Translate this page into:
Liposomal coenzyme Q10 abates inflammation, apoptosis and DNA damage induced by an overdose of paracetamol in rat's liver
⁎Corresponding author. aelhusaini@ksu.edu.sa (Ahlam Alhusaini),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Paracetamol (PCM) is widely used for its pain-relieving and antipyretic properties. However, acute intoxication of PCM remains one of the most common causes of drug-induced hepatic failure. A comparative study is conducted to evaluate the effectiveness of coenzyme Q10 (CoQ10) with that of liposomal CoQ10 (L-CoQ10) against PCM-induced liver injury.
Method
Acute liver injury was induced by a single oral dose of PCM (1000 mg/kg). CoQ10 and L-CoQ10 treatments were given orally (10 mg/kg), 1 and 12 h following PCM intoxication. Specific oxidative stress, inflammatory and apoptotic biomarkers were measured, and then proved by histopathological examination.
Results
PCM administered rats exhibited a remarkable increase in the levels of serum aminotransferase enzymes, as well as hepatic interleukin-6, C-reactive protein, malondialdehyde (MDA) and nitric oxide (NO); whereas reduced glutathione (GSH) level and superoxide dismutase (SOD) activity were decreased. BAX, Nuclear factor-kappa B (NF-κB) and cytochrome C were overexpressed, while BCL-2 was downregulated. The results of the previous parameters were supported by histopathological study of liver tissue. Using either CoQ10 or L-CoQ10 caused a significant reduction of oxidative stress by enhancing GSH and SOD activity levels and diminishing MDA and NO levels. Moreover, both CoQ10 and CoQ10 caused downregulation of pro-inflammatory cytokines and BAX, and upregulation BCL-2, and these effects mostly noticed in L-CoQ10 treated rats. Likewise, rats receiving L-CoQ10 exhibited restoration of normal hepatic architecture.
Conclusion
L-CoQ10 supplement is useful for counteracting the hepatotoxicity induced by PCM overdose, via reducing the oxidative stress, inflammation and apoptosis.
Keywords
Paracetamol
L-CoQ10
Cytochrome C
BAX
BCL-2
1 Introduction
Paracetamol (PCM) is a nonprescription analgesic and antipyretic agent. It has an excellent safety profile; however, PCM toxicity is one of the most common drug toxicity worldwide (Gunnell et al., 2000). PCM overdose can result in acute hepatotoxicity which is characterized by fatal massive hepatocyte necrosis if left untreated (Wang, 2022). The hepatic injury induced by PCM is attributed to its metabolism, as the main pathway of PCM metabolism is through cytochrome P450-mediated oxidation to produce N-acetyl-p-benzoquinone imine (NAPQI) which is a highly reactive metabolite causing liver toxicity (Alhusain, 2022). In normal doses of PCM, this potentially reactive metabolite can be converted to a non-toxic metabolite by the endogenous antioxidant, namely reduced glutathione (GSH). Nevertheless, excessive intake of PCM can increase the rate of NAPQI formation, deplete the stored GSH in the liver and eventually lead to liver dysfunction (Shinohara, 2010).
N-acetylcysteine (NACC) is the mainstay treatment of PCM toxicity used to hinder the rapid depletion of hepatic GSH as one of the main precursors for GSH synthesis thus, NACC supports the detoxification process and prevents the NAPQI accumulation (Pollman et al., 1999). For these reasons, NACC therapy is still considered the best therapeutic option for patients administered an overdose of PCM (Salom, 1998). However, intravenous administration of NACC is associated with anaphylactoid reactions; urticaria, pruritus, hypotension, headache, angioedema and bronchospasm (Yamamoto et al., 2014; Yarema, 2018). In order to manage these reactions, additional therapies like antihistamines and steroids are required, which add further burden on the liver. Receiving this antidote within hours after PCM ingestion is essential, since its effectiveness is reduced in treatment delayed cases (Athuraliya and Jones, 2009; Yang et al., 2009). Moreover, prolonged administration of NACC may slow liver regeneration and recovery after acute PCM overdose (Yang et al., 2009). An intravenous NACC regimen has a high potential for medication errors, particularly in the frequency, dosing and infusion rate (Hayes et al., 2008). Therefore, it is crucial to discover a potent, effective and safe compound that can prevent PCM overdose-induced hepatotoxicity.
Coenzyme Q10 (CoQ10), ubiquinone, is an endogenous lipophilic benzoquinone compound which acts as an electron transport in the respiratory chain of the mammalian mitochondrial membrane (Ghule et al., 2009). Over the last few years, CoQ10 gained significant research attention as a dietary supplement used for generating cellular bioenergy and preventing tisuue damage caused by oxidative stress (Akbari, 2020). The protective effects of CoQ10 have been extensively studied as it lessens the development of illnesses in different body systems including cardiovascular, digestive and central nervous systems (Qi, 2019; Spindler et al., 2009; Zozina et al., 2018) as CoQ10 deficiency has been reported in such diseases (Dhanasekaran and Ren, 2005). Arenas-Jal and colleagues (2020) reviewed published article dealing with the safety of CoQ10 and they concluded that this supplement is well tolerated, doesn’t induce serious adverse reactions and has no potential of genotoxicity (Arenas-Jal et al., 2020).
Recently, pharmaceutical sciences have developed liposomal drugs to improve drug properties, including drug tissue targeting, biocompatibility, and conjugation with proteins, peptides, and DNA (Pastor-Maldonado, 2020). Liposomes are nano-phospholipid bubbles that can diffuse through cell membranes and deliver their contents into cell cytoplasm (Torchilin, 2005). For these reasons, the current study was conducted to evaluate the efficiency of liposomal-CoQ10 (L-CoQ10) in counteracting the hepatotoxicity induced by PCM overdose in rats.
2 Materials and methods
2.1 Drugs
PCM powder was purchased from (Sigma Chemical Co., USA); CoQ10 was purchased from (Nutra Manufacturing Inc., Greenville, USA), and the marketed L-CoQ10® was obtained from Lipolife (Drakes Lane Industrial Estate, Drakes Lane, UK), and its formulation was based on an encapsulation of CoQ10 into liposomes with nano-sized vesicle (<200 nm) made from a phospholipid to ensure maximum absorption. PCM, CoQ10 and L-CoQ10 were suspended in 1% carboxymethylcellulose (CMC).
2.2 Animals and study design
Wistar albino rats weighing 200 ± 10 g were obtained from the Bio-Resource Unit, College of Pharmacy, King Saud University. The animals were kept in standard conditions and fed with standard laboratory chow and water ad libitum. They left for at least one week before starting the experiments to acclimatize the lab environment.
The rats were divided into four groups (six rats/each). The rats in the first group received a single oral dose of 1% CMC and served as a control group. Hepatotoxicity was induced in animals of the second, third and fourth groups by an oral dose of PCM (1000 mg/kg) (Kisaoglu, 2014). The third and fourth groups received either CoQ10 or L-CoQ10 (10 mg/kg, orally) at 1 and 12 h post PCM intoxication (Fouad and Jresat, 2012).
The experimental protocol was approved by the Animal Care Committee at King Saud University (Ethical number [KSU-SE-20-58]). All experimental procedures were performed in agreement with international regulations for the use of laboratory animals.
2.3 Sample preparation and biochemical studies
Twenty-four hours after PCM administration, the rats were sacrificed under anesthesia using ketamine/xylazine cocktail (0.1 ml/100 g rat wt. i.p.). Blood samples were collected and centrifuged to obtain clear sera, which were subsequently stored at −80 °C. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured colorimetrically using specific activity assay kits (Randox Laboratories Ltd., UK).
The livers were excised carefully, washed with ice-cold saline, and then homogenized in phosphate buffer. The homogenates were centrifuged and the resulting supernatants were used to determine malondialdehyde (MDA), GSH and nitric oxide (NO) levels, and superoxide dismutase (SOD) activity. The levels of interleukin-6 (IL-6) and C-reactive protein (CRP) in liver homogenates were determined by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, USA).
2.4 Determination of hepatic MDA, GSH, SOD and NO
Hepatic MDA was assayed as previously described (Ohkawa et al., 1979). Briefly, the liver tissue homogenate was mixed with TBA, SDS, and acetate buffer, then heated for one hour. Then, n-butanol was added to the mixture, centrifuged, and the absorbance of the organic layer was measured at 532 nm. GSH was determined based on the method of Ellman (Ellman, 1959). In brief, GSH reacts with 5,50-dithio-bis (2-nitrobenzoic acid) to give yellow-colored product that reads at 412 nm. SOD activity was determined in accordance with the method of Marklund and Marklund (Marklund and Marklund, 1974), in which the pyrogallol autoxidation was examined in the presence of EDTA in PH ranging from 7.9 to 10.6, and the activity of SOD was assayed based on its ability to inhibit autoxidation of pyrogallol. The determination of NO involves the measurement of its stable products NO3− and NO2−, this method was started with the reduction step of NO3− to NO2−, then NO2− was determined by the Griess reaction, and the absorbance of the product was measured at 540 nm (Green et al., 1982).
2.5 Western blot analysis for nuclear factor kappa B (NF-κB) and cytochrome C
Western blotting was utilized to determine the protein expression of NF-κB (ab16502) and cytochrome C (ab133504). The liver sample was homogenized in RIPA buffer accompanied by proteinase/phosphatase inhibitors, centrifuged, and the supernatant was collected. Protein concentration was assayed using Bradford protein assay kit (BioBasic, Markham, Canada), and 60 µg protein was subjected to 10% SDS/PAGE and electrotransferred to nitrocellulose membranes which were subsequently blocked by 5% milk. The membranes were probed with primary antibodies against NF-κB, cytochrome C, and β-actin overnight at 4 ◦C. The membranes were washed multiple times with TBST, then probed with secondary antibodies, washed again, and developed using Clarity™ Western ECL Substrate from BIO-RAD (Hercules, CA, USA). Protein bands were visualized using the ECL-Plus detection system. Positive immunoreactive bands were quantified by ImageJ and compared with control.
2.6 BAX and BCL-2 mRNA expression
Changes of BAX and BCL-2 mRNA expression levels were determined by real-time polymerase chain reaction (RT-PCR). Briefly, RNA was isolated from the frozen liver tissues using TRIzol. Following treatment with RNase-free DNase, RNA was quantified using a nanodrop. RNA was reverse transcribed into cDNA. The produced cDNA was improved using PCR master mix and the primer pairs listed in Table 1.
Oligo Name
Sequence (5′ → 3′)
BAX-F
BAX-R5-AACTTCAACTGGGGCCGCGTGGTT
5-CATCTTCTTCCAGATGGTGAGCGAG
Bcl-2-F
Bcl-2-R5-GCAGCTTCTTTCCCCGGAAGGA
5-AGGTGCAGCTGACTGGACATCT
β-actin-F
β-actin-R5-GGCCACAATGGCTGACCATTC
5-AAGGTGACAGCATTGCTTC
The PCR products were loaded in agarose gel, and the bands were visualized using UV transilluminator. The images were analyzed by ImageJ, and the values were normalized to β-actin.
2.7 DNA fragmentation
The settings used for targeted fragmentation were set up according to the original manufacturer's protocol. Briefly, the tissue samples were lysed and centrifuged to produce fragmented DNA (supernatant) and intact chromatin (pellet). The samples were treated with diphenylamine, and the absorbance of the developed color was measured at 600 nm.
2.8 Histopathological examination of liver tissue
Liver tissue samples from each animal were fixed in 4% formalin, dehydrated, and embedded in paraffin wax. Sections were cut at 4 μm, stained with hematoxylin and eosin (H&E) and visualized under a light microscope.
2.9 Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey post hoc test for multiple comparisons using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). A p value <0.05 was considered a statistically significant, and the values were expressed as mean ± SEM.
3 Results
3.1 CoQ10 and L-CoQ10 improved liver functions in PCM-induced hepatotoxicity
The current work revealed that rats who received PCM overdose showed liver injury manifested by the cellular leakage and elevation in serum AST and ALT (Fig. 1). However, concomitant administration of CoQ10 or L-CoQ10 significantly lowered liver enzymes, particularly the ALT, to a level close to controls.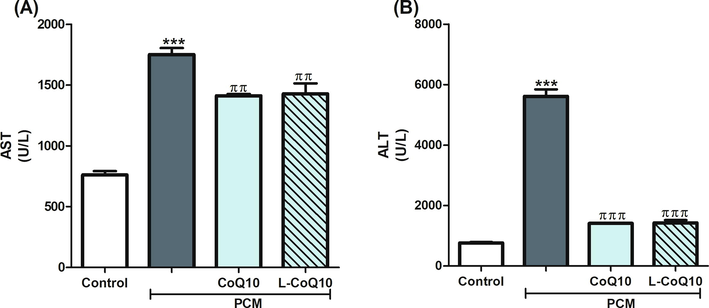
Effects of CoQ10 and L-CoQ10 on serum liver function enzymes in PCM-induced hepatotoxicity in rats. Notes: Data are presented as mean ± SEM (N = 6). ***P ≤ 0.001 vs control, ππP ≤ 0.01, πππP ≤ 0.001 vs PCM-intoxicated group.
3.2 CoQ10 and L-CoQ10 attenuated oxidative, inflammatory and apoptotic damage in PCM-induced hepatotoxicity
Further assessment of the hepatic protective effects of CoQ10 was conducted by measuring the levels of oxidative, inflammatory and apoptotic markers.
3.2.1 Oxidative markers
Both MDA and NO were significantly increased (P ≤ 0.001), whereas GSH level and SOD activity were decreased following PCM intoxication, thus indicating high oxidative damage. The ingestion of CoQ10 or L-CoQ10 ameliorated the toxic effects on PCM on the previous parameters (Fig. 2). L-CoQ10 showed a better antioxidant response than CoQ10.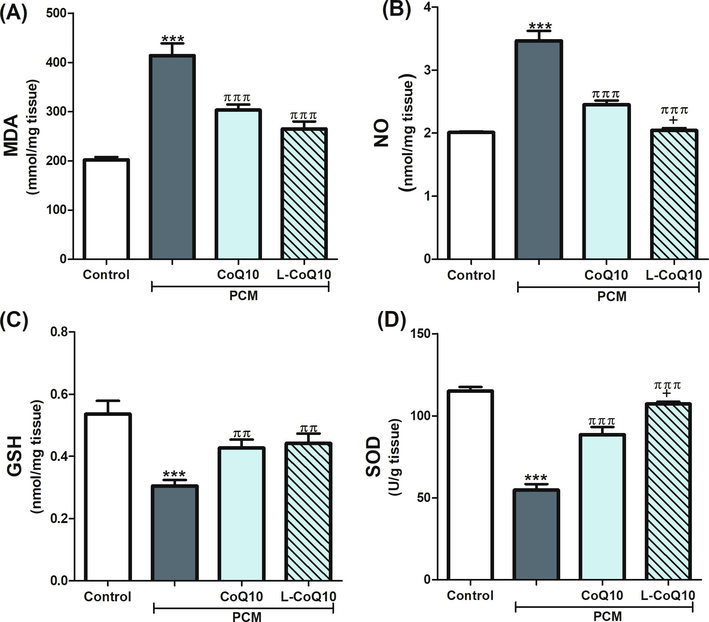
Effects of CoQ10 and L-CoQ10 on the hepatic oxidative stress markers in PCM-induced hepatotoxicity in rats. Notes: Data are presented as mean ± SEM (N = 6). ***P ≤ 0.001 vs control, ππP ≤ 0.01, πππP ≤ 0.001 vs PCM-intoxicated group and + P ≤ 0.05 vs to CoQ10 treated group.
3.2.2 Inflammatory markers
The expressions of the inflammatory biomarkers, IL-6 and CRP, were upregulated following PCM overdose but reduced in CoQ10 treated groups apparently with the liposomal form (Fig. 3). Moreover, acute intoxication with PCM in rats significantly induced the hepatic phospho-NF-κB and cytochrome C expression (P ≤ 0.001) relative to control rats. On the contrary, rats who received the antioxidants in question with PCM revealed a reduction in the expression of phospho-NF-κB and cytochrome C, notably in L-CoQ10 treated rats (Fig. 4).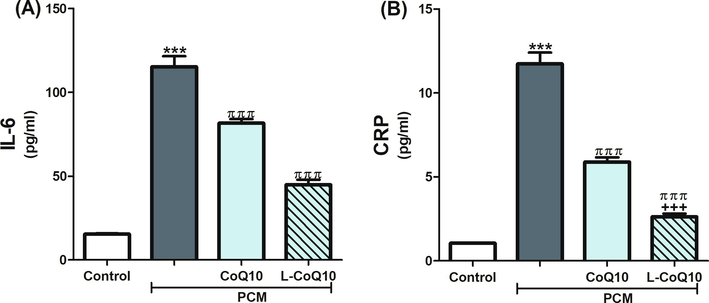
CoQ10 and L-CoQ10 reduced hepatic levels of IL-6 and CRP after PCM-induced hepatotoxicity. Notes: Data are presented as mean ± SEM (N = 6). ***P ≤ 0.001 vs control, πππP ≤ 0.001 vs PCM -intoxicated group and +++P ≤ 0.001 vs to CoQ10 treated group.
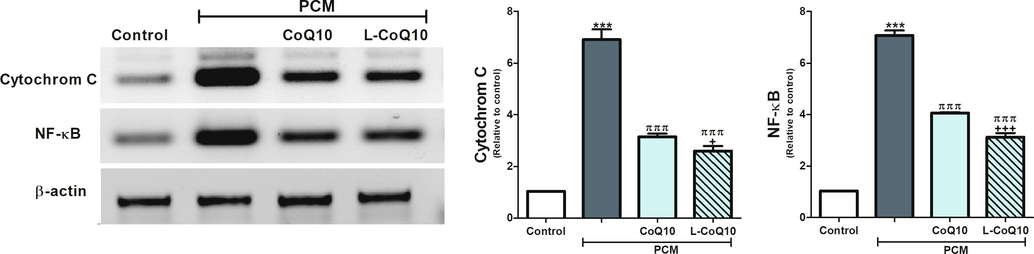
CoQ10 and L-CoQ10 attenuated cytochrome C and NF-κB overexpression in the liver of PCM-intoxicated rats. Notes: Data are presented as mean ± SEM (N = 6). ***P ≤ 0.001 vs control, πππP ≤ 0.001 vs PCM-intoxicated group, and +P ≤ 0.05, +++P ≤ 0.001 vs to CoQ10 treated group.
3.2.3 Apoptotic markers
In parallel with the previous findings, PCM overdose triggered apoptosis by causing an upregulation of BAX, and downregulation of Bcl-2 gene expression in the liver of PCM-administered rats (p < 0.001). Using of CoQ10 and L-CoQ10 showed a remarkable reduction in apoptosis by decreasing the genetic expression of BAX and increasing Bcl-2 gene expression (Fig. 5). Further analysis of the anti-apoptotic efficacy of CoQ10 and L-CoQ10 was determined by assessing the DNA fragmentation. Upon visualization of the electrophoretic pattern under UV light, a smeared DNA signal was detected in the liver of PCM intoxicated rats, indicating damaged cells, while intact genomic DNA from viable cells was detected at the top of the gel in the controls and after treatment with antioxidants (Fig. 6).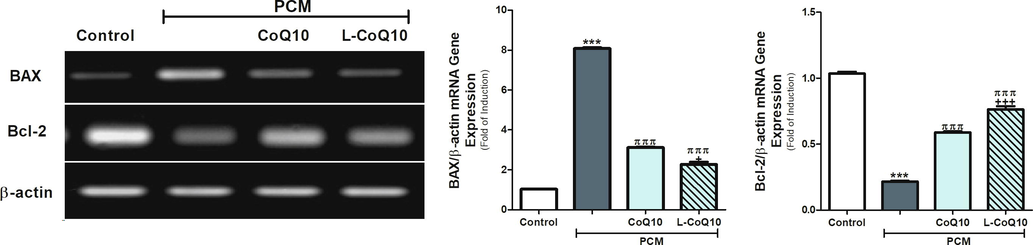
COQ10 and L-COQ10 modulated the BAX and Bcl-2 gene expression in the liver of PCM-intoxicated rats. Notes: Data are presented as mean ± SEM (N = 6). ***P ≤ 0.001 vs control, πππP ≤ 0.001 vs PCM-intoxicated group and +P ≤ 0.05, +++P ≤ 0.001 vs to CoQ10 treated group.
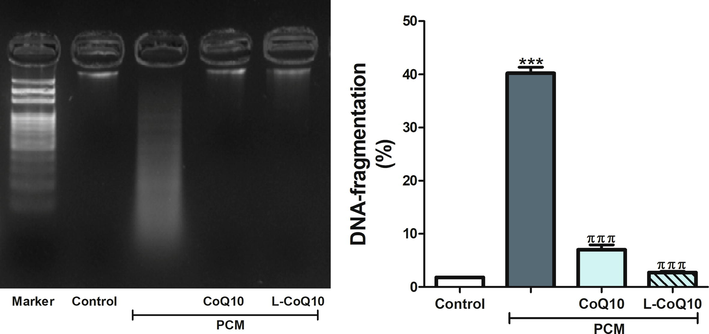
Qualitative and quantitative analysis of DNA fragmentation levels in control, PCM-intoxicated and all treated groups. Notes: Data are presented as mean ± SEM (N = 6). ***P ≤ 0.001 vs control, πππP ≤ 0.001 vs PCM-intoxicated group.
3.3 CoQ10 and L-CoQ10 prevented the histopathological changes after PCM overdose
The hepatoprotective effect of CoQ10 and L-CoQ10 was validated by the histological examination of liver sections. PCM -intoxicated rats showed congested central vein with eosinophilic plasma surrounded by degenerated and vacuolated hepatocytes; however, the treated rats with CoQ10 apparently showed normal central vein that surrounded by normal hepatocytes however few hepatocytes still showed cytoplasmic degeneration. While liver sections from rats receiving L-CoQ10 restored normal architecture of hepatic lobules and central vein, almost all hepatocytes appear of normal histological features (Fig. 7).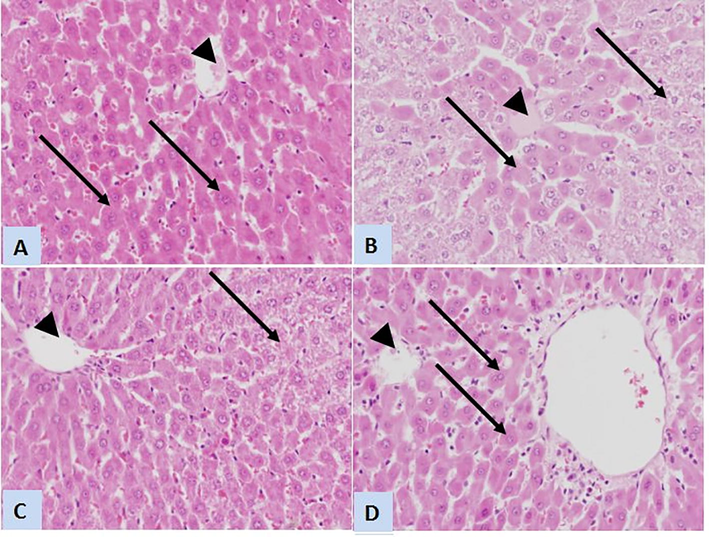
Representative photomicrographs of liver sections stained with H&E (×400) of control, PCM-intoxicated and treated rats. (A) Liver sections from control rats showing normal architecture of classic hepatic lobule with normal central vein (arrowhead) and normal hepatocytes (arrows). (B) Liver sections from rats received a toxic dose of PCM displays congested central vein with eosinophilic plasma (arrowhead) and degenerated and vacuolated hepatocytes (arrows). (C) Sections from rat treated with CoQ10 shows normal central vein (arrowhead) that surrounded by many normal hepatocytes except presence of few hepatocytes with cytoplasmic degeneration (arrow). (D) L-CoQ10 restored normal architecture of hepatic lobules and central vein (arrowhead), and almost all of hepatocytes appear of normal histological features (arrows).
4 Discussion
CoQ10 represents one of the most consumed dietary supplements, which gained a growing interest in the global healthcare trend due to its potent antioxidant activity. Therefore, we predict that CoQ10 is a valuable candidate for preventing drug-induced liver damage. The current study demonstrated that CoQ10 and L-CoQ10 at a dose of 10 mg/kg significantly reverse the acute liver failure induced by PCM toxicity. The liver has a complex and vital role in carbohydrate, protein and fat metabolism. It detoxifies many metabolic wastes, toxins and drugs to less harmful products or excretes them into bile. Additionally, it has a central role in albumin production and immunological responses. Injured hepatocytes leak certain chemicals in a range higher than average amounts, including liver enzymes. PCM overdose exhibited a marked increase in serum AST and ALT activities that indicated liver injury. It has been shown that elevated liver enzymes above the upper range of the normal are associated with increased liver-related mortality (Malakouti et al., 2017; Kwo et al., 2017). The current study revealed that treatment with either CoQ10 or L-CoQ10 retained the serum levels of these enzymes to normal after acute PCM hepatotoxicity. As previously reported, intraperitoneal administration of CoQ10 markedly protected rat liver against acetaminophen-induced liver toxicity (Fouad and Jresat, 2012). CoQ10 also normalizes the hepatic enzymes after thioacetamide hepatotoxicity (Ashkani-Esfahani, et al., 2016). In a double-blind placebo-controlled trial, the daily use of CoQ10 supplement significantly declined the serum level of aminotransferases and CRP in patients suffering from fatty liver (Farsi et al., 2016).
Oxidative stress is the main event of PCM-induced liver damage in which the scavenging of free radicals, e.g., reactive oxygen species (ROS), is reduced due to impairment in cellular energy metabolism in mitochondria, transition metals such as iron or copper, calcium homeostasis and adenosine triphosphate depletion. Oxidation of one cellular component can initiate endless oxidative events; for example, lipid peroxidation products, like MDA, could disrupt the phospholipid membrane leading to oxidation of other cellular components, including proteins, glucose and DNA, causing abnormalities in their structures and functions. PCM also activates Kupffer cells to release several inflammatory cytokines and signaling molecules, including NO, which is involved in the pathogenesis of PCM hepatotoxicity (Al-Megrin et al., 2020). Excessive NO production causes peroxynitrite radical formation, depletes intracellular GSH, and extends the oxidative damage to cell macromolecules (Alhusain, 2022). In this work, PCM significantly reduced the endogenous GSH level and SOD activity but increased MDA and NO levels reflecting oxidative stress. Fortunately, treatment with CoQ10 and L-CoQ10 effectively modulated the oxidative stress by increasing the hepatic levels of GSH and SOD and reducing MDA and NO levels. CoQ10 acts as a powerful antioxidant which prevents the initiation and propagation of lipid peroxidation in cellular membranes (Mancuso et al., 2010). Moreover, it has been shown that CoQ10 considerably suppressed lipid peroxidation and prevented the depletion of endogenous antioxidants (Fouad and Jresat, 2012).
To further investigate the mechanisms beyond PCM hepatotoxicity, some important inflammatory cytokines and apoptotic markers were measured. Liver inflammation is another pathological consequence of PCM overdose. This effect was evidenced by PCM-induced IL-6, CRP and phospho-NF-κB levels. It was previously reported that PCM administration could activate Kupffer cells to increase IL-6, TNF-α and CRP levels in mice (Wu et al., 2019). In addition, PCM overdose causes NF-κB activation with subsequent inflammatory reactions responsible for hepatic injury (Bauer, 2000). NF-κB is a pleiotropic transcription factor that controls the release of pro-inflammatory cytokines in response to oxidative stress and has a role in cell growth and apoptosis. TNF-α is linked to increased oxidative stress, ROS and reactive nitrogen species and is known to activate other inflammatory cells (Gardner, 2003). Blazka and colleagues showed remarkable upregulation in the levels of TNF-α and IL-1α in mice given PCM (Blazka et al., 1995).
Using CoQ10 and L-CoQ10 significantly attenuated the expression of IL-6, CRP and phospho- NF-κB after the acute exposure to PCM overdose. This reduction was more profound in L-CoQ10 treated group than CoQ10 group. CoQ10 has the ability to downregulate the pro-inflammatory cytokines (Jaeschke et al., 2011). A meta-analysis gave evidence about the efficacy of CoQ10 on the plasma levels of CRP, IL-6 and TNF-α in patients suffering from inflammatory diseases including metabolic, autoimmune and cardiovascular diseases. The effectiveness of CoQ10 was attributed to its ability to reduce the activity of the NF-κB signaling cascade that triggers the TNF-α and iNOS genes transcription (Gardner, 2003).
The mitochondrial apoptotic response is another mechanism of PCM-induced liver damage. Acute drug toxicity increased the expression of cytochrome C and BAX but reduced Bcl-2. BAX and Bcl-2 encode some proteins that act as pro- and anti-apoptotic regulators, respectively. The high BAX/ Bcl-2 ratio is considered an adverse prognostic marker for liver damage (McGill and Hinson, 2020). Oxidative stress and inflammation can trigger activation of Bax, which increases the permeability of mitochondrial membrane, releasing cytochrome C and activation of caspase-3. Concurrent treatment with CoQ10 and L-CoQ10 suppressed the expression of cytochrome C and restored the BAX and Bcl-2 balance which reduced cell death and improved the liver recovery after PCM overdose.
Additionally, DNA fragmentation is a subsequent event for apoptosis that has been implicated in PCM-induced liver injury. By using flurometric analysis, Salas and Corcoran detected chromatin condensation and lesion in DNA single-strand in mouse hepatocytes following PCM intoxication (Salas and Corcoran, 1997). This study supported our findings which revealed significant DNA fragmentation following an acute dose of PCM, while using of CoQ10 and L-CoQ10 diminished DNA damage.
Likewise, histopathological examination supported these results showing congestion in the central vein with eosinophilic plasma surrounded by degenerated and vacuolated hepatocytes in rats exposed to high dose PCM. In line with the previous study (Fouad and Jresat, 2012), the histopathological results revealed that CoQ10 treatment restored the normal architecture observed in the livers of control rats.
Taken together, this study demonstrates that L-CoQ10 has the ability to counteract the hepatotoxicity induced by PCM which is encountered by modulating the proteins’ levels of IL-6, CRP, cytochrome C and NF-κB and gene expression of BAX and BcL-2 in liver tissue. Furthermore, L-CoQ10 has superior actions over its native drug due to the ability of the liposome to adhere to cell membrane and release its content inside the cell, which makes L-CoQ10 a candidate drug to be used for PCM intoxicated patients.
Author contributions
AA contributed to the experimental design and writing; LF contributed to the experimental design, experimental work, and writing; LA contributed to the experimental work; NA contributed to the experimental design and writing; WS contributed to revising the manuscript; DM contributed to the experimental design; and IH contributed to the experimental design, experimental work, statistical analysis, and writing.
Formatting of funding sources
There is no grant received from funding agencies in the public, commercial, or not for-profit sectors.
Acknowledgement
This research project was supported by the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Coenzyme Q10 supplementation and oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Eur. J. Clin. Pharmacol. 2020:1-17.
- [Google Scholar]
- The potential protective effect of curcumin and α-lipoic acid on N-(4-hydroxyphenyl) acetamide-induced hepatotoxicity through downregulation of α-SMA and collagen III expression. Dose-Response. 2022;20(1)
- [Google Scholar]
- Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front. Physiol.. 2020;11:64.
- [Google Scholar]
- Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges. Compr. Rev. Food Sci. Food Saf.. 2020;19(2):574-594.
- [Google Scholar]
- Ashkani-Esfahani, S., et al., 2016. Protective effects of co-enzyme Q10 on thioacetamide-induced acute liver damage and its correlation with behavioral, biochemical, and pathological factors, Iran. Red Crescent Med. J., 18, 8.
- Prolonged N-acetylcysteine therapy in late acetaminophen poisoning associated with acute liver failure–a need to be more cautious? Crit. Care. 2009;13(3):1-2.
- [Google Scholar]
- Transcriptional activation of heme oxygenase-1 and its functional significance in acetaminophen-induced hepatitis and hepatocellular injury in the rat. J. Hepatol.. 2000;33(3):395-406.
- [Google Scholar]
- Blazka, M.E., Wilmer, J.L. Holladay, Ste. D., Wilson, R.E., Luster, M.I., 1995. Role of proinflammatory cytokines in acetaminophen hepatotoxicity, Toxicol. Appl. Pharmacol. 133, 43–52.
- The emerging role of coenzyme Q-10 in aging, neurodegeneration, cardiovascular disease, cancer and diabetes mellitus. Curr. Neurovasc. Res.. 2005;2(5):447-459.
- [Google Scholar]
- Functions of coenzyme Q10 supplementation on liver enzymes, markers of systemic inflammation, and adipokines in patients affected by nonalcoholic fatty liver disease: a double-blind, placebo-controlled, randomized clinical trial. J. Am. Coll. Nutr.. 2016;35(4):346-353.
- [Google Scholar]
- Hepatoprotective effect of coenzyme Q10 in rats with acetaminophen toxicity. Environ. Toxicol. Pharmacol.. 2012;33(2):158-167.
- [Google Scholar]
- Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1: Potential role of inflammatory mediators. Toxicol. Appl. Pharmacol.. 2003;192(2):119-130.
- [Google Scholar]
- Effect of pretreatment with coenzyme Q10 on isoproterenol-induced cardiotoxicity and cardiac hypertrophy in rats. Curr. Ther. Res.. 2009;70(6):460-471.
- [Google Scholar]
- Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem.. 1982;126(1):131-138.
- [Google Scholar]
- Use of paracetamol (acetaminophen) for suicide and nonfatal poisoning: worldwide patterns of use and misuse. Suicide Life-Threatening Behav.. 2000;30(4):313-326.
- [Google Scholar]
- Frequency of medication errors with intravenous acetylcysteine for acetaminophen overdose. Ann. Pharmacother.. 2008;42(6):766-770.
- [Google Scholar]
- Current issues with acetaminophen hepatotoxicity—a clinically relevant model to test the efficacy of natural products. Life Sci.. 2011;88(17–18):737-745.
- [Google Scholar]
- Damage induced by paracetamol compared with N-acetylcysteine. J. Chinese Med. Assoc.. 2014;77(9):463-468.
- [Google Scholar]
- ACG clinical guideline: evaluation of abnormal liver chemistries. Am. J. Gastroenterol.. 2017;112(1):18-35.
- [Google Scholar]
- Elevated liver enzymes in asymptomatic patients–what should I do? J. Clin. Transl. Hepatol.. 2017;5(4):394.
- [Google Scholar]
- Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr. Drug Targets. 2010;11(1):111-121.
- [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974
- [CrossRef] [Google Scholar]
- The development and hepatotoxicity of acetaminophen: reviewing over a century of progress. Drug Metab. Rev.. 2020;52(4):472-500.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Coenzyme q10: Novel formulations and medical trends. Int. J. Mol. Sci.. 2020;21(22):8432.
- [Google Scholar]
- Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury: influence of redox state and cell phenotype. Circ. Res.. 1999;84(1):113-121.
- [Google Scholar]
- Cancer risk among patients with type 2 diabetes: A real-world study in Shanghai, China. J. Diabetes. 2019;11(11):878-883.
- [Google Scholar]
- Calcium-dependent DNA damage and adenosine 3′, 5′-cyclic monophosphate-independent glycogen phosphorylase activation in an in vitro model of acetaminophen-induced liver injury. Hepatology. 1997;25(6):1432-1438.
- [Google Scholar]
- Protective effect of N-acetyl-L-cysteine on the renal failure induced by inferior vena cava occlusion. Transplantation. 1998;65(10):1315-1321.
- [Google Scholar]
- Silencing glycogen synthase kinase-3β inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J. Biol. Chem.. 2010;285(11):8244-8255.
- [Google Scholar]
- Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr. Dis. Treat.. 2009;5:597.
- [Google Scholar]
- Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov.. 2005;4(2):145-160.
- [Google Scholar]
- Mesenchymal stem cells protect against acetaminophen hepatotoxicity by secreting regenerative cytokine hepatocyte growth factor. Stem Cell Res. Ther.. 2022;13(1):1-14.
- [Google Scholar]
- Salvianolic acid C against acetaminophen-induced acute liver injury by attenuating inflammation, oxidative stress, and apoptosis through inhibition of the Keap1/Nrf2/HO-1 signaling. Oxid. Med. Cell. Longev.. 2019;2019
- [Google Scholar]
- Incidence and management of N-acetylcysteine-related anaphylactoid reactions during the management of acute paracetamol overdose. Eur. J. Emerg. Med.. 2014;21(1):57-60.
- [Google Scholar]
- Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit. Care. 2009;13(2):1-7.
- [Google Scholar]
- Anaphylactoid reactions to intravenous N-Acetylcysteine during treatment for acetaminophen poisoning. J. Med. Toxicol.. 2018;14(2):120-127.
- [Google Scholar]
- Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev.. 2018;14(3):164-174.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102144.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







