Translate this page into:
Macrophage plasticity enhanced by camel milk peptide attributes in wound healing in diabetic rats
⁎Corresponding author. jhattamimi@gmail.com (Jameel Al-Tamimi), jtamimi@ksu.edu.sa (Jameel Al-Tamimi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Diabetes Mellitus during wound healing alters macrophage recruitment, leading to delayed healing. The present study focuses on impact of camel milk peptide (CMP) on macrophage plasticity during their recruitment during wound healing stages. The Swiss albino rats were distributed into three groups: a normal wounded group (control), a wounded diabetic group, and a wounded diabetic group treated with CMP daily. The diabetic rats showed a significantly compromised level of monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein (MIP-1α) relative gene expression, anti-CD31, anti-Mac-3, and anti-CD68 staining than the control group. Macrophages were considerably depleted in diabetic group animals upon initiating the inflammatory phase, while the protein significantly reversed the same. Also, there was marked increase in pathogenic bacterial growth at the wound sites of diabetic rats. On the contrary, in CMP-diabetic rats, levels of pathogenic bacteria were comparable to normal control animals. Diabetic rats demonstrated a reduction in matrix metalloproteinase (MMP-9 and MMP-13) expression while increased TNF-α levels compared to control rats. In addition, they also showed significantly reduced expression of VEGF and fibroblast growth factors (FGF) genes and IL-10 during the proliferation phase. However, all these parameters exhibited a considerably reversed trend in the CMP treated diabetic rats. Hence, CMP-treated diabetic rats demonstrated a transition to the M2 phenotype, highlighting macrophage recruitment's critical role in healing of wounds in hyperglycemic rats.
Keywords
Wound healing
Macrophage
Camel milk peptide
Diabetes
- WP
-
Whey proteins
- CMP
-
Camel milk peptide
- STZ
-
Streptozotocin
- IL
-
Interleukin
- FGF
-
Fibroblast growth factors
- TNF-α
-
Tumor necrosis factor alpha
- NOS
-
Nitric oxide synthase
- VEGF
-
Vascular endothelial growth factor
- CD
-
Cluster of differentiation
- TGFβ
-
Transforming growth factor-beta
- CMP-1
-
Monocyte chemoattractant protein-1
- MIP
-
Macrophage inflammatory protein
Abbreviations
1 Introduction
Wound repair process is consist of a complex events involving the cells to undergo hemostasis, inflammation, proliferation, and maturation (van Solingen et al., 2014). Failure to occur these events in a well-timed manner can result in pathological wound healing (Boniakowski et al., 2017). An inflammatory response is essential for healing that helps degrade and ingest dead cells and foreign material to eliminate injured tissues (Chen et al., 2017). Macrophages depict a povital role in the inflammatory phase of tissue repair. These cells' plasticity mediates tissue destruction and repair functions (Kapellos and Iqbal, 2016). Macrophages remove the cells under apoptosis that facilities proliferation of the neighboring cells and restoration of tissues (Koh and DiPietro, 2011). The epigenetic reprogramming of macrophages is closely related to their plasticity and function (Boniakowski et al., 2017). Initially, pro-inflammatory macrophages (M1-like phenotypes) infiltrate the injury site to free the wound from cell debris, foreign agents, bacteria, and dead cells (Krzyszczyk et al., 2018). Activated pro-inflammatory M1 initiates inflammation by triggering pro-inflammatory cytokines and generating ROS (Landis et al., 2018). Monocytes then invade tissues, transforming into inflammatory MoMFs that secrete cytotoxic and pro-inflammatory factors, including Interleukin-1β (IL-1β), Tumor necrosis factor alpha (TNF-α), and Interleukin-6 (IL-6). The cytotoxic nitrogen intermediates further destroys target cells from L-arginine via inducible Nitric oxide synthase-2 (NOS2) (Nathan and Hibbs Jr, 1991). Anti-inflammatory cells Macrophage polarization (M2-like phenotypes) are activated in the late stages of the process to resolve inflammation and promote tissue remodeling (Landis et al., 2018).
Phenotype shifts within tissues are critical for transitioning from inflammatory to proliferative phases (Boniakowski et al., 2017). This transition involves a wave of anti-inflammatory macrophages that promotes tissue remodeling, fibrosis, and wound repair (Krzyszczyk et al., 2018). Anti-inflammatory macrophages promote the relocation and escalation of fibroblasts, endothelial cells, and keratinocytes to reinstate the dermis, epidermis, and vasculature. A signal of vascular endothelial growth factor (VEGF), is produced to stimulate the formation of blood vessels.
Macrophages differentiate into a spectrum of subpopulations in response to microenvironmental stimuli (Ramalho et al., 2018). Substantial evidence implicates macrophages as critical regulators are assigned distinct roles in wound healing in normal and diabetic subjects (Krzyszczyk et al., 2018). During diabetic wound healing, regulation of pro-inflammatory macrophage transition in response to high glucose by insulin leads to improved wound healing (Yu et al., 2019). Cluster of differentiation 31 (CD31) regulates the interaction between endothelial cells and leukocytes. Tissue macrophages, including dendritic cells, can profoundly express CD68. Monocytes are attracted in the early stage of inflammation; these then transform into inflammatory macrophages and secrete many cytotoxic factors, including TNF-α. Inflammatory macrophages release metalloproteinase to degradate the extracellular matrix in initial stages of wound healing. This creates pouches for the infiltration of inflammatory cells. In the later stages of recovery, macrophages play a crucial role in resolving inflammation. Therefore, macrophages (M2) release anti-inflammatory mediators, which play a critical role in promoting the remodeling phase of wound healing via the production of IL-10, Transforming growth factor-beta (TGFβ) and other cytokines. Vascular endothelial growth factor (VEGF) is a signal produced to stimulate blood vessel formation.
Whey proteins (WP) derived from camel milk participate in immuno-modulation, oxidative stability, and enhancement of impaired wound healing in the elderly (Ebaid, 2014, Abdel-Salam et al., 2016), the malnourished and diabetic animal models (Ebaid et al., 2011, Ahmed et al., 2015). In addition to various nutrients, camel milk contains insulin and positively impacts patients with diabetes mellitus. This study examines the effect of a CMP on wound closure in diabetic rats concerning macrophage plasticity. WP supplementation enhances immune responses and associated reactions, such as increased cell chemotaxis and proliferation (Badr et al., 2012). Also, the improvement in redox and immune parameters was pronounced by the protein (Ebaid et al., 2012).
2 Material and methods
2.1 Hydrolysis of camel whey proteins
Centrifugation of CMP at 5000g for 20 min led to production of casein by lowering the pH to 4.3. The saturation of WP supernatant was achieved by ammonium sulfate and thus the protein was collected by dialyzing for 48 h at 4 °C, and finally lyophilized as previously described (Abdel-Salam et al., 2016).
The temperature of the WP was set to 37 °C for hydrolysis and was mixed with trypsin in a ratio of 100:1. The enzyme inactivation was conducted by enhancing the samples at 100◦ C briefly followed by determination of the protein hydrolysis. The degree of hydrolysis was estimated by the ortho-phthalaldehyde based method. The main fractions retrieved from the total whey proteins by hydrolysis were the total whey protein hydrolysate, the 10 kDa and the 3 kDa fractions (Fig. 1). Fractions were tested for their bioactivity on rate of wound closure, histopathological features of cutaneous epidermal and dermal events, and redox status. Between the fractions, 3 kDa (camel milk peptide: CMP) was found superior than the 10 kDa and therefore, the earlier was selected for wound repair experiments.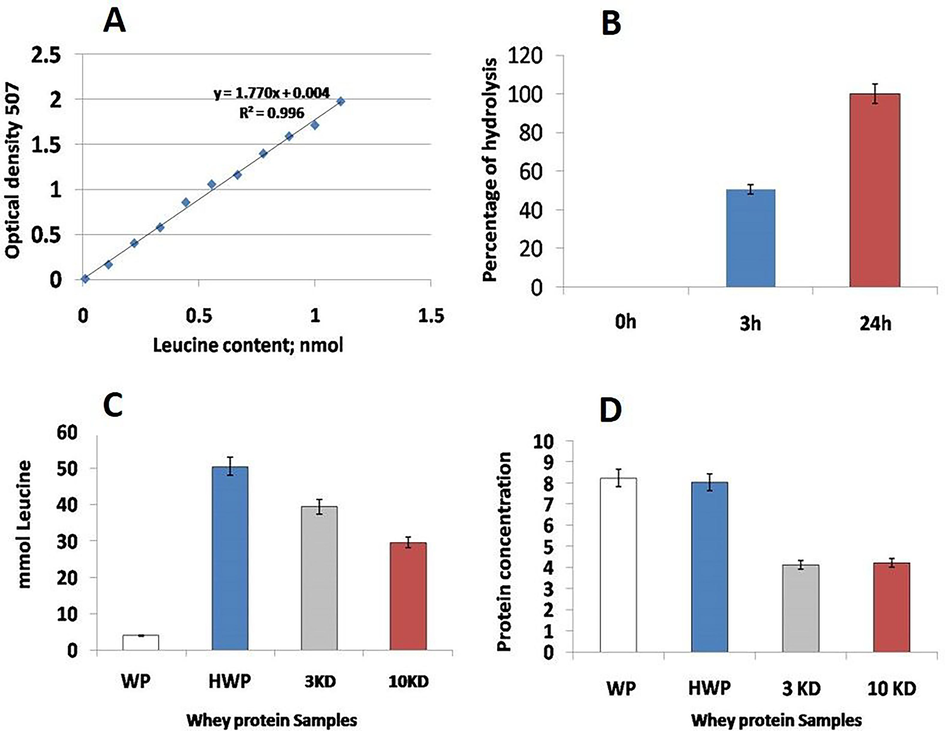
A: Standard Curve of Leucine (X: mlmol; Y: OD at 507). B: Percentage of hydrolysis degree of the whey protein samples. C: Leucine content in hydrolyzed samples of whey proteins. D: Protein contents in the whey protein hydrolsate farctions, namly, whey protein hydrolysate, 3 kDa (camel milk peptide: CMP) and 10 kDa fractions.
2.2 Experimental design
The adult albino rats (130–150 g and 6 months’ age) from the Animal House, Department of Zoology, KSU, Riyadh. Rats were distributed into four groups (n = 15). Two groups – wounded normal (CN+) and wounded diabetic (DM) animals – were administered vehicle PBS solution (1000 μL /rat/day) by intubation for one and seven days. The third group, consisting of wounded diabetic rats, was treated daily with CMP (3 kDa fraction of hydrolyzed WP) at 25 mg/kg of body weight for 7 days by gavage during wounding healing. The final group, also of wounded diabetic rats, was treated with 10 kDa of hydrolyzed WP at the same dose for 7 days by gavage during wound repair.
Camel milk was retrieved from the Alazeria farm, Saudi Arabia. As the current study didn’t involve endangered or protected species; no ethical permission was required. All procedures were conducted as per the guidelines framed by the CPCSEA and the NIH.
2.3 Diabetic models, excisional wound preparation, and total bacterial count
A freshly prepared streptozocin solution (50 mg/kg in a 0.1 mol/L citrate buffer, pH 4.5) was used to induce ddiabetes in the Swiss albino rats. Control group rats were given the same volume of citrate buffer. Diabetes (selecting glucose levels ≥ 220 mg/dl) was established by estimating fasting glucose after two weeks of STZ- dosing.
Wounds were performed (Schwentker et al., 2002) with slight modifications on shaved and sterilized back of the rats. The wound was punched with a sterile blade to form a 5 mm diameter circle on the back.
The measurement of complete bacterial count at the injured surface was conducted using a standard method (Abdelgawad et al., 2014) by taking swabs and submersed in 10 ml of ringer solution. The solutions were plated onto nutrient agar by standard incubation method.
2.4 Blood and tissue sampling
Rats were anesthetized using carbon dioxide in a designated place. The treated rats were sacrificed on day 1, 7th, and 20th after wounding and serum samples were prepared by withdrawing blood from carotid arteries. After dissection, the liver, kidney, and spleen were stored at −80 °C for later use.
2.5 ELISA estimation
Serum levels of TNF-α, Akt, SMA, Mac-3, CD31, and IL-10 of the experimental groups were determined using ELISA kits (Abcam, Cambridge, UK). Concentrations were determined by absorption at 450 nm using a spectrophotometer following the manufacturers' instructions.
2.6 Immunochemical detection of anti-CD68 and anti-TGF-β antibodies
Anti-CD68 and anti-TGF- antibody staining was performed on the sections of tissues in paraffin blocks, involving citrate buffer (0.01 M, pH 6). Remaining all steps were followed as per (Zhang et al., 2013). The cells stained with anti-CD68 and anti-TGF antibodies were counted at 20 random locations under the injured area for all the rats using a Leica image analyzer (Qwin 500).
2.7 RNA extraction and reverse transcriptase PCR (RT-PCR)
2.7.1 RNA extraction and reverse transcriptase PCR (RT-PCR)
The RNA from the samples by using an RNeasy Mini Kit (QIAGEN, Hilden, Germany) was retrieved following the associated manual instructions. The RNA extracts were used as templates for detecting the expression of MIP1-α mRNA (5′ sense primer, 5′-GCCCTTGCTGTTCTTCTCTGT-3′), antisense primer,
5′-GGCATTCAGTTCCAGGTCAGT-3′ (amplicon size: 260 bp) and Mcp-1 mRNA (sense primer, 5′-CAGGTCTCTGTCACGCTTCT-3′, antisense primer, 5′-AGTATTCATGGAAGGGAATAG-3′), and GAPDH (sense primer, 5′- CAACTCCCTCAAGATTGTCAGCAA-3′, antisense primer, 5′- GGCATGGACTGTGGTCATGA −3′ (amplicon size: 118 bp). The RT-PCR reaction volume (25 μL) contained the following components as described in our previous study (Ebaid et al., 2015); and remaining all the standard steps were followed as per Ebaid et al. (2015). The selection of primers sequences was done by pubmed database. Realtive quantification of gene expression was analyzed in comparison with the GAPDH, a housekeeping gene, as described in Applied Biosystems ® User Bulletin No. 2.
2.8 Statistics
MINITAB software (MINITAB, State College, PA, Version 13.1, 2002) was used in Statistical analysis. Data normally distributed were analyzed by one-way ANOVA supplemented with Tukey's method for pairwise comparisons. The results were depicted as mean (M) and standard deviation (SD). Only differences with p < 0.05 were considered statistically significant.
3 Results
3.1 Whey protein hydrolysis
The extent of protein hydrolysis was estimated by ortho-phthaldialdehyde. The standard curve of the leucine in the hydrolysed WP samples is presented in Fig. 1A, while the Leucine content in hydrolyzed samples of whey proteins id represented in Fig. 1B. Meanwhile the percentages of hydrolysis degree and the protein contents of samples were estimated and presented in Fig. 1C and Fig. 1D, respectively.
-
CMP effect on Macrophage (M1 activity)
-
CMP improved macrophage recruitment during the inflammatory phase
The inflammatory response to tissue wounds is critical for normal and pathological healing. Hence, the engagement of inflammatory cells is the primary innate immune system response.
-
The expression profiles of macrophage chemokines MCP-1 and MIP-1α by qRT–PCR
The efficacy of CMP on macrophage recruitment was examined using tissues from wounds excised on days one through day seven post-wounding. Tissues were assessed for effect on expression of macrophage chemokines, MCP-1 and MIP-1α by qRT–PCR (Fig. 2). The MCP-1 mRNA level was not much changed over a time either in the control or in diabetic groups. MIP-1α relative gene expression decreased on day 1 compared to controls for all diabetic groups (DM; DM + CMP and DM + 10 kDa). CMP significantly increased relative gene expression of MIP-1α at day seven compared to control wounded tissue (Fig. 2).
Estimation of Mac-3 and CD31 expression by ELISA
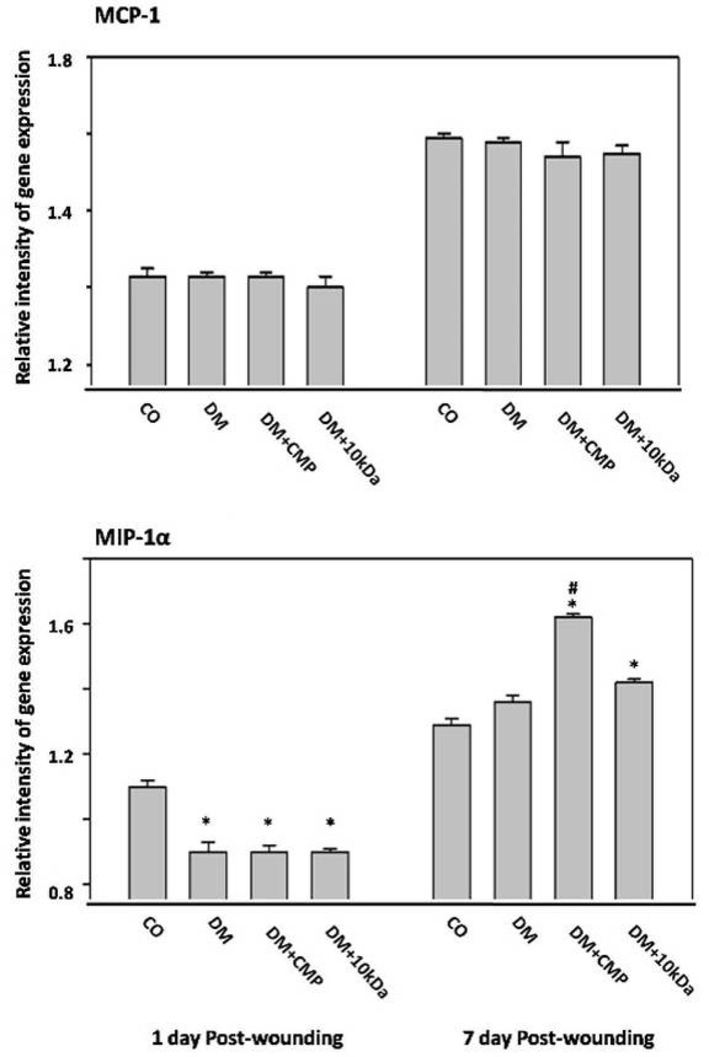
- The expression profiles of macrophage chemokines MCP-1 and MIP-1a by qRT-PCR Wound tissue is excised from days one through seven post-wounding. MCP-1 (no significant group differences were found from day one to day seven post-wounding) and MIP-1a gene expression were measured and compared to the housekeeping gene GAPDH using qRT–PCR. shown are means ± SD. *indicates a significant difference with the control group. # indicates a significant difference with the diabetic group.
Further, supernatants of homogenized tissues were furnished for immunodetection with a specific antibody against murine Mac-3 (Fig. 3). Macrophages needed to initiate an inflammatory phase required for tissue repair showed significant depletion in the DM group. Macrophage numbers significantly improved following the Treatment with CMP (DM + CMP group), even though macrophage protein was still considerably lower in the DM + CMP group as compared to the control group.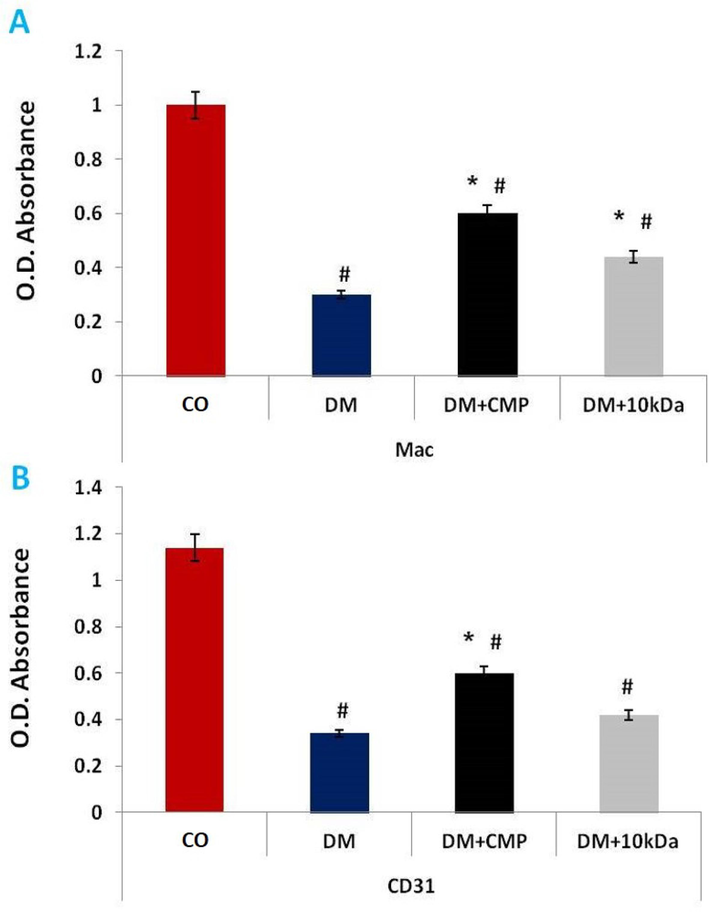
ELISA estimation of reactivity of anti-Mac-3 (A) and anti-CD31 (B) antibodies. The values shown are means ± SD. *indicates a significant difference with the control group. # denotes a statistically significant difference with the diabetic group.
CD31 is found on the surface of endothelial cells, platelets, macrophages and lymphocytes and shows an expression pattern similar to Mac-3 expression in diabetic animals with CMP treatment (Fig. 3).
CD68 expression on macrophages at the diabetic wound site
CMP enhanced macrophage recruitment in diabetic wounds was confirmed in paraffin sections from wounded skin to assess macrophage immunostaining with anti-CD68 antibody. CD68 is a phagocytic marker. Macrophage recruitment (anti-CD68 staining) was significantly less in DM rats compared to the control group (Fig. 4). CMP was found to reverse this decrease in the number of macrophages significantly. Interestingly, no significant group differences were found on days one through seven post-wounding (data not shown), while macrophage numbers increased in diabetic animals on day 20 of wounding.
-
Improved macrophage recruitment restored the number of bacteria
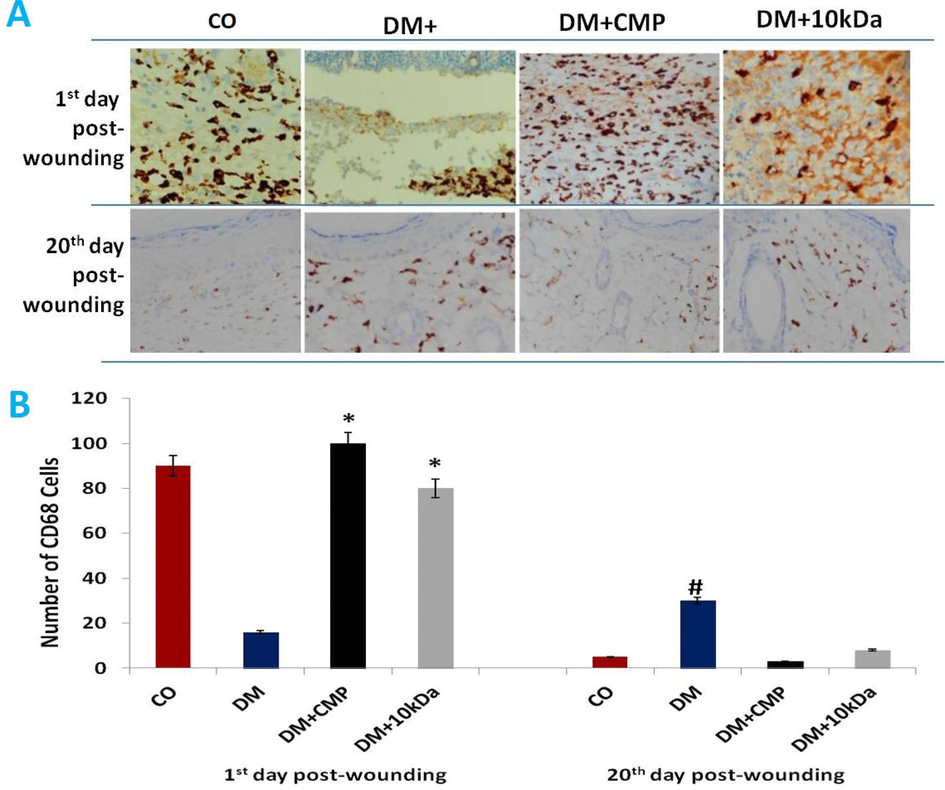
- A: Skin sections stained with anti-CD68 antibodies to show macrophage activity (brown-stained cells). Wound sections (5 µm) were immune-stained with a monoclonal antibody specific for macrophages (anti-CD68 antibody) and counterstained with hematoxylin (400X). B: Histograms depict the number of cells at the beginning and twenty days after wounding. shown are means ± SD. * indicates a significant difference with the diabetic group. # indicates a significant difference with the control group.
The efficacy of CMP in countering lower macrophage recruitment at wound sites was addressed by examining several pathogenic bacteria. The number of these bacteria was significantly higher (3-fold) in diabetic rats at wounds than in control animals (Fig. 5). Treatment with CMP significantly restored macrophage recruitment in the inflammatory phase, and the numbers of pathogenic bacteria returned to normal as defined by control group rats.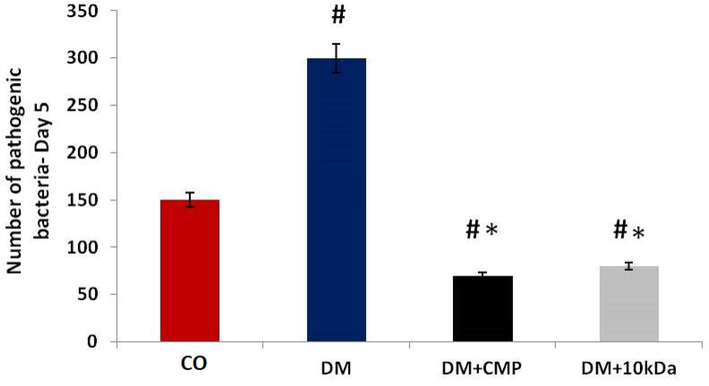
The number of pathogenic bacteria in wounded tissues from different rat groups. The bacterial count from the injured surface was measured by using a viable cell counting method. The values shown are means ± SD. * indicates a significant difference with the diabetic group, and # indicates a significant difference with the control group.
3.2 CMP restored the inflammatory markers at the diabetic wound site
-
TNF-α
The concentration of TNF-α significantly increased in DM group rats as compared to the control group. In CMP-treated diabetic group (DM + CMP) animals, levels of TNF-α were restored to near normal as defined by control animals. TNF- levels in CMP-treated animals remained slightly higher than in control animals, though there was no statistically significant difference (Fig. 6).
MMP-9 and MMP-13
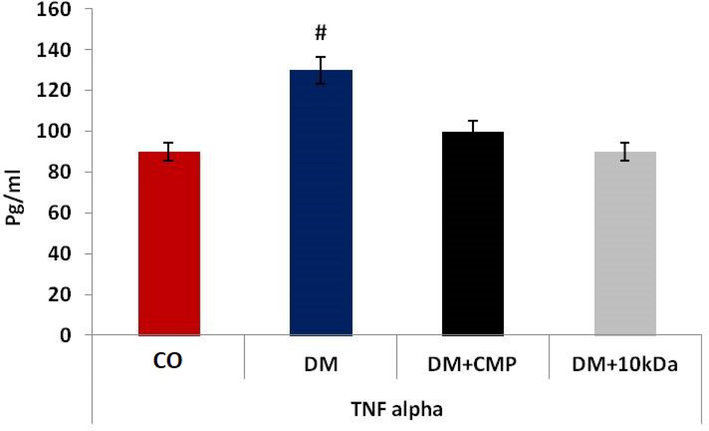
- Inflammatory mediators during wound healing. ELISA estimation of TNF-α. The values shown are means ± SD. * indicates a significant difference with the control group, and # indicates a significant difference with the diabetic group.
CMP significantly reversed the downregulation of MMP-9, although it remained significantly higher than in control rats. However, there was no any prominent differences in expression among groups on 7th day of wounding (Fig. 7).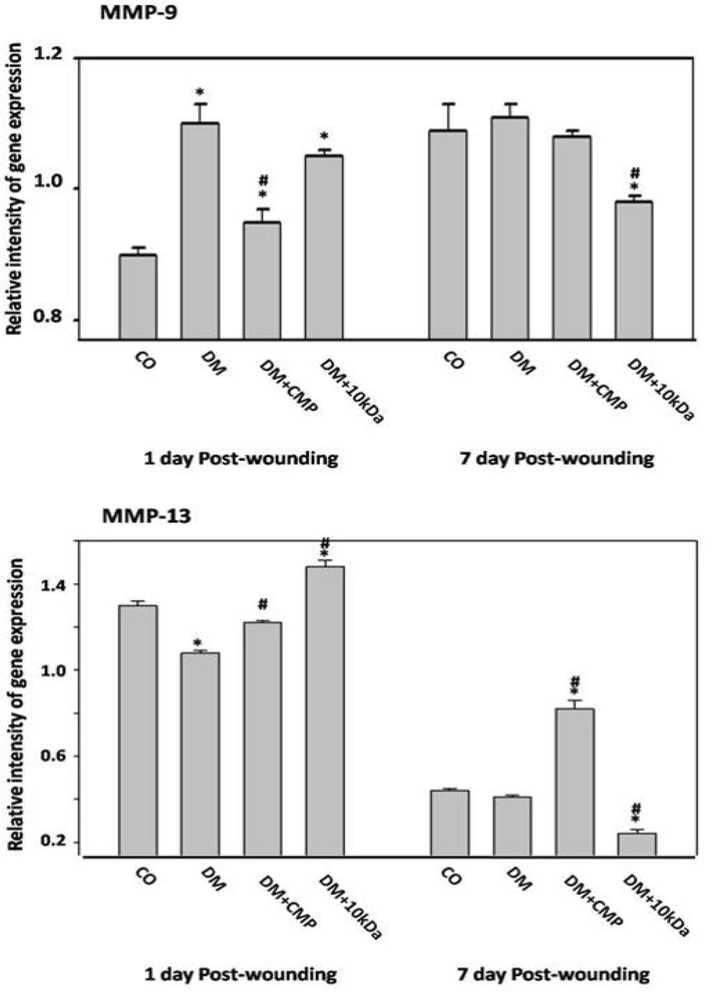
Inflammatory mediators during wound healing. Expression profiles of MMP-9 and MMP-13 by qRT–PCR. Gene expression of MMP-9 and MMP-13 in excised wound tissue on days 1 through day 7 post-wounding were measured and compared to the housekeeping gene GAPDH using qRT–PCR. The values shown are means ± SD. * indicates a significant difference. # indicates a significant difference.
Reduced gene expression of MMP-13 in comparison to control rats was observed in DM animals (Fig. 7). Treatment of diabetic rats with CMP significantly reversed this downregulation. Interestingly, Treatment of diabetic rats with CMP was found to considerably upregulate gene expression of MMP-13 in comparison to control rats on day seven post-wounding. This result runs parallel to the increment of macrophage (MIP-1α expression) recruitment at day seven post-wounding in the diabetic rats treated with CMP (Fig. 2).
-
CMP effect on macrophages in the remodeling phase (M2 activity)
-
IL-10 and TGF-β
We estimated IL-10 by ELISA to assess the role of macrophage M2 in remodeling. CMP significantly increased the level of IL-10 one day after wounding compared to the control rats. However, by the beginning of final remodeling, day seven post-wounding, levels of IL-10 also decreased (Fig. 8). Treatment of diabetic rats with CMP restored IL-10 to levels observed in control rats. Macrophages failed to tranform from the inflammatory to the proliferative phase. This failure may lead to a state of chronic, unresolved inflammation in the injured tissue in diabetic animals. No significant alteration in TGF- levels was observed on day 1 and 20 post-wounding (Fig. 8).
Gene expression of FGF and VEGF, and the tissue granulation
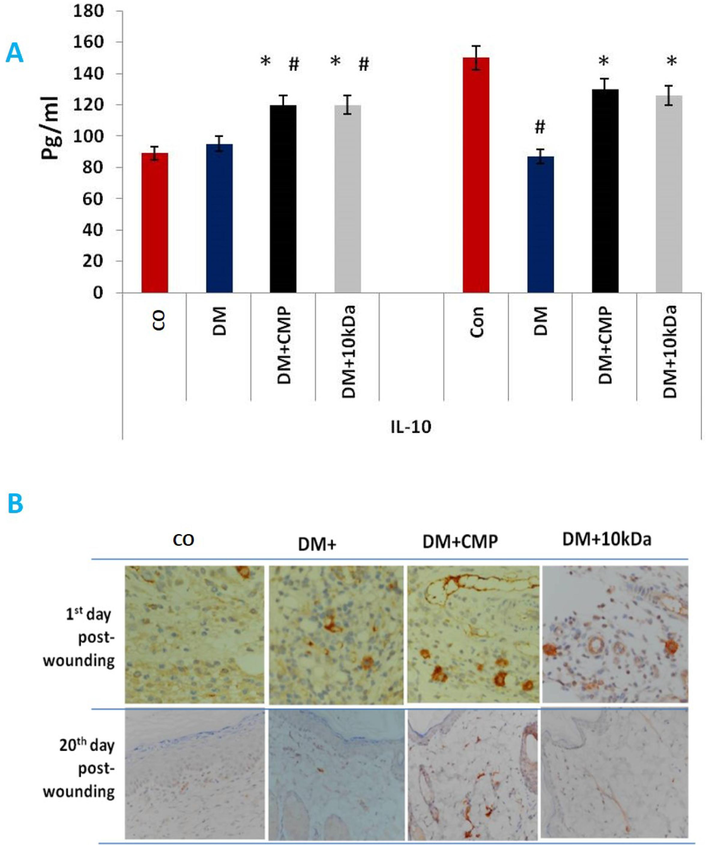
- Anti-inflammatory cytokines released by M2 macrophages during the remodeling phase of wound healing. IL-10 (A) protein was estimated by ELISA. B: Anti-TGF-antibodies stained skin sections to show macrophage activity. An anti-TGF- antibody was used to stain wound sections (5 m) and hematoxylin was used to counterstain them (400X).. The values shown are mean ± SD. * indicates a significant difference with the diabetic group. # indicates a significant difference with the control group.
On day one (inflammatory phase), no significant changes in FGF gene expression among rat groups were found. As with FGF, with the proliferation period of wound repair, rats treated with CMP showed more prominent upregulation of FGF expression than observed in diabetic rat groups (Fig. 9).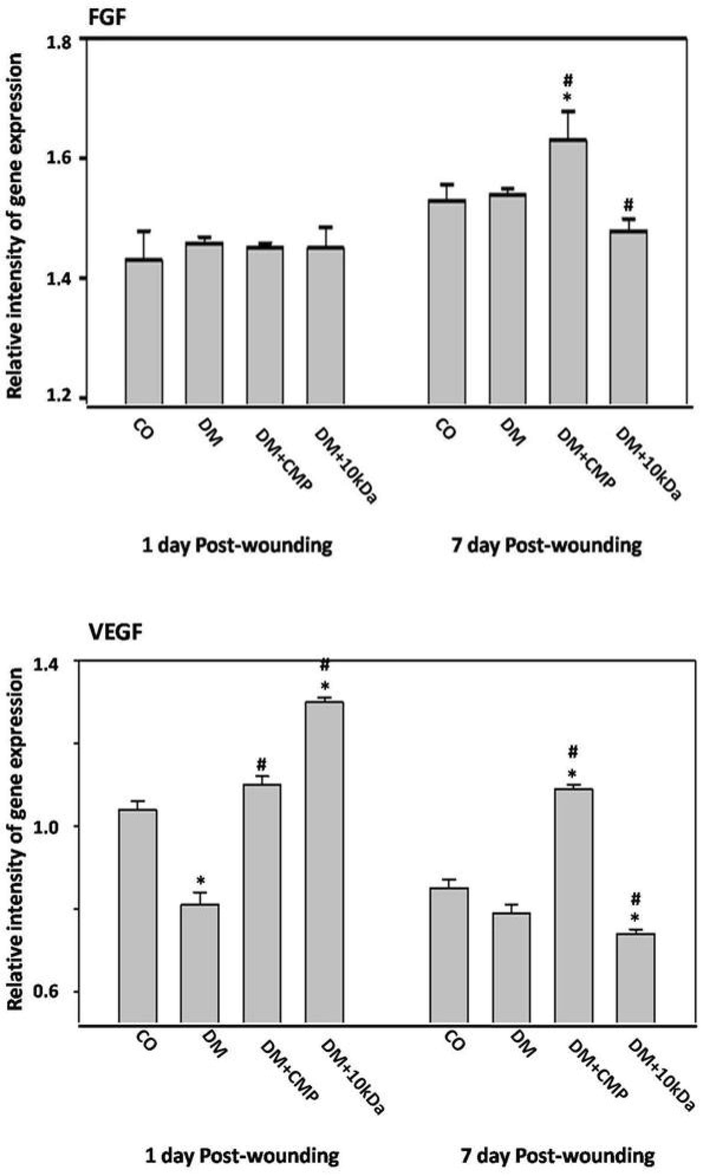
The expression profiles of macrophage promotion of the remodeling phase; fibroblast growth factor (FGF), and the vascular endothelial growth factor (VEGF) by qRT–PCR. Tissue is excised from wounds on days one through seven post-wounding. FGF and VEGF gene expression were measured and compared to the housekeeping gene GAPDH using qRT–PCR. The data represents mean ± SEM. * indicates a significant difference from the control group. # indicates a significant difference with the diabetic group.
Diabetes significantly impaired VEGF gene expression in diabetic rats at both the inflammatory and proliferation phases compared to control rats. CMP substantially upregulated VEGF gene expression compared to diabetic and control animals (Fig. 9).
Impairment of macrophage recruitment in the proliferation phase in diabetic rats suppressed critical growth factors needed for tissue granulation and wound contraction. Thus, examination of wounded skin tissue revealed a delay in collagen deposition (non-developed light fibers; Fig. 8), blood vessel formation, and an abundance of fibroblasts in the dermal tissue of the diabetic rats. However, Treatment with CMP enhanced tissue granulation compared to the non-treated diabetic rats (Fig. 9).
Wound healing process is summarized in Fig. 10. Prolonged diabetic recovery is due to expression of inflammatory cytokines, and macrophages do not transition from pro-inflammatory to reparative anti-inflammatory phenotypes. CMP enhanced phagocytosis by neutrophils and wound pathogens by macrophages. The phagocytic process mediates macrophage transition and produces growth factors in the proliferation phase. CMP also supports antioxidant glutathione production and provides different amino acids for collagen formation.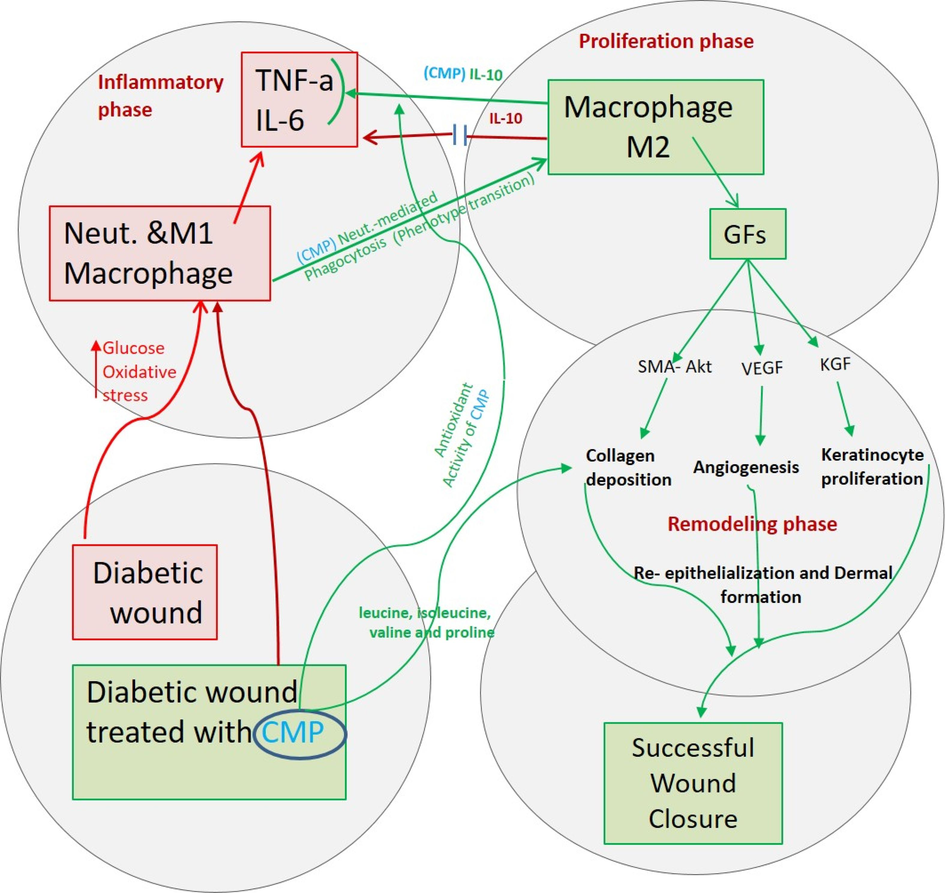
A diagrammatic figure summarizes prolonged diabetic wound healing (red lines) due to the secretion of inflammatory cytokines and that macrophages cannot transition from a pro-inflammatory to a reparative anti-inflammatory phenotype. In contrast, CMP enhances the phagocytosis of neutrophils and wound pathogens by macrophages. Phagocytosis mediates macrophage transition to the anti-inflammatory phenotype (green lines), which produces growth factors needed for the proliferation phase of healing. CMP supports antioxidant glutathione production and provides different amino acids required in collagen formation (blue lines). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4 Discussion
Recently, we reported that WP enhanced wound closure by alleviating the chronic inflammation, depletion of oxidative stress and elevation of the redox status in diabetic rats (Ebaid et al., 2013). We treated diabetic animals with wounds with a selected peptide enzymatic hydrolysate of camel whey proteins. This peptide improved wound healing by helping regulate redox status and immune response (Ebaid et al., 2015). We found that delayed wounds remarkably increased the toxicity with the expression of CD80 and CD86 on polymorphonuclear neutrophils. CMP peptide downregulated CD80 and CD86, and chemoattractants in diabetic animals (Ebaid et al., 2020). Here, we have interest to reveal on the role of CMP (3 kDa: CMP, camel milk peptide) on the recruitment of macrophages associated with diabetic wound healing. Also, the wound healing showed visible signs on 14th day while most of the wounds were entirely closed by 22 days.
Macrophages are essential for wound healing, especially during the proliferative phase. At this point, they are activated to produce TGF-β1, leading to improved angiogenesis and epithelialization through stimulation of keratinocytes (Douglas, 2010). Hence, depletion in levels of macrophages results in impaired wound healing (Mirza et al., 2009). The first cells that migrate to wound sites are the neutrophils, and these cells remove microorganisms and are, in turn, consumed by macrophages. This process starts the inflammation of the wound healing stage (Mishra et al., 2008, Okizaki et al., 2015). Both macrophages (Okizaki et al., 2015) and neutrophils (Ebaid, 2014, Fouda et al., 2016) are significantly reduced in diabetic mice. The current study reveals the similar pattern of results, where levels of macrophages in the DM group were notably decrease as compared to the control. The numbers of macrophages were elevated considerably by CMP treatment of DM group animals. CMP likely enhances wound healing by increasing macrophage recruitment and promoting appropriate immune responses.
Monocytes are attracted to wound sites, where they transform into inflammatory macrophages. Lack of sufficient numbers of macrophages early after wounding may cause severe hemorrhage, chronic wound closure, and delayed tissue maturation (Okizaki et al., 2015).
Macrophages were significantly deficient in the DM group rats at first, but their numbers increased on day five after wounding (Fig. 4). In DM, macrophages generally remain in an inflammatory phenotype, leading to tissue injury that does not normally resolve (Boniakowski et al., 2017). Pro-inflammatory macrophages (M1) persist in cases of prolonged healing without transforming to anti-inflammatory phenotypes (M2) (Fig. 10). This lack of transition is assumed for the disturbed tissue repair (Krzyszczyk et al., 2018). The pathologic phenotype is thus characterized by stalling of healing before the resolution phase of tissue repair.
Additionally, residual iron in the wound surface gathers during the local inflammatory macrophages and promotes TNF- production, preventing phagocytosis-mediated conversion of inflammatory macrophages to reparative phenotypes (Voll et al., 1997). We found that TNF-α levels increased significantly in DM rats compared to control animals. TNF-α inhibited the normal transition of pro-inflammatory macrophages to anti-inflammatory phenotypes in diabetic rats.
Blocking the inflammatory mediators is necessary to promote the healing process. CMP treatment reduced levels of TNF-α in diabetic rats to normal levels in the control animals. Blocking TNF-α and other inflammatory mediators can shift toward anti-inflammatory phenotypes. (Voll et al., 1997) suggest that activated macrophages inhibit TNF-expression and increase the release of anti-inflammatory cytokines in the presence of apoptotic cells. These findings entail the general secretion trend of GFs, such as VEGF, KGF, and FGF, required for the proliferation on day seven post-wounding in the CMP-treated diabetic animals.
Downregulation of CD-31 is an essential marker of diabetic endothelial cell dysfunction and endothelial cell death (Thomas et al., 2008). In rats, the microvascular density of blood vessels containing cells immune-stained against CD31 was low compared with a control group (Zhang et al., 2013). We obtained similar results for diabetic animals in which a low level of CD31 was seen compared to a non-diabetic control group. This decrease was reversed in CMP-treated diabetic rats, indicating that CMP may restore normal endothelial cells, leading to improved angiogenesis and wound healing. These beneficial effects of CMP may promote the transition of M1 to anti-inflammatory macrophages in diabetic wounds.
Usually, the macrophages during wound repair display enhanced expression of a surface tyrosine kinase involved in the phagocytosis of the cells undergoing programmed cell death. These molecules can produce VEGF and TGF-β (Crane et al., 2014). Phagocytosis of apoptotic cells augments other tissue repair processes. The activated macrophages with apoptotic cells increased the release of anti-inflammatory cytokines (Voll et al., 1997). These findings are in accord with our study in which expression of IL-10 was upregulated in normal and CMP-treated diabetic wounds seven days post-wounding. IL-10 enhances the downregulation of pro-inflammatory cytokines (Moore et al., 2001). Expression of IL-10 in diabetic group animals was higher compared to normal controls as an attempt to down-regulate elevated pro-inflammatory cytokines. Similarly, (Ramalho et al., 2018) found that in people with diabetes, systemic levels of IL-10 are upregulated. In the present study, IL-10 was also high in the inflammatory phase of wounds in diabetic animals. However, high levels of IL-10 might decrease macrophage infiltration, consequently delaying healing (Kimura et al., 2013). This mechanism may underlie the inhibition of macrophage recruitment in diabetic wounds. Besides, IL-10 knock-down mice exhibit an accelerated rate of macrophage infiltration followed by enhanced healing (Eming et al., 2007). The present study showed that CMP down-regulated IL-10 in diabetic animals to a normal level and retained typical macrophage infiltration. Overall, the effect of CMP was to promote normal healing. At the beginning of the final remodeling phase, levels of IL-10 declined in diabetic wounds, perhaps due to the continuation of inflammatory responses by macrophages.
During remodeling, macrophages elicit matrix metalloproteinases (MMPs) for degradation of the provisional extracellular matrix leading to apoptosis, allowing the skin to restore its health devoid of any sign of wound (Vannella and Wynn, 2017). This process may explain the upregulation of MMP-13 e expression by CMP treatment of diabetic rats on day seven post-wounding. This result parallels macrophage (MIP-1a expression) increment at day seven post-wounding in diabetic rats treated with CMP (Fig. 2b). Still, treatment of diabetic rats with CMP significantly improved MMP-13 expression compared to untreated diabetic rats.
Recently, our results provided evidence that WP can reverse the autoimmunity factor in DM1 by downregulation of autoreactive T cells and TNF-α and Fas. Consequently, it results in enhanced functionality of pancreatic ß cell (Ebaid, 2014). Several studies have found that WP decreased glycemia and improved insulin response (Lacroix et al., 2014). Mouse pancreatic islets incubated with whey increased insulin secretion (Salehi et al., 2012). WP is rich in isoleucine, leucine and valine, which along with its bioactive peptides may indirectly affect the glycemic response (Pal et al., 2013). It is most likely that CMP may be rich in amino acids activating insulin secretion.
5 Conclusion
Our results confirm that macrophages perform a main role in both the inflammatory and remodeling phases of wound repair in diabetes (Fig. 10). Hence, delayed macrophage transition to anti-inflammatory phenotypes in diabetes affects: 1) secretion of anti-inflammatory cytokines and chemokines; 2) initiation of angiogenesis; 3) proliferation and migration of keratinocytes; and 4) formation of collagen fibers. Further, CMP enhanced wound closure by supporting macrophage transition to M2, limiting prolonged inflammation, and producing growth factors needed for normal proliferation during wound healing. CMP also encouraged the restoration of neovascularization and keratinocyte migration. These data may facilitate scathing insights for futuristic dierty intervention for enhancement of wound healing in diabetes. Poor healing appears to be a multifactorial process. Research has not yet wholly elucidated the pathogenesis of impaired wound healing in diabetes, and failure to transition from the inflammatory to remodeling phases may be one underlying cause. Pathways programing the macrophage phenotypes in tissue can dictate the therapeutic strategies in the future. Activating macrophage transition to anti-inflammatory phenotypes may be clinically helpful in avoiding wound healing complications in diabetic patients.
Acknowledgments
This work was supported by Researchers Supporting Project number (RSP2024R366), King Saud University, Riyadh, Saudi Arabia.
Availability of data and materials
All data of this study are included in this published article.
Financial disclosure
This work was supported by Researchers Supporting Project number (RSP2024R366), King Saud University, Riyadh, Saudi Arabia.
Ethical approval
The Animal Ethics Committee of KSU approved the study protocol (KSU-SE-20-40), Riyadh.
Consent for publication
The authors generated all the data from our experiments conducted in the laboratory. We have all consented to the publication the data presented in this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym.. 2014;100:166-178.
- [Google Scholar]
- Enhancement of wound healing by un-denatured camel whey proteins in protein malnourished mice. Pakistan J. Zool.. 2016;48
- [Google Scholar]
- Up-regulation of Hsp72 and keratin16 mediates wound healing in streptozotocin diabetic rats. Biol. Res.. 2015;48(1)
- [Google Scholar]
- Modulation of immune cell proliferation and chemotaxis towards CC chemokine ligand (CCL)-21 and CXC chemokine ligand (CXCL)-12 in undenatured whey protein-treated mice. J. Nutr. Biochem.. 2012;23(12):1640-1646.
- [Google Scholar]
- Macrophage-mediated inflammation in normal and diabetic wound healing. J. Immunol.. 2017;199:17-24.
- [Google Scholar]
- The monocyte to macrophage transition in the murine sterile wound. PLoS One. 2014;9(1)
- [Google Scholar]
- Neutrophil depletion in the early inflammatory phase delayed cutaneous wound healing in older rats: improvements due to the use of un-denatured camel whey protein. Diagn. Pathol.. 2014;9:1-12.
- [Google Scholar]
- Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis.. 2011;10(1):235.
- [Google Scholar]
- Bioactivity of Samsum ant (Pachycondyla sennaarensis) venom against lipopolysaccharides through antioxidant and upregulation of Akt1 signaling in rats. Lipids Health Dis.. 2012;11(1):93.
- [Google Scholar]
- Limiting prolonged inflammation during proliferation and remodeling phases of wound healing in streptozotocin-induced diabetic rats supplemented with camel undenatured whey protein. BMC Immunol.. 2013;14:1-13.
- [Google Scholar]
- Camel milk peptide improves wound healing in diabetic rats by orchestrating the redox status and immune response. Lipids Health Dis.. 2015;14(1)
- [Google Scholar]
- Accelerated wound closure in mice deficient for interleukin-10. Am. J. Pathol.. 2007;170(1):188-202.
- [Google Scholar]
- Wound healing of different molecular weight of hyaluronan; in-vivo study. Int. J. Biol. Macromol.. 2016;89:582-591.
- [Google Scholar]
- Epigenetic Control of Macrophage Polarisation and Soluble Mediator Gene Expression during Inflammation. Mediators Inflamm.. 2016;2016:1-15.
- [Google Scholar]
- Delayed wound healing due to increased interleukin-10 expression in mice with lymphatic dysfunction. J. Leukocyte Biol.. 2013;94:137-145.
- [Google Scholar]
- Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med.. 2011;13
- [Google Scholar]
- The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol.. 2018;9:419.
- [Google Scholar]
- M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Curr. Pharm. Des.. 2018;24(20):2241-2249.
- [Google Scholar]
- Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol.. 2009;175(6):2454-2462.
- [Google Scholar]
- Diabetic delayed wound healing and the role of silver nanoparticles. Dig J Nanomater Bios.. 2008;3:49-54.
- [Google Scholar]
- Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol.. 2001;19(1):683-765.
- [Google Scholar]
- Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol.. 1991;3(1):65-70.
- [Google Scholar]
- Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed. Pharmacother.. 2015;70:317-325.
- [Google Scholar]
- Impaired wound healing in type 1 diabetes is dependent on 5-lipoxygenase products. Sci. Rep.. 2018;8(1)
- [Google Scholar]
- Novel KIR3DL1 alleles and their expression levels on NK cells: convergent evolution of KIR3DL1 phenotype variation? J. Immunol.. 2008;180:6743-6750.
- [Google Scholar]
- Improved repair of dermal wounds in mice lacking micro RNA-155. J. Cell Mol. Med.. 2014;18:1104-1112.
- [Google Scholar]
- Mechanisms of organ injury and repair by macrophages. Annu. Rev. Physiol.. 2017;79(1):593-617.
- [Google Scholar]
- Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J. Cell. Physiol.. 2019;234(4):4217-4231.
- [Google Scholar]
- The antidiabetic drug metformin inhibits the proliferation of bladder cancer cells in vitro and in vivo. Int. J. Mol. Sci.. 2013;14(12):24603-24618.
- [Google Scholar]







