Translate this page into:
Biosynthesized copper oxide nanoparticles (CuO NPs) enhances the anti-biofilm efficacy against K. pneumoniae and S. aureus
⁎Corresponding author. mythumithras@gmail.com (Balasubramanian Mythili Gnanamangai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this experimental study was designed to synthesis the CuO NPs using medicinal plant of Moringa oleifera for eradicate the biofilm formation. In result, the UV-spectrum of the synthesized CuO NPs subjected to the range between 200 and 600 nm. Among these, the prominent absorbance peak at 430 nm indicated the peak was held to the copper oxide nanoparticle (CuO NPs). The particle size of biosynthesized CuO NPs varied from 90 nm to 250 nm with more than 50% distribution appearing between 130 nm and 170 nm. In addition, it was further confirmed by PSA, SEM, TEM and SAED of irregular shape and also crystalline nature. All the synthesis and characterization results were clearly supported to the result and confirmed that the synthesized nanoparticle is CuO NPs. Further, the biosynthesized CuO NPs was performed against biofilm forming K. pneumoniae, S. aureus and A. baumannii. The anti-bacterial activity result was shown with 20 mm, 14 mm and 18 mm zone of inhibition against K. pneumoniae, S. aureus and A. baumannii. The minimum biofilm inhibition concentration result was more evident, that the CuO NPs has excellent anti-biofilm activity at 1000 µg/mL concentration. Furthermore, the decreased survival rate of the CuO NPs was observed at 1000 µg /mL concentration by liquid survival degradation assay. Overall, all the results were clearly confirmed that the CuO NPs has excellent anti-biofilm ability against K. pneumoniae, S. aureus and A. baumannii.
Keywords
Copper oxide nanoparticles
Green synthesis
Anti-biofilm activity
Minimum biofilm inhibition concentration
Biofilm survival assay
1 Introduction
Copper oxide nanoparticles (CuO) are nanoparticles containing oxidized to form Cu ions. There are several methods of producing CuO NPs, like physical, chemical and biological methods. Biological methods are preferred over other methods as they are environmentally friendly. So they are referred to as green methods. Of the biological methods, metal ions are reduced to metal oxide mostly by either plant extracts or microbes. Microbial methods are ones which makes use of primary or secondary metabolites of micro-organisms for the reduction of metal oxide. The importance of green synthesis of nanoparticles is discussed by Akintelu et al. (2021). Based on the previous statement, the economic, product purity, biocompatibility and reaction requirement conditions were more advantages for green synthesis methods over chemical synthetic, and also physical synthesis procedures (Nithiyavathi et al., 2021; Rajivgandhi et al., 2019a,b).
Plant extracts are excellent source of enzymes which is responsible for redox reaction responsible for the formation of metal oxide nanoparticles from their bulk counterparts. The general reaction for the formation of metal oxide nanoparticle (References). This process stabilizes metal ions without the use of harmful chemicals or extreme physical conditions. A wide variety of plants were used for copper nanoparticle green synthesis. A comparative analysis of Letchumanan et al. (2021), evaluated various plant based methods along with physical and chemicals methods for synthesis of CuO NPs. Each method has its own advantages and disadvantages. Plant based methods are cheap, eco-friendly and makes use of renewable resources. Some of the disadvantages mentioned for plant based metal nanoparticle synthesis include batch to batch variability in size, shape and yield along with less control over synthetic systems.
Ananda Murthy et al. (2020) utilized indigenous medicinal plant Hagenia abyssinica (Brace) JF. Gmel for the synthesis of CuO NPs. They utilized leaf extracts for biosynthesis of CuO NPs and found bioactive nanoparticles with potential medical application. Similarly Ghosh et al. (2020) reported multiple applications of CuO NPs synthesized using biodiesel plant called Jatropha curcas leaves. Apart from anti-microbial properties, they were evaluated electrical and magnetic properties of CuO NPs. Recently, Mali et al. (2020), documented that the Celastrus paniculatus leaf extract mediated CuO NPs has excellent antifungal and photocatalytic activity compared with physical and chemical methods. The aqueous extract of Nerium oleander leaf aqueous mediated CuO NPs has excellent anti-bacterial activity against various pathogen (Gopinath et al., 2020).
Extensive studies are available for CuO synthesis using plant extracts. Hence the study was designed to understand the formation of CuO NPs, its types, characteristics and utility across various industries. In this study copper acetate was selected to use as a starting material. Copper acetate was chosen to eliminate residual components such as halides or sulfur containing materials which might interfere with nanoparticle purity and integrity if those inorganic salts were employed. Acetate salts leave little of no residue as the redox reaction takes care of liberating the available counter ions in the plant extract mixture. Upon drying, the settlements can be directly used without needing for further purification steps. Previously, Chung et al. (2017) was also synthesized CuO NPs from copper acetate using plant of Eclipta prostrata leaves extract and it has excellent biological properties against various bacteria.
As a source of plant material, Moringa oleifera (MO) leaves were utilized in this study. Due to availability and known medicinal properties, the plant source was decided. Finished product would be CuO NPs, which was intended for biomedical application and it will be acted as an efficient biomaterial in future drug delivery.
2 Materials and method
2.1 Moringa olifera (MO) leaf powder preparation
MO leaves were collected for this study. Leaves were inspected visually to make sure they are devoid of infections and pests. They were carefully separated from their stems and washed multiple times with running water to remove dust and other impurities. The cleaned leaves were allowed to dry completely by keeping them in shade at room temperature for 3 days. It was then made into a fine powder using lab scale blender. The powdered leaf material was properly stored in dry containers till further usage.
2.2 Leaf extract preparation
30 g of ground MO powder was taken in a beaker and 100 mL of distilled water was added to it. The solution was allowed to stir using magnetic beads at 250 rpm in room temperature for three hours. It was then filtered using Whatman No. 1 filter paper.
2.3 Synthesis of CuO NPs
From the filtered solution 50 mL was taken in a beaker and 0.2 M copper acetate solution was added to it. Then the solution was left to dissolve overnight at room temperature in a shaker incubator. After about 16 h, the solution was completely dried and the sediments were collected and the CuO NPs were dried at 100 °C. It was then stored in brown bottles (Merugu et al., 2021).
2.4 Characterization of CuO NPs
The synthesized CuO NPs was subjected to the following methods for characterization. UV–Visible spectroscopy, Particle size analysis, Scanning electron microscopy, Transmission electron microscopy, Energy dispersive X-ray analysis and Selective area electron diffraction. These results will help in understanding the purity, size, shape, uniformity and distribution of biosynthesized nanoparticles.
2.5 Anti-bacterial activity of CuO NPs
The anti-bacterial activity of the CuO NPs was effectively performed against biofilm producing bacteria by agar well diffusion method (Naseer et al., 2021). Briefly, the 24 h culture of biofilm producing bacteria K. pneumonia, S. aureus and A. baumannii were taken on cotton swab and spread evenly on freshly prepared muller hinton agar plates. Then, the wells were cut and then added the synthesized CuO NPs at the concemtration of 10–100 µg/mL into the wells, and kept into incubator for one-day incubation. After one day incubation, the zone of inhibition around the synthesized copper oxide nanoparticles was calculated based on the measuring scale in diameter Baig et al. (2020).
2.6 Minimum biofilm inhibition concentration of synthesized CuO NPs
The 24 h old bacterial cultures of K. pneumonia, S. aureus and A. baumannii were added into freshly prepared tryptic soy broth into 96-well plate to detect the adherent bacteria detection. This liquid medium based inhibition assay was used in this study based on the previously reported protocol of Hashim Khan et al. (2019). Briefly, the freshly prepared tryptic soy broth was evenly added into all the wells in the 100 µL volume. Next 10 µL of pathogens culture was added in every well and it was shaken well, then the plate was allowed for 1 h incubated. Then, different concentration (100–1000 µg/mL) of synthesized CuO NPs was added in the respective wells. Then shaken the plate gently and seen whether the nanoparticle sample was diluted into the cultures containing solution or not. After clear dilution, the plate was put into incubator with 37 ℃ and allowed to treat one day time interval. Other side, the pathogen plus culture without treatment with nanoparticle of the well was performed as a control. After successful treatment with one day time interval, the turbidity of the control and treated wells of the plate was seen in naked eye. The wells showing increased turbidity due to dead cells was considered as a response expected due to nanoparticle inhibition and it was compared to untreated control well with no turbidity. Then, this result was cross checked using inhibition percentage detection based on the previously reported evidences of Rajivagndhi et al. (2019). In percentage of biofilm inhibition, both treated and untreated wells containing culture was analyzed on O.D of 550 nm and interpreted each other. The lowest and highest inhibition effect was identified in particular concentrations, and highest inhibition with lowest concentration exhibited result concentration was fixed as a minimum biofilm inhibition concentration.
2.6.1 Biofilm survival assay
In biofilm survival experiment, the selected bacterial culture was intended to analyze the bacterial survival after treatment with CuO NPs by UV-spectroscopy with the help of XTT solution. In this experiment was completely followed by Kim et al. (2017), and step by step protocol was available below. Initially, the tryptic soy agar medium was filled into 24-well plate and 250 µg/mL of pathogen plus different diluted concentration of CuO NPs after sonication were inoculated into the wells. In addition, without addition of CuO NPs with above used procedure containing well was performed as control. The mixtures were shaked each other and plates were maintained incubation using 37 ℃ for 24 h. Then, the plate was taken and added XTT solution in equal volume in all the wells. Then, the plate was maintained 1 h incubation with 37 °C set up incubator. After added PBS solution, the 50 µg/mL of menadione acetate solution was added in to all the wells and shaken the plate clearly and maintained into the 37 °C set up incubator for 1 h. Finally, the formed and unformed results of the treated and untreated wells were read on UV-spectrometer at 540 nm. Then, the calculation was made after compared the control and tested values (Khaled et al., 2021).
3 Results and discussion
3.1 Biosynthesis of CuO NPs
Fig. 1a depicts, the formation of CuO NPs using MO leaf extract. Copper acetate solution which was blue in color turned to brown, indicating the formation of CuO NPs. Not much of literature is available for using copper acetate as a precursor in plant based nanoparticle synthesis. However, some of the research articles quote about using copper acetate for synthesis of CuO NPs by thermal decomposition methods (Adner et al., 2013). Lastovina et al. (2016) prepared CuO NPs from copper acetate by following three methods which employ physical, chemical and combination methods.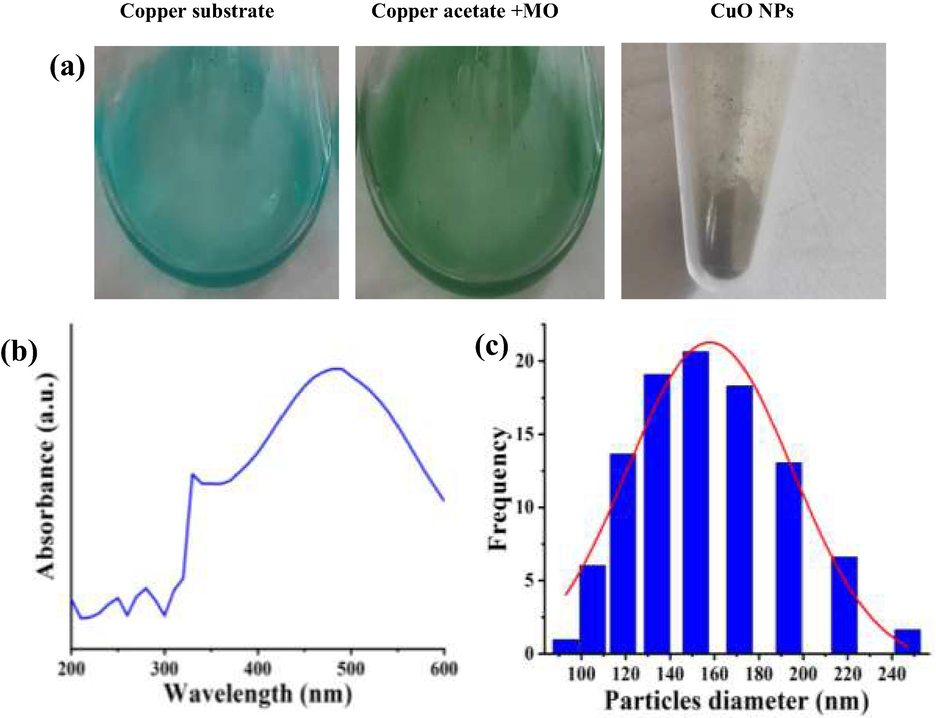
Biosynthesis method of CuO NPs using Moringa olifera leaves (a), UV–vis spectroscopy (b) and PSA frequency analysis (c) of CuO NPs using Moringa olifera leaves.
3.2 Characterization of CuO NPs
3.2.1 UV–Visible spectroscopy
The UV–visible spectrum of reconstituted CuO NPs in distilled water is shown in Fig. 1b. The spectrum was read from 200 nm to 600 nm using a quartz cuvette of path length 1 cm. It showed prominent absorbance peak at 430 nm. Some of the chemically synthesized CuO NPs produces electromagnetic radiation absorption at around 500 nm, which they mention as reaction dependent and could vary with the type of reagents used (Zhao et al., 2022; Lee et al., 2011).
3.2.2 Particle size analysis
The particle size of biosynthesized CuO NPs varied from 90 nm to 250 nm with more than 50% of frequencies appearing between 130 nm and 170 nm as shown in Fig. 1c. The particles could be irregular shaped, resulting in broad range of particle size distribution. However it cannot be ascertained based only on PSA graphs. Additional characterization methodologies need to be addressed to understand the size and shape. Chemical and physical methods could yield uniform particles with lesser range of size distribution as the leverage on control of reaction exists with the researcher. Biological methods cannot be taken in a similar fashion. Hence a broader range of particle distribution is expected in this case. Nagar and Devra, (2018) used neem leaves to biologically synthesize CuO NPs and the mean particle size was observed as 48 nm.
3.2.3 Electron microscopy (SEM and TEM)
Fig. 2 shows the electron microscopic images of CuO NPs synthesized using MO leaves. Fig. 2a-d show, the SEM images at various magnifications, indicating the topology of synthesized CuO NPs. They seemed to be irregular shaped with varying size distribution, which concurs with the observation from particle size analysis. TEM also confirmed the inference of irregular shaped CuO NPs as observed while testing with other characterization tools (Fig. 3a-d). Recently, Dashtizadeh et al. (2021), synthesized CuO NPs by two methods such as green chemistry and leaf extract method. They noticed that chemical synthesis method yielded smaller particles (less than 50% lesser in dimension) compared to the leaf extract method. They produced CuO NPs of mean size of 100 nm which corroborates with our observation. Jahan et al. (2021) was revealed that the biosynthesized CuO NPs has uniform size in TEM micrograph image and it highly similar to present result of biosynthesized CuO NPs. Also, Usha et al. (2017) reported spherical nanoparticles of uniform size while using Tulasi plant extract for biosynthesis of CuO NPs. Uniform distribution of CuO NPs of about 10 nm was observed by Ghosh et al. (2020) using Jatropha curcas leaf extracts.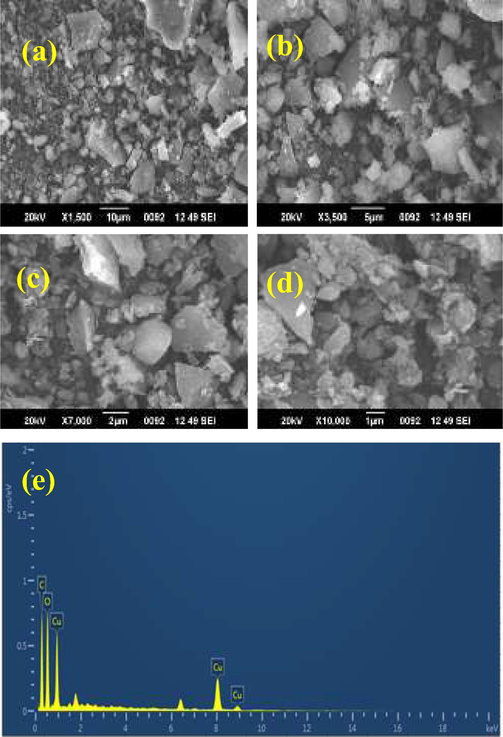
Various magnification of biosynthesized CuO NPs in SEM (a-d) and EDAX profile of CuO NPs using Moringa olifera leaves (e).
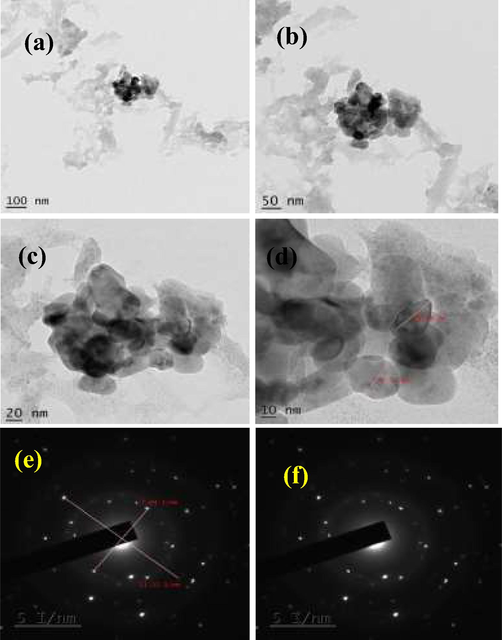
Various magnification of biosynthesized CuO NPs in TEM pictograph (a-d) and SAED profile (e, f) of CuO NPs using Moringa olifera leaves.
3.2.4 Energy dispersive X-ray examination
EDAX analysis of biosynthesized nanoparticles yielded prominent copper peaks upon elemental analysis. Few oxygen peaks were observed; however, they were insignificant compared to the copper peaks as shown in Fig. 2e. This proves that the biosynthesized CuO NPs are highly pure and could be taken for any kind of applications, be it medical or food application as it doesn’t contain residual species.
3.2.5 Selected area diffraction
Fig. 3e, f show, the SAED profile of biosynthesized CuO NPs. The presence of bright spots indicates that the particles are crystalline in nature. Since background haze is not observed, the nanoparticles synthesized are not amorphous. The visualization of concentric rings in the profile shows the presence of polymorphic forms with irregular shapes as reported in PSA inference. The observation is in agreement with Anandha Murthy et al. (2020) as they observed 6 concentric rings indicating planar rings with interplanar spacing calculated which corresponded to Cu and CuO nanoparticles. Chung et al. (2017) illustrated that CuO NPs synthesized using leaf extracts of Eclipta prostrata had various crystal sized of different orientation however of uniform size. Recent report of Nithiyavathi et al. (2021) and Sharma et al. (2019) documented with supportive evidences of biosynthesized CuO NPs with their SAED profile.
3.2.6 Anti-bacterial activity of synthesized CuO NPs
Based on the 24 h completed plate result, the synthesized copper oxide nanoparticle was very effective against biofilm producing tested K. pneumonia, S. aureus and A. baumannii. All the bacteria were highly sensitive to copper oxide nanoparticle at 1000 µg/mL concentration. In 500 µg/mL and 1000 µg/mL concentrations, the zones were shown 6 mm, and 16 mm zone of inhibition against K. pneumonia, 8 mm and 18 mm zones of inhibition against S. aureus and 4 mm and 18 mm zones of inhibition against A. baumannii were observed respectively. A study using synthesized nanoparticles by Asamoah et al. (2020), exhibited 26 mm and 30 mm zones of inhibition against S. aureus and A. baumanni were also observed respectively. In addition, the resulted concentration of 100 µg/mL showed the inhibition role against both the bacteria of K. pneumonia S. aureus and A. baumanni. The inhibition range of concentration and their respective zones were available in Fig. 4. In addition, the zone of inhibition against all the K. pneumonia, S. aureus and A. baumanni in muller hinton agar plate was exhibited in Fig. 4a, b, c. Based on the above said result, the result was suggested that the synthesized has efficient anti-bacterial activity against tested K. pneumonia, S. aureus and A. baumanni at increasing concentration. This increasing concentration study format is highly useful to current research to inhibit the multi-drug resistant pathogens in very lowest concentration (Rajeshkumar et al., 2021). The result was most accordance with previous report of Sasidharan et al. (2020) and biosynthesized copper oxide nanoparticles very effective against multi drug resistant bacteria. Mechanistically, the negative charges of the bacterial surface were attractive to positive charges of the synthesized (Sharmila et al., 2018) and lysis the cell wall initially, and then enter into extracellular lipids, polysaccharides and other granular parts. Finally, the CuO NPs was enter into the nucleolus of the bacteria and altered the function of bacteria, and it leads to complete death (Bhushan et al., 2019; Fernandez et al., 2020).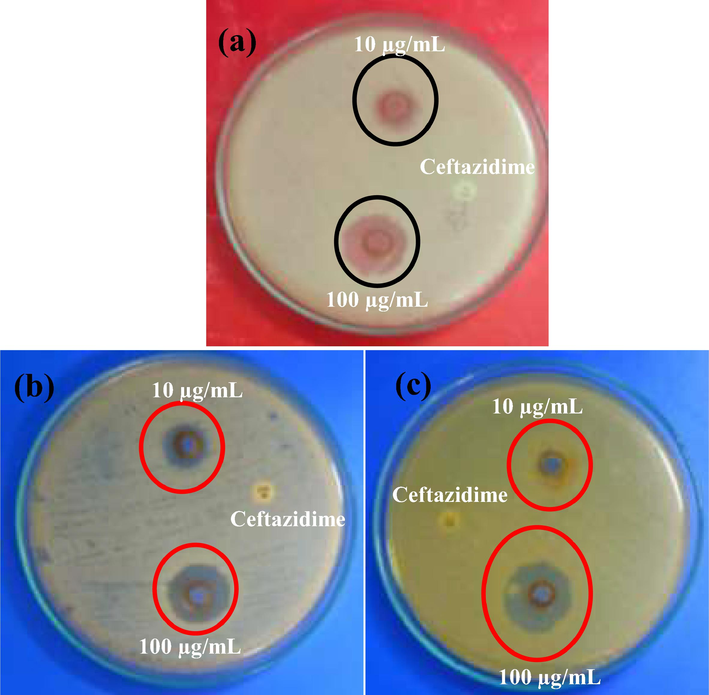
Anti-biofilm activity of CuO NPs against biofilm producing bacteria K. pneumoniae (a), S. aureus (b), and A. baumannii (c) using agar well diffusion method.
3.2.7 Minimum biofilm inhibition concentration of CuO NPs
The confirmation evidences of anti-bacterial activity of agar well diffusion experiment was done by liquid medium based spectrophotometric analysis and confirmed by Chen et al., (2019). In liquid medium, the more turbidity was observed at 1000 µg/mL concentration and it was very effective against both the bacteria. When nanoparticle enters into the bacterial cell wall, the theichoic acid was resembled to foreign particles and helped to target the lysis in bacterial body. The intracellular was damaged continuously, and lost their pathogenicity. Then, the other antigenicity particles were also lost and it affected entire bacteria. Continuous disturbance of bacteria could lead to death. This statement was agreed by previous report of Lotha et al. (2019) and gram positive bacteria virulence factors were damaged by Copper oxide nanoparticles. Caires et al., (2020), also agreed this result and internal parts of the bacteria was lost. As same as, the virulence factors of quorum sensing, exopolysachharide, biofilm production, enzyme inactivation and others. In this result, the more turbidity of the 1000 µg/mL concentration was highly powerful against multi drug resistant bacteria. In addition, the O.D value of the 100–1000 µg/mL concentrations were compared with untreated control O.D values and shown highest inhibition concentration against tested K. pneumonia, S. aureus and A. baumannii. In 1000 µg/mL concentration, bacteria were shown high damage and exhibited 94% (Fig. 5a), 89% (Fig. 5b) and 95% (Fig. 5c) of inhibition. Finally, the result was proved that the synthesized copper oxide nanoparticles were very efficient against gram positive and gram negative bacteria. At the same time, both the bacteria were more suppressed at increasing concentration and the result was also dependent on concentration. In addition, some of the other recent reported green synthesis CuO NPs using various plant sources was tabulated in Table 1.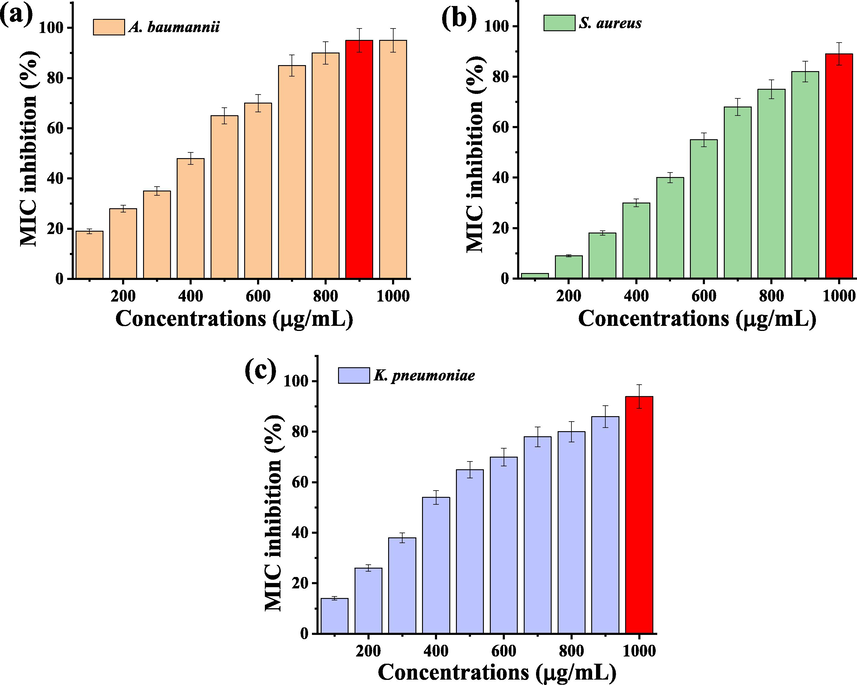
Minimum inhibition concentration of CuO NPs against biofilm producing bacteria of K. pneumoniae (a), S. aureus (b) and A. baumannii (c).
S. No
Plant source
Biological activities
Reference
1
Leaf extract of Ageratum houstonianum
Antibacterial and photocatalytic activities
Chandraker et al., 2020
2
Leaf extract of Ocimum sanctum
Anti-bacterial activities
Jayadev and Krishnan, 2021
3
Leaf extract of Cissus vitiginea
Antioxidant and antibacterial activity
Wu et al., 2020
4
Seed extract of Caesalpinia bonducella
Antibacterial activity
Sukumar et al., 2020
5
Fruit pulp of Moringa oleifera
Antimicrobial activities
Merugu et al., 2021
6
Leaf extract of Nerium oleander
Antibacterial activity
Gopinath et al., 2014
3.2.8 Biofilm survival eradication assay
The complete eradication of biofilm formation using CuO NPs was available in Fig. 6 at different concentration. The result of biofilm survival inhibition assay was suggested that the CuO NPs has anti-biofilm efficiency against biofilm producing K. pneumonia, S. aureus and A. baumannii. The result of O.D value was exhibited with 92% (Fig. 6a), 90 (Fig. 6b) and 95% (Fig. 6c) of inhibition for 1000 µg/mL, and 14%, 18% of inhibition was observed at 10 µg/mL concentrations. The biofilm producing intracellular materials were absent in this process. It may be shown with absence of pathogenicity only due to the arrest of virulence factors. In this assay result was clearly stated may or may not the antigenicity of the biofilm producing K. pneumonia was destroyed, but the pathogenicity was completely arrested due to the effect of CuO NPs. The survival rates were depended on increasing concentration. The concentration dependent biofilm survival inhibition assay against various bacteria was effectively seen in Fig. 6a, b, c. Recently, Chaieb et al. (2011), reported that the virulence factors inhibition was more important in biofilm inhibition study. Especially, the inhibition of QS, polysaccharide, enzymes, exopolysaccharide and other virulence factors were inhibited by CuO NPs. When we see in the muller hinton agar plates, the culture was grown smartly after treatment with CuO NPs (Data not shown). It was suggested that the CuO NPs of this study was highly inhibited the biofilm production due to the eradication of pathogenicity factors.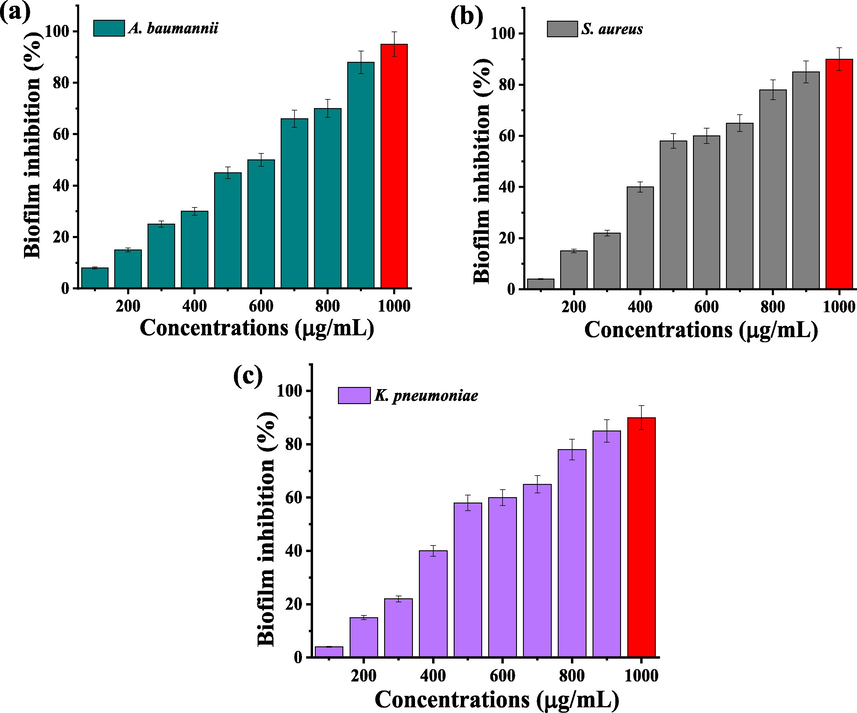
Biofilm survival assay of biofilm producing bacteria K. pneumoniae (a), S. aureus (b) and A. baumannii (c) after treatment with biosynthesized CuO NPs.
4 Conclusion
Biosynthesis of copper nanoparticle was carried out using Moringa Oleifera leaf extract. The method yielded a highly pure copper nanoparticle which was then characterized by UV–Visible spectroscopy, PSA, SEM, TEM, EDAX and SAED. The bioactivity of the synthesized copper nanoparticle was evaluated using pathogenic bacterial strains namely K. pneumoniae, S. aureus and A. baumannii. Based on the anti-microbial activity, minimum biofilm inhibition concentration and biofilm survival assay of the result, the biofilm producing bacteria were highly compromised to biosynthesized CuO NPs. In addition, the biosynthesized copper nanoparticle was highly eradicating the bacterial biofilms effectively at 1000 µg/mL. Thus such highly pure CuO NPs synthesized using MO leaf extract is intended to as a nanocoating material for biomedical application and it will be acted as an efficient biomaterial in future drug delivery.
Acknowledgements
The authors express immense gratitude to Dr. R. Gopalakrishnan, Principal of K. S. Rangasamy College of Technology (Autonomous), Tiruchengode, Tamil Nadu for the financial assistance. The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/70), King Saud University, Riyadh, Saudi Arabia. Acknowledgement are also due to DST–FIST (fund for infrastructure for science and technology) FIST NO: 368 (SR/FST/College-235/2014 dated November 21, 2014), DBT STAR and DBT PG College Scheme (BT/HRD/September 11, 2018 and BT/HRD/July 01, 2020) and TNSCST Tamil Nadu for their instrumental catalyses and financial support rendered to carry out the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A straightforward approach to oxide-free copper nanoparticles by thermal decomposition of a copper(I) precursor. Chem. Commun.. 2013;49:6855-6857.
- [CrossRef] [Google Scholar]
- Green chemistry approach towards the synthesis of copper nanoparticles and its potential applications as therapeutic agents and environmental control. Curr. Res. Green Sustain. Chem.. 2021;4:100176.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of zinc and copper oxide nanoparticles and their antibacteria activity. Results Mater.. 2020;7:100099.
- [CrossRef] [Google Scholar]
- Single step production of high-purity copper oxide-titanium dioxide nanocomposites and their effective antibacterial and anti-biofilm activity against drug-resistant bacteria. Mater. Sci. Eng., C. 2020;113:110992.
- [CrossRef] [Google Scholar]
- Study of synthesis, structural, optical and magnetic characterizations of iron/copper oxide nanocomposites: A promising novel inorganic antibiotic. Mater. Sci. Eng., C. 2019;96:66-76.
- [CrossRef] [Google Scholar]
- Effective killing of bacteria under blue-light irradiation promoted by green synthesized silver nanoparticles loaded on reduced graphene oxide sheets. Mater. Sci. Eng., C. 2020;113:110984.
- [Google Scholar]
- XTT assay for evaluating the effect of alcohols, hydrogen peroxide and benzalkonium chloride on biofilm formation of Staphylococcus epidermidis. Microb. Pathog.. 2011;50(1):1-5.
- [Google Scholar]
- Chandraker, S.K., Lal, M., Ghosh, M.K., Tiwari, V., Ghorai, T.K., Shukla, R. 2020. Green synthesis of copper nanoparticles using leaf extract of Ageratum houstonianum Mill. and study of their photocatalytic and antibacterial activities. Nano express. 1, 010033, https://doi.org/10.1088/2632-959X/ab8e99.
- Preparation and antibacterial activities of copper nanoparticles encapsulated by carbon. New Carbon Mater.. 2019;34(4):382-389.
- [CrossRef] [Google Scholar]
- Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp. Ther. Med.. 2017;14(1):18-24.
- [CrossRef] [Google Scholar]
- Phytosynthesis of copper nanoparticles using Prunus mahaleb L. and its biological activity. Mater. Today Commun.. 2021;27:102456.
- [CrossRef] [Google Scholar]
- Green synthesis, characterization, catalytic and antibacterial studies of copper iodide nanoparticles synthesized using Brassica oleracea var. capitata f. rubra extract. Chem. Data Collect.. 2020;29:100538.
- [CrossRef] [Google Scholar]
- Ghosh, M.K., Sahu, S., Gupta, I., Ghorai, T.K. 2020. Green synthesis of copper nanoparticles from an extract of Jatropha curcas leaves: characterization, optical properties, CT-DNA binding and photocatalytic activity. RSC Adv. 10, 22027, https://doi.org/10.1039/d0ra03186k.
- Synthesis of Copper Nanoparticles from Nerium oleander Leaf aqueous extract and its Antibacterial Activity. Int. J. Curr. Microbiol. App. Sci.. 2014;3(9):814-818.
- [CrossRef] [Google Scholar]
- Facile microwave-mediated green synthesis of non-toxic copper nanoparticles using Citrus sinensis aqueous fruit extract and their antibacterial potentials. J. Drug Delivery Sci. Technol.. 2021;61:102172.
- [CrossRef] [Google Scholar]
- Green synthesis of copper nanoparticles and its characterization. J. Sci. Res.. 2021;65:1.
- [CrossRef] [Google Scholar]
- Anti-biofilm activity of LC-MS based Solanum nigrum essential oils against multi drug resistant biofilm forming P. mirabilis. Saudi J Biol Sci. 2021;28:302-309.
- [CrossRef] [Google Scholar]
- Bactericidal potential of silver-tolerant bacteria derived silver nanoparticles against multi drug resistant ESKAPE pathogens. Biocatal. Agric. Biotechnol.. 2019;18:100939.
- [CrossRef] [Google Scholar]
- Inhibitory activity of hinokitiol against biofilm formation in fluconazole-resistant Candida species. PLoS ONE. 2017;12(2):e0171244.
- [CrossRef] [Google Scholar]
- Copper-based nanoparticles prepared from copper(II) acetate bipyridine complex. J. Serb. Chem. Soc.. 2016;81(7):751-762.
- [Google Scholar]
- Lee, H.J., Lee, G., Jang, N.R., Yun, J.H., Song, J.Y., Soo, K.M. 2011. Biological synthesis of copper nanoparticles using plant extract. Technical Proceedings of the 2011 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech 2011. 1. 371-374.
- Plant-based biosynthesis of copper/copper oxide nanoparticles: an update on their applications in biomedicine, mechanisms, and toxicity. Biomolecules. 2021;11:564.
- [CrossRef] [Google Scholar]
- Biogenic phytochemicals (cassinopin and isoquercetin) capped copper nanoparticles (ISQ/CAS@CuNPs) inhibits MRSA biofilms. Microb. Pathog.. 2019;132:178-187.
- [CrossRef] [Google Scholar]
- Mali, S.C., Dhaka, A., Githala, C.K., Trivedi, R., 2020, Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties, Biotechnol. Rep., 27, e00518, https://doi.org/10.1016/j.btre.2020.e00518.
- Synthesis, characterization and antimicrobial activity of bimetallic silver and copper nanoparticles using fruit pulp aqueous extracts of Moringa oleifera”. Mater. Today:. Proc.. 2021;44:153-156.
- [CrossRef] [Google Scholar]
- Synthesis of green copper nanoparticles using medicinal plant Hagenia abyssinica (Brace) JF. Gmel. leaf extract: antimicrobial properties. J. Nanomater. 2020;2020:1-12.
- [Google Scholar]
- Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys.. 2018;213:44-51.
- [CrossRef] [Google Scholar]
- Facile green synthesis of copper oxide nanoparticles for the eradication of multidrug resistant Klebsiella pneumonia and Helicobacter pylori biofilms. Int. Biodeterior. Biodegrad.. 2021;159:105201.
- [CrossRef] [Google Scholar]
- Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. J. Infect. Public Health. 2021;14:1893-1902.
- [CrossRef] [Google Scholar]
- Environment friendly synthesis copper oxide nanoparticles and its antioxidant, antibacterial activities using Seaweed (Sargassum longifolium) extract. J. Mol. Struct.. 2021;1242:130724.
- [CrossRef] [Google Scholar]
- Biologically synthesized copper oxide nanoparticles enhanced intracellular damage in ciprofloxacin resistant ESBL producing bacteria. Microb. Pathog.. 2019;127:267-276.
- [CrossRef] [Google Scholar]
- Synthesis of silver and copper oxide nanoparticles using Myristica fragrans fruit extract: Antimicrobial and catalytic applications. Sustainable Chem. Pharm.. 2020;16:100255.
- [CrossRef] [Google Scholar]
- Green synthesis and characterization of copper nanoparticles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J. Microbiol. Methods. 2019;160:107-116.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of CuO nanoparticles using Bauhinia tomentosa leaves extract: Characterization and its antibacterial application. J. Mol. Struct.. 2018;1165:288-292.
- [CrossRef] [Google Scholar]
- Green-synthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extract and their applications. ACS Omega. 2020;5(2):1040-1051.
- [Google Scholar]
- Biosynthesis and characterization of copper nanoparticles from tulasi (Ocimum sanctum L.) leaves. Int. J. Curr. Microbiol. Appl. Sci.. 2017;6(11):2219-2228.
- [Google Scholar]
- Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol.. 2020;48(1):1153-1158.
- [Google Scholar]
- Biological synthesis of copper oxide nanoparticles using marine endophytic actinomycetes and evaluation of biofilm producing bacteria and A549 lung cancer cells. J. King Saud Univ. Sci.. 2022;34(3):101866.
- [CrossRef] [Google Scholar]







