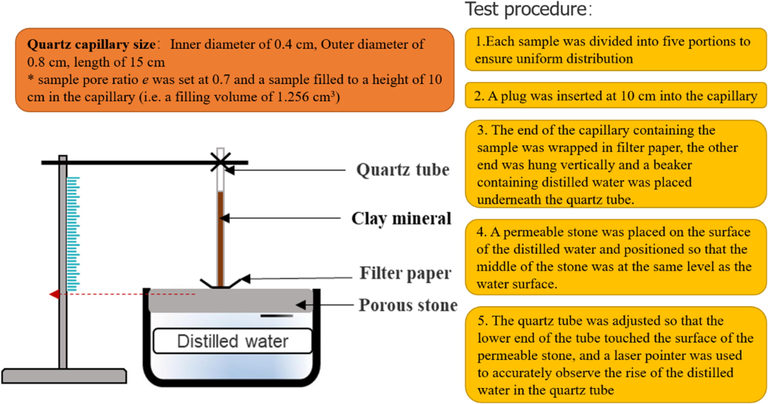
- Device and test procedure for measuring contact angle of powdered mineral pigments.
2.3 Determination of isothermal hygroscopic curves for powder pigments and pigment layers
Under constant temperature, the isothermal hygroscopic curve of the material is obtained by taking the relative humidity (RH) as abscissa and the equilibrium moisture content of the material corresponding to different RH as ordinate. Therefore, this study calculated the equilibrium moisture content for pigment samples by measuring the dry mass of each sample and the mass after hygroscopicity saturation under different RH conditions. The samples and detailed testing procedures are shown in Fig. 3. Different RH conditions were controlled by the saturated salt solution in the sealed chamber, and room temperature was maintained at 23 °C during moisture adsorption.
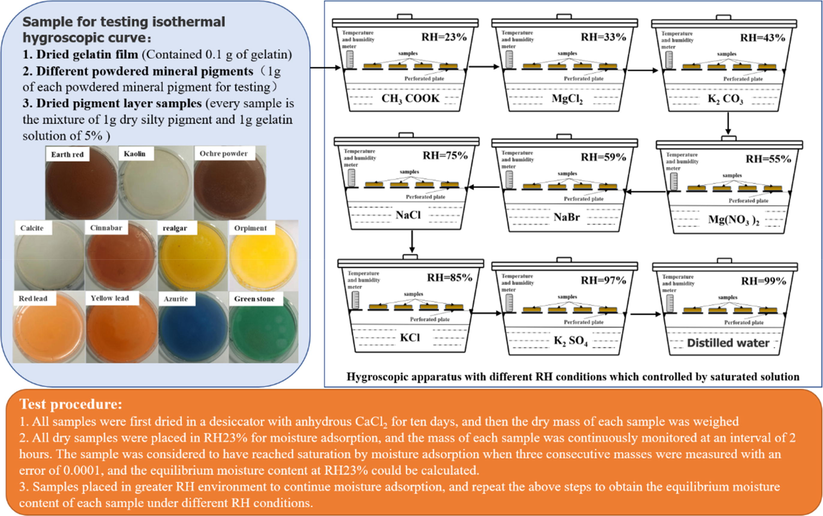
- Sample, test device and test procedure for isothermal hygroscopic curve.
2.4 Determination of specific surface area and pore characteristics
The specific surface area and pore characteristics of the different powder pigments and the pigment layer samples were determined using nitrogen adsorption apparatus (Tristar II 3020) from Micromeritics company. The TriStar II 3020 is a fully automatic three-station specific surface area and porosity analyzer designed to measure material-specific surface area, pore size distribution, and volume.
3 Result and discussions
3.1 Hydrophilic
Theoretically, a material with a contact angle >90° is considered hydrophobic, in fact when the contact angle is >65° the material can already be considered hydrophobic (Zhao, 2000; Julie et al., 2022). The H2 -t curves of different powder mineral pigments are straight lines (Fig. 4). Table 3 shows the results obtained from the calculations. The Highest hydrophilic minerals are Earth red and Ochre powder and it is followed by Kaolinite, Calcite, Azurite, and Green stone. The contact angles of Cinnabar, Red lead, Yellow lead, Realgar, and Orpiment can be considered to be largely non-hydrophilic.
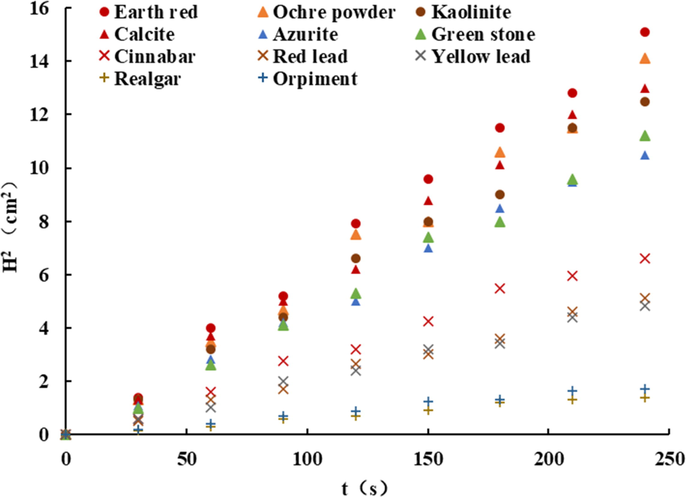
- Test results of contact angle determination of powdered mineral pigments (H2 -t).
| Mineral pigments | Powdered mineral pigments | 5% gelatin pigment layer | |||
|---|---|---|---|---|---|
| H2 -t curve slope | Contact angle () | Specific surface area (m2 /g) |
Total volume of microporosity (cm3 /g) (0.1–200 nm) |
Total volume of microporosity (cm3 /g) (0.1–200 nm) |
|
| Earth Red | 0.0633 | 33.2 | 58.28 | 0.275 | 0.174 |
| Kaolinite | 0.0532 | 45.3 | 18.12 | 0.142 | 0.092 |
| Ochre powder | 0.0581 | 39.8 | 4.56 | 0.091 | 0.090 |
| Calcite | 0.0559 | 42.3 | 1.89 | 0.014 | 0.014 |
| Azurite | 0.0451 | 53.4 | 1.76 | 0.013 | 0.013 |
| Green stone | 0.0471 | 51.5 | 1.74 | 0.009 | 0.009 |
| Cinnabar | 0.0287 | 67.7 | 0.83 | 0.006 | 0.006 |
| Realgar | 0.0215 | 73.5 | 0.81 | 0.004 | 0.004 |
| Orpiment | 0.0204 | 74.4 | 0.79 | 0.004 | 0.004 |
| Red lead | 0.0062 | 85.3 | 0.54 | 0.002 | 0.001 |
| Yellow lead | 0.0075 | 84.3 | 0.49 | 0.002 | 0.001 |
*surface tension value of 7.238·10-4N-m−1 and viscosity of water is 9.57·10-2 Pa-s during counting process (Ning and William, 2012).
3.2 Isothermal hygroscopic curves
Gelatin film has a very strong water vapor adsorption capacity and can reach a saturation hygroscopic capacity of 45% at RH99% (Fig. 5(a)). Earthen red and Kaolinite have strong hygroscopic properties and Ochre powder has moderate hygroscopic properties, similar to Kaolinite in the low humidity range (<RH60%), but not very high in the high humidity range (>RH60%). Calcite, Azurite, and Green stone have a certain ability to absorb water vapor but can not absorb much moisture (Fig. 5(b)). The rest of the mineral pigments have no water vapor adsorption capacity and the amount of moisture adsorption in them cannot be measured. The moisture adsorption of the pigment layer is slightly higher than that of the corresponding powdered pigments at all humidity levels because the gelatin in the pigment layer absorbs a certain amount of water vapor (Fig. 5(c)). Fig. 5(d) also shows the difference between the saturated moisture adsorption of the pigment layer and the saturated moisture adsorption of the powdered mineral pigment. The amount of gelatin in the various pigment layer samples was 0.05 g. Gelatin's saturated moisture absorption in the pigment layer was estimated to be around 1.8 percent%. This difference is less than the theoretical moisture adsorption of gelatin for Earthen red and Kaolinite, indicating that the addition of gelatin in the pigment layer affects the vapor adsorption process of these two pigments; however, this difference is equal to the theoretical moisture adsorption of gelatin for other types of mineral pigments, indicating that the addition of gelatin has no effect on the water vapor adsorption process of these two pigments.
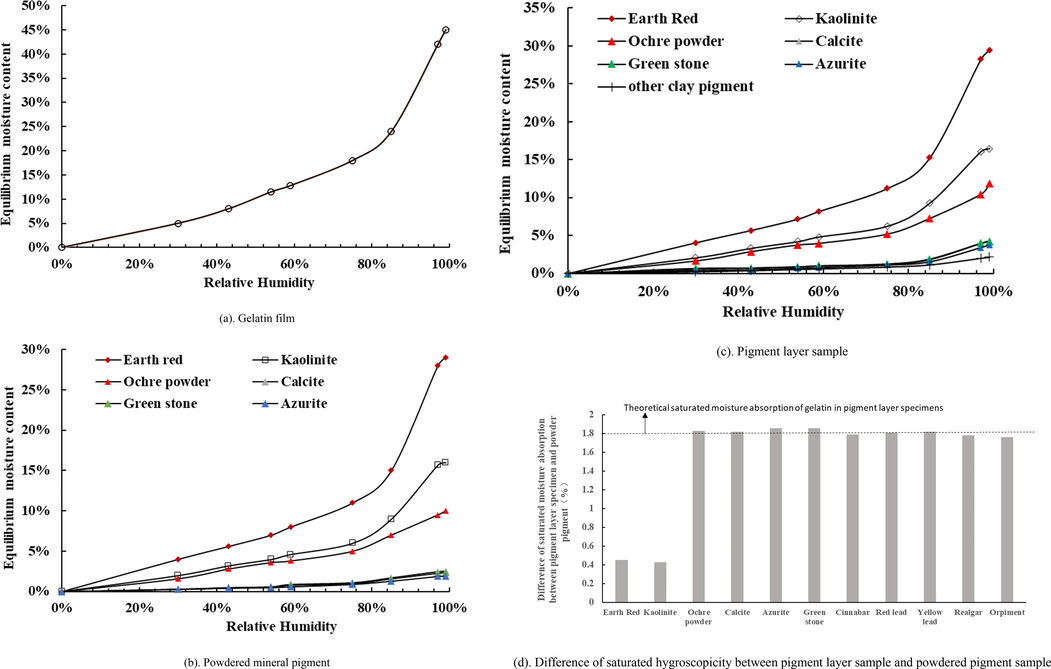
- Measurement results of isothermal hygroscopic curves.
3.3 Specific surface area and pore characteristics
Clay mineral pigments, such as Earthen red and Kaolinite, have a large specific surface area and microporous volume, whereas other mineral pigments have a smaller specific surface area and lack microporosity (Table 3). In addition, when comparing the results of the powdered mineral pigments with the pigment layers obtained after mixing with gelatin, it can be seen that for mineral pigments such as Earthen red and Kaolinite, the addition of gelatin reduces the volume of microporosity in the mineral pigments, indicating that gelatin molecules can invade and fill the microporosity. However, due to the small amount of microporosity in other types of mineral pigments, the addition of gelatin has no significant effect on the distribution of microporosity, and gelatin only fills the larger pores or binds the pigment particles.
3.4 Water vapor adsorption characteristics of different mineral pigments
In combination with the above test results, common mineral pigments can be classified into three categories according to their water vapor adsorption characteristics.
Firstly, mineral pigments containing clay minerals, such as Earthen red and Kaolinite, are highly hydrophilic and have a large microporous volume in them, suggesting that the water vapor adsorption of this type of mineral pigment is a combination of particle surface adsorption and capillary condensation. Theoretically, the total volume of microporosity is equal to the total amount of water that can be adsorbed by capillary condensation. The total volume of microporosity of both Earth red and Kaolinite accounts for >80% of their saturation moisture adsorption (i.e. moisture adsorption at RH99%), indicating that the majority of water adsorbed by these two mineral pigments comes from capillary condensation and that microporosity characteristics are the main factor affecting water vapor adsorption (Ning and William, 2012). More serious deterioration processes may be induced in this group of mineral pigments due to the relatively significant moisture adsorption under the influence of water vapor in the external environment.
Secondly, Ochre powder and carbonate mineral pigments (Calcite, Azurite, Green stone) have a certain capacity for water vapor adsorption but are not very hygroscopic, their water vapor adsorption occurs mainly on the surface of the particles. The main component in Ochre powder is hematite, a mineral consisting of free oxides containing trivalent Fe, which exhibits strong hydrophilicity due to the positive charge on its particle surface and its ability to adsorb water molecules with polarity (Qian, 2015). The carbonate mineral pigments (Calcite, Azurite, and Green stone) are somewhat hydrophilic but not very strong. There are electron exchange and chemical bonding between water molecules and calcium carbonate (Medeiros et al., 2007; Lardge et al., 2009; Chai et al., 2018), this is one of the reasons that carbonate mineral pigments have certain hygroscopicity. Previous research also suggested that there is a stable adsorption layer on the surface of ideal carbonates, which decreases as the distance between the water molecules and the surface of the particles increases (Wang et al., 2005; Wolthers et al., 2012).
Finally, mineral pigments that are poorly hydrophilic and also have a small microporous volume can be considered to have little ability to absorb water vapor. This category includes Cinnabar, Realgar, Orpiment, Red lead, and Yellow lead. These pigments do not usually deteriorate due to the physical adsorption of water vapor by the material itself. However, during the blending of pigments using gelatin solutions, the gelatin solution is less malleable between the mineral pigment particles, which may result in the gelatin molecules not bonding as tightly to the pigment particles as other mineral pigments, and causing the pigment particles falling out.
The isothermal sorption curves of the first two types of hygroscopic powdered mineral pigments, and gelatin films alone (Fig. 5(a) & Fig. 5(b)), can be further described by theoretical models of sorption curves. The main existing theoretical models used to describe the isothermal sorption curves of materials include Langmuir, Freundlich, Fritz-Schlender, Redlich-Peterson, and the BET multilayer sorption isotherm (Yan, 1986; Xie et al., 2019), the expressions and parameters of each model and fitting results are shown in Table 4. It is found that the BET adsorption model fitted the isothermal hygroscopic curves of Earth red and Kaolinite with the best fit results, both of which yielded correlation coefficients >0.99 (Table 4). The isothermal sorption curves for Ochre powder, Calcite, Azurite, Green stone, and gelatin films are fitted better when using the Freundlich sorption isotherm, all correlation coefficients >0.98. This model, although was a purely empirical formula initially and was later theoretically derived to give physical meaning to its parameters, and the derivation process be assumed to water vapor adsorption does not occur over the entire surface (Vannice, 2005). This also accords with the actual situation of the vapor adsorption process of these kinds of pigments.
| Isothermal adsorption theoretical model | Expressions | Parameters |
|---|---|---|
| Langmuir |
|
Q: amount of moisture absorbed.: Relative humidity k,: isothermal constant |
| Freundlich |
|
Q: amount of moisture absorbed.: Relative humidity k, n: isothermal constants |
| Fritz-Schlunder |
|
Q: amount of moisture absorbed.: Relative humidity k,, b1, b2: isothermal constants |
| Redlich-Peterson |
|
Q: amount of moisture absorbed.: Relative humidity k,, b: isothermal constant |
| BET Multilayer Adsorption Isotherm |
|
Q: amount of moisture absorbed.: Relative humidity qm, c, n: isothermal constants |
| Mineral pigments or gelatin | Fitting results | Correlation coefficient |
| Earth Red |
qm = 0.02808; c = 2.9777E44; n = 20.57225 |
0.9916 |
| Kaolin |
qm = 0.01624; c = -2.0034E44; n = 19.70999 |
0.9928 |
| Ochre powder | Q = kφn k = 0.09768; n = 1.73747 |
0.9801 |
| Calcite | Q = kφn k = 0.0253; n = 2.24681 |
0.9863 |
| Green Stone | Q = kφn k = 0.02407; n = 2.33093 |
0.9847 |
| Azurite | Q = kφn k = 01925; n = 2.15901 |
0.9843 |
| Gelatin film | Q = kφn k = 0.43804; n = 2.52982 |
0.9823 |
3.5 Effect of gelatin on the adsorption of water vapor from mineral pigments
Pigment layers with Earthen red containing varying concentrations (2% −10%) of gelatin were generated to examine its pore properties in order to further investigate the effect of the cementing material content on the microporosity of clay mineral pigments. Simultaneously, the viscosity of gelatin solutions of various concentrations were evaluated using a Brookfield viscometer to analyze the gelatin solutions' flow characteristics. To characterize the effect of different gelatin concentrations on the microporosity in the pigment, define the degree of gelatin microporosity filling in the Earthen red pigment layer sample. The gelatin filling degree is the ratio of the difference in microporous volume between the pigment layer and the powder pigment to the powder pigment's microporous volume. It has been shown that as the gelatin concentration increases, viscosity also increases (Fig. 6). This indicates that more gelatin molecules will be able to invade the micropores as the gelatin concentration increases, but when the concentration reaches a value, the gelatin solution is unable to invade the micropores. This may be related to the higher value of viscosity in gelatin solution with a bigger concentration, which increases the resistance of gelatin invading the pores (Lin, 2018).
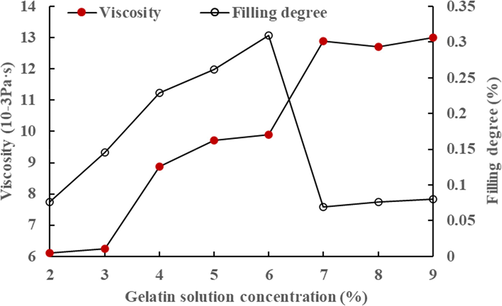
- Viscosity of gelatin solution and micropore filling degree of gelatin to pigment layer.
4 Conclusions
In our results, investigated in this research study, common inorganic mineral pigments are classified into three types based on their water vapor adsorption capacity: 1) mineral pigments containing clay minerals usually have the strongest water vapor adsorption capacity and good hydrophilicity, and the water vapor adsorption mainly comes from the combined effect of particle surface adsorption and capillary condensation, and their isothermal adsorption curves can be expressed using the BET multilayer isothermal adsorption equation. (2)) Ochre powders containing hematite and carbonate minerals pigments (including Calcite, Azurite, and Green stone) have a certain water vapor adsorption capacity and hydrophilicity, and the water vapor adsorbed mainly comes from the surface action of the particles. However, due to the low content of microporosity in them, capillary condensation is not obvious. The isothermal hygroscopic curves can be described using the Freundlich theoretical model. 3) Cinnabar, lead compounds (Red lead and Yellow lead), Realgar and Orpiment are no longer hydrophilic and have little capillary condensation ability due to their low microporous content, and therefore have little water vapor sorption capacity. A higher concentration of gelatin can fill more microporosity in clay mineral pigments, however, as the gelatin concentration continues to increase, there is essentially no influence on the microporosity in mineral pigments, which may be related to a change in the viscosity of the gelatin solution.
Based on the findings of this study, painted artworks with inorganic mineral pigments containing clay minerals should be given special consideration under the influence of changing environmental humidity, with measures taken to reduce the amount of water vapor in the environment. For painted artworks made with other mineral pigments, the lower hygroscopic properties of the pigments allow for a reduction in humidity control, but there is still a need to focus on monitoring the humidity in the environment.
Acknowledgements
The first author is grateful to the School of History and Culture, Lanzhou University for a post-doctoral researcher (Award No: 267779, dated 2020-09-24) for the financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Colourful earth: Iron-containing pigments from the Hellenistic pigment production site of the ancient agora of Kos (Greece) J. Archaeolog. Sci.: Rep.. 2019;26:101843
- [Google Scholar]
- First-principles study on the adsorption of H2O molecule on CaCO3 (104) surface. J. Atom. Mol. Phys.. 2018;35(6):1075.
- [Google Scholar]
- A grand canonical monte carlo study of capillary condensation in mesoporous media: effect of the pore morphology and topology. J. Chem. Phys.. 2004;121(8):3767-3774.
- [Google Scholar]
- Gypsum, mirabilite, and thenardite efflorescences of tuff stone in the underground environment. Environ. Earth Sci.. 2022;81(8):1-12.
- [Google Scholar]
- Maya blue pigments derived from clay minerals – science direct. Nanomater. Clay Minerals 2019:627-661.
- [Google Scholar]
- Surface properties of cork: Is cork a hydrophobic material? J. Colloid Interface Sci.. 2022;608(Part 1):416-423.
- [CrossRef] [Google Scholar]
- Assessment of cleaning techniques and its effectiveness for controlling biodeterioration fungi on wall paintings of Maijishan Grottoes. Int. Biodeterior. Biodegrad.. 2022;171
- [Google Scholar]
- Applicability of Washburn capillary rise for determining contact angles of powders/porous materials. J. Colloid Interface Sci. 2013 397(Complete):169–176
- [Google Scholar]
- Investigation of the interaction of water with the Calcite (10.4) surface using ab initio simulation. J. Phys. Chem. C. 2009;113(17):7207.
- [Google Scholar]
- Simulation experiment research on the Glue blending Process of Mogao Grottoes murals. Xi 'an: Northwest University; 2017.
- Moisture Adsorption Mechanism of Earthen Plaster Containing Soluble Salts in the Mogao Grottoes of China. Stud. Conserv.. 2018;64(3–4):159-173.
- [Google Scholar]
- Experimental study on the moisture adsorption characteristics and mechanism of the earthen plaster in the Mogao Grottoes of Dunhuang. Lanzhou: Lanzhou University; 2020.
- Recent research on natural pigments stabilized by clay minerals: A review. Dyes Pigm.. 2021;190(20):109322
- [Google Scholar]
- Protection of silk Road Grottoes frescoes with painted Sculptures. Beijing: Science Press; 2005.
- Effect of Liquid Viscosity and Surface Wettability on Droplet Impact Dynamics. Chengdu: Southwest Jiaotong University; 2018.
- Electronic and optical properties of CaCO3 Calcite, and excitons in Si@ CaCO3 and CaCO3@ SiO2 core-shell quantum dots. J. Phys. D Appl. Phys.. 2007;40(18):5747.
- [Google Scholar]
- Mechanics of unsaturated soils. Beijing: Higher Education Press; 2012.
- Experimental study on a specific surface area of red clay. Guilin: Guilin University of Technology; 2015.
- Crystallization Behavior and Damage Potential of Na2SO4 -NaCl Mixtures in Porous Building Materials. Cryst. Growth Des.. 2020;20(9):5974-5985.
- [Google Scholar]
- Applicability of the modified Washburn method to contact angle measurements of calcium carbonate. Ceramica.. 2018;64(370):197-206.
- [Google Scholar]
- Adsorption and Desorption Processes. US: Springer; 2005.
- Wang, Jianwei., Kalinichey, A G., Kirkpatrick, R J., et al., 2005. Structure, energetics, and dynamics of water adsorbed on the muscovite (001) surface: A molecular dynamics simulation. Journal of Physical Chemistry B.109(33): 1589.
- Electron microprobe studies of chemical reactions in ancient painted murals: The Beni Hasan Tombs, Egypt. Can. J. Chem.. 2011;66(66):2348-2361.
- [Google Scholar]
- Calcite surface structure and reactivity: molecular dynamics simulations and macroscopic surface modelling of the calcite-water interface. PCCP. 2012;14(43):15145-15157.
- [Google Scholar]
- A prediction model for isothermal adsorption curves based on adsorption potential theory and adsorption behaviour of methane on granular coal. Energy Fuels. 2019;33:1910-1921.
- [Google Scholar]
- Adsorption and Coagulation. Beijing: Science Press; 1986.
- Application of mineral pigments in ancient China. Metallic Ore Dress. Abroad. 1990;27(1):2.
- [Google Scholar]
- Investigation of ancient wall paintings in Mogao Grottoes at Dunhuang using laser-induced breakdown spectroscopy. Opt. Laser Technol.. 2019;120:105689
- [Google Scholar]
- Zhao, Zhenguo., 2000. Contact Angle and its Application in Surface Chemistry. Chemical Research and Application, 2000, 12(4):370-374.
- The influence of water molecules on the stability of mineral green pigments in Chinese ancient painting. Chem. Phys. Lett.. 2019;731:136592
- [Google Scholar]







