Translate this page into:
The role of thiazole acetate derivatives on isolated heart and blood vessels in experimental rats
⁎Corresponding author. a.alamri@tu.edu.sa (Abdulhakeem Alamri),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background and objectives
Though various drugs are available in the market for cardiovascular diseases, search for an efficacious and patient friendly drug is an ongoing process. The effect of synthesized thiazole compounds on isolated rat heart and isolated rat blood vessel to determine their potential as newer therapeutic agents.
Materials and methods
Six thiazole acetate compounds were examined for their function on isolated heart and blood arteries utilizing standardized experimental models after being characterized and identified in the laboratory. Anesthetized Wistar albino rats' hearts were removed and placed on a Langendorff setup. The percentage change in developed tension and heart rate owing to injecting each thiazole acetate compound in the developed tension and heart rate were seen and recorded in an isolated heart that was mounted in a retrograde fashion using Krebs Henseleit solution. Similarly, anesthetized rats' thoracic aortas were separated, and responses to the standard drug phenylephrine and different thiazole acetate derivatives were examined. The pharmacological effect of the specific thiazole compound was determined by the percentage change in contractile response.
Result
Each of the six thiazole acetate derivatives was injected at 1 nM, 10 nM, 100 nM, 1 M, 10 M, and 100 M concentrations. All six (T1 to T6) chemicals investigated in the present study had no significant effect on developed tension or heart rate. There was a no significant change in the percentage contraction after injecting 1 nM 3 nM,10 nM, 30 nM, 100 nM, 300 nM, 1 M, 3 M, 10 M, and 30 M,100 M,300 M (cumulative dose) of T1, T3, T4, T5, and T6, while T2 (- ethyl-4-methyl-2-aminothiazole) showed contractile effect in the form of vasoconstrictor activity, which was 30 % of that produced by phenylephrine.
Interpretation and conclusion
Thiazole acetate derivatives did not show any cardiovascular effects except ethyl-4-methyl-2-aminothiazole, which showed a slight vascular contractile response as compared to phenylephrine. A more thorough examination of the utilization of thiazole derivatives is required to establish their potential significance in the cardiovascular system.
Keywords
Cardiovascular
Aorta
Isolated blood vessel
Isolated heart
Langendorff set-up
Krebs Henseleit solution
Thiazole acetate
1 Introduction
Despite advances in diagnosis and availability of newer and better medications, the prevalence of cardiovascular disorders continues to rise. The incidence and prevalence is on the rise in the Middle Eastern countries resulting in high incidence of morbidity and mortality (Alhabib et al., 2020). There are many causes for the increased incidence of cardiovascular events. Atherosclerosis is the most prevalent cause of cardiovascular event such as myocardial infarction (MI), which produces an imbalance in supply and demand in the myocardial, leading in hypoxia and cellular waste accumulation, which can lead to myocyte death owing to ischemia-induced free radical formation (Kumar et al., 2016; Asdaq et al., 2021). Despite this, the pathophysiology of MI remains a mystery. Inflammation and necrosis, on the other hand, have been identified as key factors to MI in several investigations (Othman et al., 2017; Goyal et al., 2015). One of the important causes is the adverse effect of non-cardiovascular drugs (Raj et al., 2009).
Thiazole derivates are reported for a number of pharmacological effects. Their range of effects is numerous and includes anti-inflammatory, antidiabetic, anticancer, antimicrobial and anti-HIV activities to name a few (Dawood et al., 2021). Search for newer activities for this group of compounds is increasing as more and more activities are being reported (Dahal et al., 2021; Pereira et al., 2022; Raveesha et al., 2022; Svirčev et al., 2022). Due to their diverse chemical, physical, and pharmacological properties, derivatives of thiazole have always aroused the curiosity of synthetic and biological chemists. By altering the thiazole ring at various positions, a range of novel compounds with a varied spectrum of therapeutic potentials such as antioxidant, anti-tubercular, antibacterial, antifungal, diuretic, anti-anticancer, and anti-inflammatory effects were generated (Abdu-Rahem et al., 2021). Thiazole, as a nucleus or fused ring, is a fundamental component of antibiotics, which are natural penicillin-like drugs. However, there are very few reports on the adverse effects of compounds.
A number of synthesized thiazole-acetate derivatives were procured from the synthetic chemistry department of our institute. These compounds were screened for potential effects on the cardiovascular system using isolated rat heart model and isolated rat blood vessel preparation. The involvement of different receptors in their effects was also determined.
2 Methodology
2.1 Animals
Wistar rats weighing between 150 g and 250 g aged 3–7 months were used in the study. The animals were maintained at room temperature with free access to food (Standard pellet diet, Amrut feed, Bangalore, India) and tap water. All other standard conditions were maintained in the animal house as per the guidelines of the institutional animal ethical committee. The experimental protocol was approved by the Institutional Ethical Committee (KCP/IAEC-15).
2.2 Chemicals
Reagents and chemicals of analytical grade procured from different suppliers were used in the study. For all the drugs, a stock solution of 10 mM was prepared in 1 ml of ethanol by homogenization. Cyclo mixer was used for homogenization and diluted to 10 ml of distilled water. In case of compounds that were not soluble in 1 ml of ethanol, an additional 0.5 ml of ethanol was added. The list of compounds and abbreviations are given in Table 1.
No.
Abbreviation
Name
Molecular weight
1.
T-1
5-ethyl-4-methyl-2-aminothiazole-5-acetate
186
2.
T-2
Ethyl-4-methyl-2-aminothiazole
186
3.
T-3
2-acetamido-4-methyl-aminothiazole-5-acetate
228
4.
T-4
Ethyl-2-succinamidethiazole-4-acetate
285
5.
T-5
Ethyl-2-Acetoamidothiazole-4-acetate
227
6.
T-6
2-Acetamido-4-methylthiazole-5- carboxylic acid
213
2.3 Isolated heart preparation
The heart was perfused ex-vivo using a modified Langendorff’s setup. The set up was made of a preheating coil that warms the physiological salt solution before it enters the heart. It was connected to a three-way (˦) inlet and a three-way (˦) outlet. The flow of physiological salt solution to the heart can be controlled through lower three way outlet. Latex rubber tubing was connected between the lower outlet and cannulated heart for injection of drugs. The three-way design of the outlet helps in removing any air bubbles that may be trapped during administration of drugs. The isolated heart is kept warm in a chamber covered by temperature controlled water heated jacket at 37 °C. The set up is made up in such as way water circulated to and fro between the water bath and the jacket. The upper three-way inlet also helps in preventing the entry of air bubbles into the preheating coil and subsequently to the heart (Fig. 1). It takes about 15 sec before the drug reaches the heart and, in most instances, the effect passes off in few minutes as the drugs are washed through. The physiograph was used for recording of the rate of heart rate.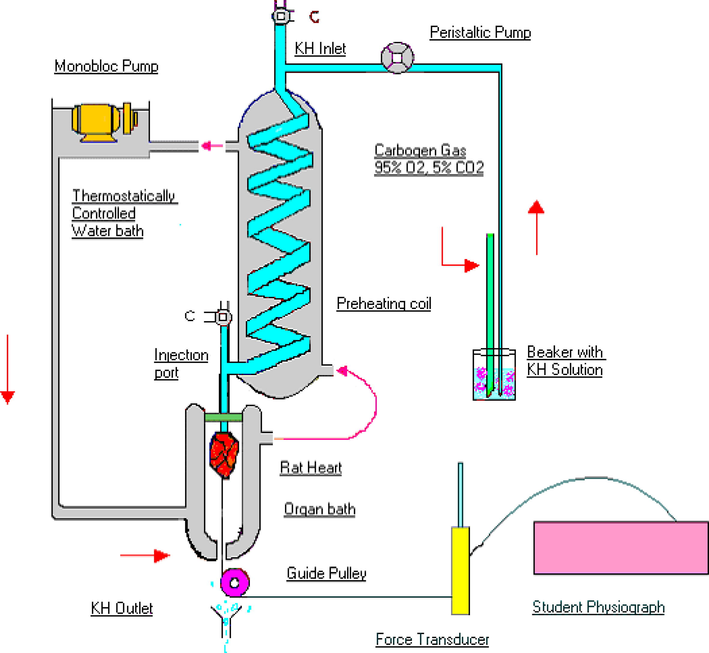
Schematic diagram of modified Langendorff’s apparatus.
Rats were deeply anaesthetized by administering a combination of ketamine hydrochloride (65 mg/kg i.p.) and xylazine (7.5 mg/kg i.p.)(Levin-Arama et al., 2016). To prevent intravascular clotting during isolation and perfusion of heart, anticoagulant (heparin – 100 U) was injected through intraperitoneal route 15 min before administration of ketamine/xylazine cocktail. Heart with at least 1 cm of aorta attached was removed as quickly as possible and placed in a China dish containing Kreb’s Henseleit’s (KH) solution at room temperature that was saturated with carbogen (95 % O2 and 5 % CO2). Perfusion of heart was done in retrograde route via aorta using a peristaltic pump (Bailey and Ong, 1978). The flow rate of physiological salt solution was kept at 5 ml/min using a peristaltic pump. To record the contraction, the apex of the heart was connected to a transducer using a thread and a pulley. After all the connections were secured, heart was allowed to adapt to this artificial set up for 10 min. An initial recording of contractions was done for 15 min before administration of the compounds. Starting from the lowest concentration, 0.1 ml of diluted synthesized compounds was injected and the effect of various concentrations of compounds was studied.
Developed tension, which is the tension in grams developed during the contraction at the optimum resting tension of the heart was noted. Total developed tension was calculated by measuring the contractions recorded in millimeter above the resting tension, converting them (each gram corresponds to 8 mm) into gram unit and adding to it the gm of resting tension maintained to give the total developed tension. Percentage change in developed tension was calculated by considering the normal response as 100 % and percentage increase/decrease from this contractile response in the presence of various synthetic drugs gives the percentage change in developed tension. Similar to resting tension, heart rate was calculated. Based upon the response produced by different compounds, their effect on heart was studied further to know their agonist property.
2.4 Isolated blood vessel study
The set up for mounting isolated aortic blood vessel preparation used in the lab consisted of a jacketed tissue bath (Fig. 2). It has a cylindrical tube of 10 ml capacity that was used for suspending the isolated aortic ring in which KH solution was maintained at 37 ± 0.5 °C.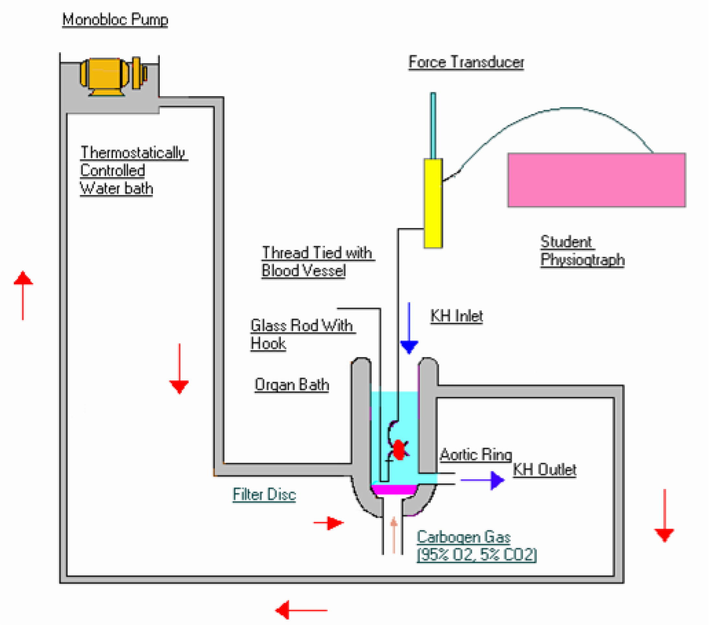
Schematic diagram of isolated blood vessel preparation.
The rats were anesthetized using ketamine and xyalzine as mentioned above. Aorta was isolated and it was mounted in an organ bath that was perfused with KH salt solution aerated with carbogen gas (95 % O2 and 5 % CO2). The isolated blood vessel was suspended in KH solution under controlled temperature in the inner cylindrical bath with the help of two stainless steel hooks, one fixed to the tissue holder while the other freely moving hook is connected to the vessel was then suspended in10 ml jacked tissue bath containing KH solution gassed with carbogen maintained at a temperature of 37 ± 0.5 °C and pH of 7.4 under a resting tension of 1 g using stainless steel hooks. The isolated blood vessel was then connected to isometric force displacement transducer (T-305 Ft-1202). The tissue was attached to the force transducer and the tension was slowly increased till it equals that of 1-gram weight originally hung. After the equilibration period, the recorder pen was set to the baseline and the responses were recorded.
After an equilibration period of 15 min, the isolated blood vessel was primed using a sub maximal dose of phenylephrine (PHE) − 1 µM allowing a contact period of 1–2 min and then washed thoroughly with the KH solution. The tissue was further allowed to equilibrate for 1-1½ hr before drug application. During the total equilibrium period of 1-1½ hr, the KH solution was changed several times before recording the concentration response curve by cumulative addition of PHE. The different concentrations of PHE chosen were 10 nM, 30 nM, 100 nM, 300 nM, 1 µM, 3 µM, 10 µM and 30 µM. Dose response curve (DRC) by cumulative addition (0.1 µM, 0.3 µM, 1 µM, 3 µM, 10 µM, 30 µM, 100 µM, 300 µM, 1000 µM, and 3000 µM) of synthetic compounds were determined. Based on the response of the synthetic compounds, further experiments were carried out to know whether these responses are receptor mediated or non receptor mediated in the presence of standard antagonist.
3 Results
The cardiovascular effects of thiazole acetate derivatives- T-1, T-2, T-3, T-4, T-5, and T-6 were studied using isolated rat heart and blood vessel preparations. In the isolated rat heart the effect of T-1, T-2, T-3, T-4, T-5 and T-6 was assessed using parameters such as the developed tension (grams) and heart rate (beats per minute). Of all the tested compounds, T5 and T6 decreased heart rate at the maximum concentration used (Table 2). All Values are mean ± SEM, n = 6.
Treatment
Concentration
Developed tension (% response)
Heart rate (% response)
Adrenaline
6 µM
63.7 ± 18.19
22.60 ± 4.98
Acetylcholine
1 µM
−10.55 ± 6.58
−13.40 ± 2.49
T1
1 nm
0
0
10 nm
0
0
100 nm
0
0
1 μm
0
0
10 μM
0
0
100 μM
0
0
T2
1 nm
0
0
10 nm
0
0
100 nm
0
0
1 μm
0
0
10 μM
0
0
100 μM
0
0
T3
1 nm
0
0
10 nm
0
0
100 nm
0
0
1 μm
0
0
10 μM
0
0
100 μM
0
0
T4
1 nm
0
0
10 nm
0
0
100 nm
0
0
1 μm
0
0
10 μM
0
0
100 μM
0
0
T5
1 nm
0
0
10 nm
0
0
100 nm
0
0
1 μm
0
0
10 μM
0
0
100 μM
0
−1.63 ± 0.34
T6
1 nm
0
0
10 nm
0
0
100 nm
0
0
1 μm
0
0
10 μM
0
0
100 μM
0
−5.50 ± 0.97
The maximum contractile effect was obtained at 100 ηM PHE, which was considered as 100 %. The tissue was then allowed to relax for 1 h. The effect of different compounds for contractile response was observed at 1 nM 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, 1 µM, 3 µM, 10 µM, and 30 µM,100 µM,300 µM (cumulative dose). Only T2 was active in inducing contraction of isolated blood vessels (Table 3). A comparison of contractile response of T2 and PHE is shown in Fig. 3. PRA was effective in antagonizing the effect of PHE completely while such an antagonism was not observed with T2. PRA was effective in antagonizing the effect of lower concentrations of T2 and it is ineffective in antagonizing the effect of higher c oncentrations of T2 (Table 4). All Values are mean ± SEM, n = 6. All Values are mean ± SEM, n = 6.
Treatment
Concentration
Contractile response (%)
PHE
1 nM
30.95 ± 12.74
3 nM
52.38 ± 11.94
10 nM
92.85 ± 5.83
30 nM
97.61 ± 1.94
100 nM
100 ± 0.00
300 nM
100 ± 0.00
T-1
0.001mcM-300mcM
0.00 ± 0.00
T2
1 nM
0.00 ± 0.00
3 nM
3.33 ± 3.33
10 nM
20.87 ± 6.51
30 nM
28.87 ± 5.73
100 nM
30.26 ± 5.71
300 nM
30.26 ± 5.71
T-3
0.001mcM-300mcM
0.00 ± 0.00
T-4
0.001mcM-300mcM
0.00 ± 0.00
T-5
0.001mcM-300mcM
0.00 ± 0.00
T-6
0.001mcM-300mcM
0.00 ± 0.00
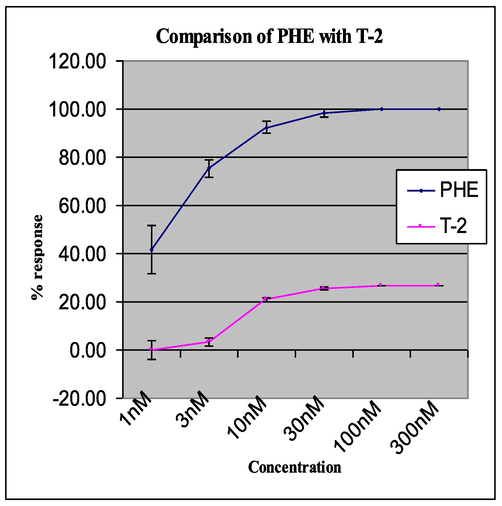
Comparison of PHE and T-2.
Treatment
Concentration
Contractile response (%)
PHE
1 nM
62.06 ± 11.74
3 nM
83.81 ± 8.47
10 nM
95.24 ± 4.77
30 nM
100.00 ± 0.00
100 nM
100.00 ± 0.00
300 nM
100.00 ± 0.00
T2
10 nM
15.87 ± 9.67
30 nM
27.30 ± 3.91
100 nM
27.30 ± 3.91
300 nM
27.30 ± 3.91
PRA (1 µM) + PHE
1 nM
0.00 ± 0.00
3 nM
0.00 ± 0.00
10 nM
0.00 ± 0.00
30 nM
4.76 ± 4.77
100 nM
15.08 ± 8.30
300 nM
15.08 ± 8.30
PRA (1 µM) + T2
10 nM
0.00 ± 0.00
30 nM
6.67 ± 6.67
100 nM
28.41 ± 6.74
300 nM
33.97 ± 3.32
4 Discussion
In most developed countries, cardiovascular illnesses constitute the main cause of morbidity and mortality. Pharmaceuticals, illicit substances, and pollutants can all add to the overall cardiovascular burden, thus they need to be addressed (Mladěnka et al., 2018). The vascular system is also physiologically related to the heart; therefore, its functions are intertwined (e.g., endothelial dysfunction and subsequent hypertension could result in a damage to the heart, and vice versa). Drugs that predominantly cause heart rhythm abnormalities can eventually lead to decreased heart hemodynamic function, and so on.
Studies about the functioning of heart are being carried out for four centuries. However, in the past few years, this research has seen a paradigm shift. Experiments using isolated heart (Ex-vivo) and isolated cell lines are becoming increasingly used (Watanabe and Okada, 2018). Studying the effect of new molecules on contraction and relaxation helps in screening of new molecules. The present study is a small step in screening of synthetic compounds.
Thiazole acetate derivatives (T-1, T-2, T-3, T-4, T-5 and T-6) were screened for cardiovascular effect by observing the changes in developed tension and heart rate. Thiazole acetate derivatives did not produce any changes in both developed tension and heart rate of isolated rat heart. Hence, it can be said that these thiazole acetate derivatives that were screened do not have any agonistic activity on isolated rat heart preparation.
Further, thiazole acetate derivatives were screened on isolated blood vessel, results show T-2 produced contractile response up to 30 % when compared to α1- adrenergic agonist PHE. Mechanism of normal contractile process is due to binding of α1- adrenergic agonist to adrenergic receptors, which activates phospholipase C, further increase the formation of diacylglycerol activates protein kinase C, and phosphorylates a wide range of effector protein. Inositol triphosphate interacts with receptor that releases calcium from intracellular stores and forms complex with myosin resulting in contractile process (Bylund, 2013).
These α-adrenergic receptor mediated effects are blocked by α1 selective adrenergic antagonist like prazosin but in our study, blocking action of contractile response by T-2 was not produced by prazosin. Hence, it can be concluded that the agonistic activity of T-2 may not be through α1- adrenergic receptors mediated mechanism as PHE. Further studies will provide the exact mechanism of action or T-2. Other Thiazole acetate derivative (T-1, T-2, T-3, T-4, T-5 and T-6) failed to show any response on blood vessel up to 300 μM concentration.
Thiazole acetate derivatives (T-1, T-2, T-3, T-4, T-5, and T-6) have been shown to possess marked anti-inflammatory and analgesic activity in a previous study (Jajoo, 2001). However, T-2 produced slight contraction of isolated rat blood vessel. Hence it can be seen as a future probability as anti-inflammatory/ analgesic drug without cardiovascular complication (except for T-2).
5 Conclusion
Based on the findings of this investigation, we believe that five of the six thiazole acetate derivatives (T-1, T-3, T-3, T-4, T-5, and T-6) have no effect on blood vessels or the heart and are hence unlikely to cause cardiovascular problems. As a result, after the necessary clinical trials, these compounds can be employed as analgesics and anti-inflammatory agents in patients with cardiovascular disorders. Nonetheless, they are candidates for additional pharmacological investigation.
Acknowledgement
The author MK is thankful to the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University, Al-Kharj, Kingdom of Saudi Arabia for providing support.
Funding
Abdulhakeem Alamri would like to acknowledge Taif university for support No. TURSP (2020/288). Syed Mohammed Basheeruddin Asdaq wishes to express his gratitude to AlMaarefa University in Riyadh, Saudi Arabia, for providing support (TUMA-2021-1) to do this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and medicinal attributes of thiazole derivatives: A review. Syst. Rev. Pharm.. 2021;12(1):290-295.
- [Google Scholar]
- Demographic, behavioral, and cardiovascular disease risk factors in the Saudi population: Results from the Prospective Urban Rural Epidemiology study (PURE-Saudi) BMC Public Health. 2020;20:1-14.
- [CrossRef] [Google Scholar]
- Cardioprotective potential of garlic oil and its active constituent, diallyl disulphide, in presence of carvedilol during chronic isoprenaline injection-mediated myocardial necrosis in Rats. Molecules. 2021;26(17):5137.
- [Google Scholar]
- Krebs-Henseleit solution as a physiological buffer in perfused and superfused preparations. J. Pharmacol. Methods. 1978;1:171-175.
- [CrossRef] [Google Scholar]
- Dahal, S., Cheng, R., Cheung, P.K., Been, T., Malty, R., Geng, M., Manianis, S., Shkreta, L., Jahanshahi, S., Toutant, J., Chan, R., Park, S., Brockman, M.A., Babu, M., Mubareka, S., Mossman, K., Banerjee, A., Gray-Owen, S., Brown, M., Houry, W.A., Chabot, B., Grierson, D., Cochrane, A., 2021. The Thiazole-5-Carboxamide GPS491 Inhibits HIV-1, Adenovirus, and Coronavirus Replication by Altering RNA Processing/Accumulation. Viruses 2022, Vol. 14, Page 60 14, 60. https://doi.org/10.3390/V14010060.
- Novel bis-thiazole derivatives: synthesis and potential cytotoxic activity through apoptosis with molecular docking approaches. Front. Chem.. 2021;9:565.
- [CrossRef] [Google Scholar]
- Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int. J. Mol. Sci.. 2015;16(11):27457-27469.
- [Google Scholar]
- Synthesis and Studies Of Pharmacology Properties Of Sulphur Nitrogen Heterocyclic System. Rajiv Gandhi University of Health Sciences; 2001.
- Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm. Res.. 2016;65(8):613-622.
- [Google Scholar]
- Subcutaneous compared with intraperitoneal ketamine-xylazine for anesthesia of mice. J. Am. Assoc. Lab. Anim. Sci.. 2016;55:794.
- [Google Scholar]
- Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev.. 2018;38(4):1332-1403.
- [Google Scholar]
- Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction. Eur. J. Pharmacol.. 2017;794:27-36.
- [Google Scholar]
- Pereira, P.S., Rodrigues Costa, A., Julyanne, T., De Oliveira, S., Vinícius, C., Oliveira, B., Do, M., Alves De Lima, C., Ferreira De Oliveira, J., Kim, B., Coutinho, H.D.M., Duarte, A.E., Kamdem, J.P., Gonçalves Da Silva, T., 2022. Neurolocomotor Behavior and Oxidative Stress Markers of Thiazole and Thiazolidinedione Derivatives against Nauphoeta cinerea. Antioxidants 2022, Vol. 11, Page 420 11, 420. https://doi.org/10.3390/ANTIOX11020420.
- Cardiovascular effects of noncardiovascular drugs. Circulation. 2009;120:1123-1132.
- [CrossRef] [Google Scholar]
- Synthesis, molecular docking, antimicrobial, antioxidant and anticonvulsant assessment of novel S and C-linker thiazole derivatives. Chem. Phys. Lett.. 2022;791:139408
- [CrossRef] [Google Scholar]
- Design, synthesis, and biological evaluation of thiazole bioisosteres of goniofufurone through in vitro antiproliferative activity and in vivo toxicity. Bioorg. Chem.. 2022;121:105691
- [CrossRef] [Google Scholar]
- Langendorff perfusion method as an ex vivo model to evaluate heart function in rats. Methods Mol. Biol.. 2018;1816:107-116.
- [CrossRef] [Google Scholar]







