Translate this page into:
Sinensetin mitigates polystyrene nanoplastics induced hepatotoxicity in albino rats: A biochemical and histopathological study
⁎Corresponding author. asmaashraf@gcuf.edu.pk (Asma Ashraf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polystyrene nanoplastics (PS-NPs) are environmental pollutants that induce oxidative stress (OS) in multiple organs particularly, liver. Sinensetin (SNS) is a naturally present flavones that shows diverse pharmaceutical properties i.e., anti-oxidant, anti-inflammatory and anti-apoptotic. Therefore, the present study was designed to evaluate the therapeutic role of SNS against PS-NPs induced hepatotoxicity. 48 rats were distributed into 4 groups i.e., control, PS-NPs (50 µgkg−1) treated, PS-NPs + SNS (50 µgkg−1 + 20 mgkg−1) co-treated and only SNS (20 mgkg−1) treated group. PS-NPs intoxication reduced the activities of catalase (CAT), glutathione-S-transferase (GST), superoxide dismutase (SOD), glutathione peroxidases (GPx) glutathione reductase (GSR) and glutathione (GSH) level, whereas increased the levels of ROS and MDA. Additionally, PS-NPs increased the levels of liver serum marker enzymes i.e., alanine transaminase (ALT), alkaline phosphatase (ALP) and aspartate aminotransferase (AST). Moreover, the level of inflammatory makers such as nuclear factor-kappa B (NF-κB), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1 beta (IL-1β) and cyclooxygenase-2 (COX-2) activity were increased following the PS-NPS exposure. The intoxication of PS-NPs elevated Caspase-3, Bax and Caspase-9 levels, while reducing the Bcl-2 level. Furthermore, the exposure of PS-NPs induced significant histopathological damages in hepatic tissue of rats. However, the supplementation of SNS considerably improved the PS-NPs induced damages as well as histological changes due to its hepatoprotective, anti-inflammatory, anti-apoptotic and anti-oxidant nature.
Keywords
Polystyrene nanoplastics
Sinensetin
Hepatic damage
Oxidative stress
Apoptosis
1 Introduction
Plastic is a potential environmental pollutant and a critical problem of the 21st century due to its tendency to persist in the environment for a long time span. The annual plastic production has been raised up to 400 million tons throughout the world (Gu et al., 2020; Ahmad et al., 2023). Plastics materials are converted into smaller plastics of 1–100 nm, named as “nanoplastics” (NPs) by UV radiation, mechanical abrasion, hydrolysis, and biodegradation (Gigault et al., 2018). NPs have been reported in multiple food products i.e., tea bags, honey, milk, drinking water and table salt (Prata et al., 2020). Human are exposed to NPs through ingestion, inhalation as well as dermal contact (Yong et al., 2020). Due to small size, NPs can penetrate into the cell membrane and decreases the activities of anti-oxidant enzymes. It is reported that NPs have the ability to cause genotoxicity, reproductive toxicity, neurotoxicity as well as inflammatory responses (Sharma et al., 2021).
Multiple kind of plastics are reported in environment such as polystyrene (PS), polyvinyl chloride (PVC) and polyethylene (PE) (Andrady, 2011). One of the most common form of plastics is PS (Gelbke et al., 2019). PS is mainly used for the manufacturing of various type of products i.e. bio imaging, personal care products, disposable cups, plates, cutleries, automobile tires and synthetic textiles (Zheng et al., 2019). PS-NPs are the environmental toxicants that are reported in various pharmaceutical products, cosmetics, biomedical and electronic materials (Koelmans et al., 2015). Exposure to PS-NPs causes OS, apoptosis and inflammation, which leads to renal, brain, heart and lungs damages (Sarasamma et al., 2020; Jung et al., 2020). PS-NPs can enter into body of rats and may accumulate in liver, spleen and lymph node (Pitt et al., 2018). PS-NPs have ability to damage the hepatic tissue and also disturb hepatic metabolism as well as induce OS in liver that results in increased levels of liver serum markers i.e., AST, ALT and ALP (Bouteraa et al., 2020). According to a previous study the exposure of PS-NPs also induces histopathological damages in liver of rats due to inflammation and OS (Pitt et al., 2018).
Flavonoids have received a lot of attention due to their anti-oxidants, anti-allergic, anti-inflammatory and anti-bacterial activities (Saraei et al., 2019). Sinensetin (SNS) is a flavone that is present in Orthosiphon aristatus and various citrus fruits. It is reported to display strong therapeutic properties i.e., anti-inflammatory and anti-oxidant (Nasri et al., 2017). Therefore, the present study was planned to evaluate the mitigative role of SNS against PS-NPs induced hepatotoxicity in rats.
2 Material and methods
2.1 Chemicals
PS-NPs and SNS were acquired from Sigma-Aldrich (Germany).
2.2 Animals
Fourty eight albino rats having weight 180–220 g (6–8 weeks old) were used and kept in the animal house of University of Agriculture Faisalabad. 12 h light/dark cycle, standard temperature (25 ± 2 °C) and humidity (45 ± 5 %) were maintained. Moreover, commercial food and water was provided to the rats. All the rats were handled in compliance with the protocols of European Union of Animal Care and Experimentation (CEE Council 86/609).
2.3 Experimental design
48 rats were allocated into 4 groups (n = 12). First group was designed as control group. The second group was exposed to PS-NPs (50 µgkg−1). Third group was co-treated with PS-NPs + SNS (50 µgkg−1 + 20 mgkg−1). Fourth group was treated with only SNS (20 mgkg−1). All the doses were given orally on daily bases through oral gavage. The experiment was conducted for thirty days. On the last day, the rats were sedated by using 6 mgkg−1 of xylazine and 60 mgkg−1 of ketamine, decapitated and blood was stored in sterile containers for further assessment. Liver was excised and cut into 2 equal parts; one part was kept in zipper bag at −80 °C for further assessment. While second part was fixed in formalin (10 %) for the histological examination.
2.4 Assessment of antioxidants
CAT activity was evaluated by the procedure outlined by Chance and Maehly (1955). SOD activity was appraised by using the technique of Nishikimi et al., (1972). GST activity was measured according to the method of Couri and Abdel-Rahman (1979). To estimate GSR activity the method of Carlberg and Mannervik (1975) technique was followed. The protocol outlined by Sedlak and Lindsay (1968) was used to appraise the level of GSH. While Lawrence and Burk (1976) method was followed to examine GPx activity.
2.5 Assessment of oxidative stress markers
The level of ROS was appraised by using the method of Hayashi et al., (2007). For measuring MDA content, Ohkawa et al. (1979) approach was followed.
2.6 Assessment of liver function enzymes
The levels of liver function enzymes i.e., AST (ab263883), ALP (ab287823) and ALT (ab285264) were assessed by using ELISA kits (MA, Abcam, USA), according to the manufacturer’s instructions.
2.7 Inflammatory markers analysis
The levels of IL-1ß (CSB-E08055r), NF-κB (CSB-E13148r) IL-6 (CSB-E04640r), TNF-α (CSB-E07379r) and COX-2 activity (CSB-E13399r) were evaluated by using the rat ELISA kits. The analysis was performed by using Elisa plate reader in accordance with the company's instructions (Bio-Tek, Winooski, VT, USA).
2.8 Apoptotic markers analysis
The levels of Bax (CSB-EL002573RA), Bcl-2 (CSB-E08854r), Caspase-3 (CSB-E08857r) and Caspase-9 (CSB-E08863r) were evaluated via ELISA kits (Cusabio Technology Llc, Houston, TX, USA) in compliance with the manufacturer’s instructions.
2.9 Histopathological examination
Liver tissues were fixed in 10 % formaldehyde solution, dehydrated using the increasing concentrations of alcohol and encased in the paraffin. 4–5 μm thick sections were cut with the help of a microtome and staining was performed by using Hematoxylin-Eosin. Lastly, a compound microscope (Nikon, Japan), connected with a micro-photographic system was used to examine the slides.
2.10 Statistical analysis
Data were displayed as Mean ± SEM. The whole data were examined using one way ANOVA and Tukey's test. Significance level was set at P < 0.05.
3 Results
3.1 Protective effect of SNS on antioxidant profile
PS-NPs intoxication induced a significant (p < 0.05) reduction in the activities of anti-oxidant enzymes i.e., CAT, SOD, GPx, GST, GSR and GSH level in PS-NPs administered animals in comparison to the control animals. Nevertheless, SNS supplementation with PS-NPs significantly increased the anti-oxidants activities as compared to PS-NPs exposed animals. Moreover, no remarkable difference was noted in the anti-oxidants activities between only SNS supplemented and control groups (Table 1). Values are shown on the basis of Mean ± SEM. The values with different superscripts are significantly different from other groups.
Parameters
Groups
Control
PS-NPs
PS-NPs + SNS
SNS
CAT (Umg−1 protein)
9.4 ± 0.22a
4.8 ± 0.12c
7.9 ± 0.13b
9.5 ± 0.23a
SOD (Umg−1 protein)
8.37 ± 0.28a
4.09 ± 0.22c
6.48 ± 0.12b
8.44 ± 0.29a
GPx (Umg−1 protein)
19.54 ± 0.73a
8.05 ± 0.56c
14.16 ± 0.67b
19.89 ± 0.84a
GSR (nM NADPH oxidized/min/mg tissue)
7.16 ± 0.21a
2.26 ± 0.19c
5.27 ± 0.31b
7.19 ± 0.23a
GST (nM/min/mg protein)
27.39 ± 1.06a
11.42 ± 0.82c
21.60 ± 0.74b
27.64 ± 1.18a
GSH (μM/g tissue)
16.04 ± 1.03a
6.57 ± 0.16c
11.51 ± 0.54b
16.09 ± 1.16a
ROS (Umg−1 tissue)
1.24 ± 0.60a
6.85 ± 0.16c
2.05 ± 0.08b
1.19 ± 0.08a
MDA (nmol/mg protein)
0.60 ± 0.12a
3.14 ± 0.04c
1.73 ± 0.07b
0.63 ± 0.13a
3.2 Protective effect of SNS on oxidative stress markers
PS-NPs intoxication significantly (p < 0.05) increased the level of ROS and MDA in PS-NPs exposed group as compared to the control group. Nevertheless, the supplementation of SNS in co-administrated group (SNS + PS-NPs) significantly reduced the level of MDA and ROS in comparison to PS-NPs exposed group. Furthermore, ROS and MDA levels in SNS only supplemented and control animals were comparable (Table 1).
3.3 Protective effect of SNS on liver function enzymes
The intoxication of PS-NPs significantly (p < 0.05) increased the levels of ALT, ALP and AST as compared to control animals. Nevertheless, the supplementation of SNS in co-administrated group significantly lowered the levels of these enzymes in contrast to PS-NPs exposed animals. Furthermore, only SNS supplemented group exhibited the level of these markers close to control animals (Table 2). Values are shown on the basis of Mean ± SEM. The values with different superscripts are significantly different from other groups.
Parameters
Groups
Control
PS-NPs
PS-NPs + SNS
SNS
ALT (U/L)
42.98 ± 1.69a
84.99 ± 1.58c
53.92 ± 0.87b
42.80 ± 1.78a
AST (U/L)
74.22 ± 2.11a
175.52 ± 4.95c
96.94 ± 2.05b
73.45 ± 2.39a
ALP (U/L)
134.23 ± 5.33a
357.18 ± 6.16c
179.32 ± 5.27b
131.30 ± 3.58a
3.4 Protective effect of SNS on inflammatory indices
The levels of NF-κB, IL-1β, TNF-α, IL-6 and COX-2 activity were significantly (p < 0.05) increased following the PS-NPs administration as compared to the control group. Nevertheless, SNS supplementation reduced the level of above-mentioned markers in SNS + PS-NPs supplemented animals in comparison to PS-NPs exposed animals. However, in only SNS treated animals these levels were near to control animals (Table 3). Values are shown on the basis of Mean ± SEM. The values with different superscripts are significantly different from other groups.
Parameters
Groups
Control
PS-NPs
PS-NPs + SNS
SNS
NF-κB (ngg−1 tissue)
14.66 ± 0.68a
65.59 ± 2.08c
23.72 ± 1.31b
14.44 ± 0.58a
TNF-α (ngg−1 tissue)
8.21 ± 0.28a
27.97 ± 1.42c
12.21 ± 0.93b
8.16 ± 0.29a
IL-1β (ngg−1 tissue)
25.89 ± 0.79a
83.13 ± 0.87c
35.49 ± 0.93b
25.45 ± 0.85a
IL-6 (ngg−1 tissue)
7.16 ± 0.34a
26.45 ± 0.49c
12.51 ± 0.96b
7.10 ± 0.37a
COX-2 (ngg−1 tissue)
22.26 ± 1.08a
74.63 ± 1.44c
33.19 ± 1.15b
21.99 ± 0.99a
3.5 Protective effect of SNS on apoptotic markers
The exposure of PS-NPs significantly (p < 0.05) increased the levels of Caspase-3, Bax and Caspase-9, whereas reducing the Bcl-2 level in PS-NPs administered animals as compared to control animals. However, SNS treatment lowered (p < 0.05) the levels of Caspase-3, Bax and Caspase-9, while increasing the Bcl-2 level in co-administrated (PS-NPs + SNS) animals in contrast to PS-NPs administered animals. Moreover, no remarkable change was seen in the levels of apoptotic markers in only SNS supplemented and control animals (Table 4). Values are shown on the basis of Mean ± SEM. The values with different superscripts are significantly different from other groups.
Parameters
Groups
Control
PS-NPs
PS-NPs + SNS
SNS
Bcl-2 (ng/mg Protein)
17.29 ± 0.61a
3.22 ± 0.17c
12.00 ± 1.02b
17.38 ± 0.68a
Bax (ng/mg Protein)
1.54 ± 0.15a
8.20 ± 0.32c
2.84 ± 0.12b
1.51 ± 0.12a
Caspase-3 (pg/mL)
1.79 ± 0.09a
15.18 ± 0.84c
2.76 ± 0.11b
1.77 ± 0.09a
Caspase-9 (pg/mL)
2.56 ± 0.13a
14.43 ± 0.42c
3.60 ± 0.14b
2.51 ± 0.11a
3.6 Protective effect of SNS on the histology of hepatic tissues
The intoxication of PS-NPs induced significant (p < 0.05) damages in hepatic tissues i.e. disrupted central venules, detrimental alterations in hepatocytes nuclei and Kupffer cells as well as dilated sinusoids in comparison to control group. However, the co-treatments of PS-NPs and SNS significantly recovered these injuries in hepatic tissues as compared to PS-NPs exposed group. Moreover, the histological profile in only SNS supplemented group was similar to control group (Fig. 1).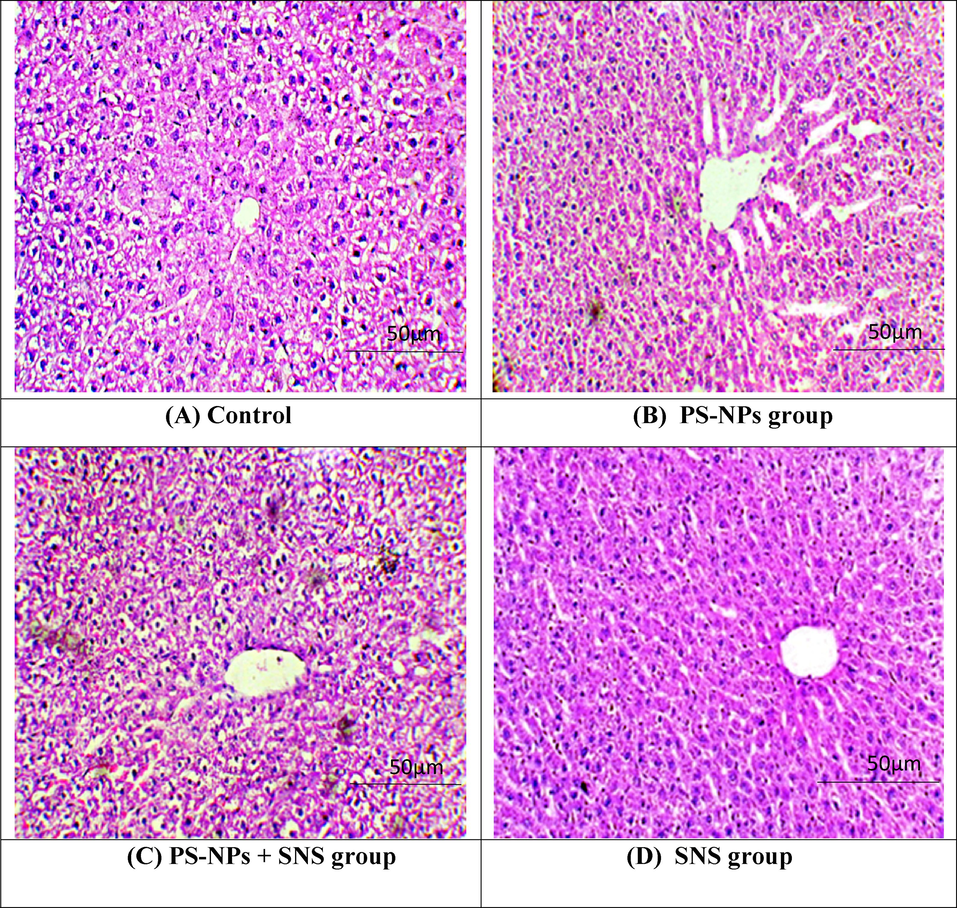
Photomicrographs of rat hepatic tissues. (A) Control group presenting normal histology. (B) PS-NPs intoxication prompted adverse alterations in liver i.e., unclear hepatocyte boundaries, congested central veins, degenerated hepatocytes, necrotic cells with nuclear dissolution and dilated sinusoid. (C) PS-NPs + SNS group displayed restored histology of liver tissues. (D) SNS group showing normal histology almost as in the control rats. PS-NPs: Polystyrene nanoplastics; SNS: Sinensetin; S: Sinusoids; CV: Central vein; KC: Kupffer cell; N: Nucleus; H: Hepatocytes.
4 Discussion
The present study was planned to evaluate the therapeutic role of SNS against PS-NPs induced hepatic damage in rats. The exposure to PS-NPs induces hepatotoxicity by inducing OS, lipid peroxidation (LP) as well as increasing the level of inflammatory markers (Mohamed et al., 2019). Additionally, PS-NPs exposure affects the structure of hepatic system as well as impairs liver parenchymal cells (Kazemi et al., 2016). PS-NP exposure to mammals occurs through inhalation and ingestion. Because PS-NPs are so prevalent in the environment, their exposure is almost inevitable. Moreover, it is reported that humans are exposed to PS-NPs via the aquatic food chain. Furthermore, PS-NPs have also been found in salt, water (Ossmann et al., 2018), air, beer and honey (Barria et al., 2020). SNS is an important naturally occurring flavonoid that is present in the leaves of Orthosiphon aristatus that possesses potential therapeutic effects i.e., anti-bacterial, anti-oxidant, hepato-protective and anti-inflammatory activities (Hu et al., 2021).
The exposure to PS-NPs significantly decreased the activities of anti-oxidants i.e., CAT, SOD, GPx, GST, GSR and GSH level, while increasing the levels of MDA and ROS. The endogenous-antioxidant-enzymes are considered as the first line of defense that safeguards the macromolecules (proteins, lipids, and DNA) by lowering ROS production. CAT facilitates the transformation of H2O2 into H2O and O2 (Safhi et al., 2016). SOD helps in the transformation of O2 into H2O2 (Stinghen et al., 2014). GSR converts glutathione disulfide into GSH that protects the cells from OS by decreasing H2O2 and other peroxides (Deponte, 2013). GST plays multiple functions, but generally helps in the process of detoxification (Allocati et al., 2018). OS is caused by an imbalance in the anti-oxidants and pro-oxidants that in turn causes hepatotoxicity (Ijaz et al., 2023). MDA, a marker of lipid peroxidation (LP), is overproduced when there is an increase in free radicals. LP is a reliable indicator of OS and antioxidant status (Alvi et al., 2022). However, our findings suggested that SNS-administration significantly reduced the levels of MDA and ROS by increasing the activities of anti-oxidative enzymes due to its anti-oxidative nature.
In the current investigation, PS-NPs intoxication induced a significant increase in the levels of ALT, AST and ALP, which showed severe hepatic injury. The measurement of the aforesaid liver function enzymes in the blood is one of the routinely used methods for analyzing the liver activity (Ulasoglu et al., 2019). Previous research has shown that OS disrupts the membrane integrity of hepatocytes that leads to the increased levels of hepatic function marker enzymes in the blood (Pratibha et al., 2006). However, SNS supplementation reduced the levels of these enzymes. The release of hepatic enzymes was thought to be prevented by the palliative effect of SNS on the hepatocytes’ membranes due to its hepato-protective property.
The intoxication of PS-NPs increased the levels of NF-κB, IL-6, TNF-α, IL-1β and COX-2 activity. NF-κB stimulation has a pivotal role in inflammatory cytokines expressions that indicates acute inflammation and other diseases associated with excessive ROS. NF-kB stimulation encourages the production of IL-1β, TNF-α, IL-6 via gene up-regulation that results in inflammation (Cai et al., 2022). COX-2 is also an inflammatory indicator that performs a pivotal role in inflammation (Ju et al., 2022). However, the supplementation of SNS decreased the levels of inflammatory markers that may be attributed to its anti-inflammatory nature.
PS-NPs exposure increased Bax, Caspase-3 and Caspase-9 levels, on the other hand decreased the Bcl-2 level. Bcl-2 and Bax are the members of Bcl-2 family (Santana, 2018). Apoptotic marker Bax triggers the cell death. Contrarily, Bcl-2 prevents the cells from apoptotic cell death (Opferman and Kothari, 2018). An increase in Bax level and a decrease in Bcl-2 induces the liberation of cytochrome C into cytoplasmic matrix that activates the Caspase-9 and eventually stimulates Caspase-3 that chops up cellular proteins; as a result, it induces change in cell structure and prompts apoptosis. (Chipuk and Green, 2009). However, SNS administration reduced the levels of Bax, Caspase-3 and Caspase-9, while increasing the Bcl-2 level, that may be due to its anti-apoptotic nature.
Our findings revealed that the administration of PS-NPs induced severe histopathological abnormalities in liver such as, disrupted central venules, detrimental alterations in hepatocytes nuclei and Kupffer cells as well as dilated sinusoids. These damages were triggered by PS-NPs via the production of excessive free radicals and LP. Free radicals have the potential to destroy macromolecules and cause oxidative damage, which ultimately disrupts cellular processes and results in the degeneration of hepatic tissues (Bouteraa et al., 2020). However, the supplementation of SNS significantly improved the histomorphological damages due to its hepatoprotective, anti-oxidant, anti-apoptotic and anti-inflammatory activities.
5 Conclusion
Our findings indicated that SNS demonstrated significant attenuative potential against PS-NPs-induced liver damage. SNS supplementation significantly recovered the levels of hepatic function enzymes, anti-oxidant enzymes activities, MDA and ROS levels, inflammatory indices, levels of apoptotic markers and histopathological damages due to its hepatoprotective, anti-oxidant, anti-apoptotic and anti-inflammatory nature. Taken together, the results of the current study indicated that SNS might be used as a curative agent to cure hepatic damage.
Acknowledgment
This work was funded by Researchers Supporting Project number (RSP2024R191), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
None.
References
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-induced Nephrotoxicity in Rats. Pak. Vet. J.. 2023;43(3):623-627.
- [Google Scholar]
- Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7:1-15.
- [Google Scholar]
- Nephroprotective Effects of Delphinidin against Bisphenol A Induced Kidney Damage in Rats. Pak. Vet. J.. 2022;43(1):189-193.
- [Google Scholar]
- Effect of nanoplastics on fish health and performance: a review. Mar. Pollut. Bull.. 2020;151:110791
- [Google Scholar]
- Cellular Apoptosis, Mitochondrial Swelling, Permeability and Cytochrome-C Level After (Feo)-Nps nanoparticles exposure and protective role of diferuloylmethane in rats liver. Acta Scientifica Naturalis.. 2020;7:140-154.
- [Google Scholar]
- Polyguanine alleviated autoimmune hepatitis through regulation of macrophage receptor with collagenous structure and TLR4-TRIF-NF-κB signalling. J. Cell. Mol. Med.. 2022;26:5690-5701.
- [Google Scholar]
- Carlberg INCER and Mannervik BENGT, 1975. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 250, 5475-5480.
- PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle. 2009;8:2692-2696.
- [Google Scholar]
- Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol.. 1979;3:451-460.
- [Google Scholar]
- Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. Gen. Sub.. 2013;1830:3217-3266.
- [Google Scholar]
- Gelbke, H.P., BantonM, Block, C., Dawkins, G., Eisert, R., Leibold, E., Pemberton, M., Puijk, I.M., Sakoda, A., Yasukawa, A., 2019. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem. Toxicol. 124, 151–167.
- Nanoplastics impair the intestinal health of the juvenile large yellow croaker Larimichthys crocea. J. Hazard. Mater.. 2020;397:122773
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res Genet Toxicol Environ Mutagen. 2007;631:55-61.
- [Google Scholar]
- The regulatory effects of citrus Peel powder on liver metabolites and gut flora in mice with non-alcoholic fatty liver disease (NAFLD) Foods. 2021;10:3022.
- [Google Scholar]
- Tectochrysin Attenuates Cisplatin-induced Hepatotoxicity by Restoring Biochemical, Inflammatory and Histological Profile in Rats. Pak. Vet. J.. 2023;43(2):366-370.
- [Google Scholar]
- Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. b.. 2022;12:2790-2807.
- [Google Scholar]
- Neurotoxic potential of polystyrene nanoplastics in primary cells originating from mouse brain. Neurotoxicology. 2020;81:189-196.
- [Google Scholar]
- Nonylphenol induces liver toxicity and oxidative stress in rat. Biochem. Biophys Res. Commun.. 2016;479:17-21.
- [Google Scholar]
- Nanoplastics in the aquatic environment. Cham: Critical review. Marine Anthropogenic Litter. Springer International Publishing; 2015. p. :325-340.
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun.. 1976;71:952-958.
- [Google Scholar]
- Palliative effects of zinc sulfate against the immunosuppressive, hepato-and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus) Aquac. 2019;504:227-238.
- [Google Scholar]
- Antioxidant, antiinflammatory, and analgesic activities of Citrus reticulata Blanco leaves extracts: an in vivo and in vitro study. Phytothérapie. 2017;46:1-13.
- [Google Scholar]
- The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun.. 1972;46:849-854.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Anti-apoptotic BCL-2 family members in development. Cell Death Differ.. 2018;25:37-45.
- [Google Scholar]
- Small-sized microplastics and pigmented particles in bottled mineral water. Water Res.. 2018;141:307-316.
- [Google Scholar]
- Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio) Aquat. Toxicol.. 2018;194:185-194.
- [Google Scholar]
- Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ.. 2020;702:134455
- [Google Scholar]
- Enzymatic studies of cisplatin induced oxidative stress in hepatic tissue of rats. Eur. J. Pharmacol.. 2006;532:290-293.
- [Google Scholar]
- Cadmium-induced nephrotoxicity via oxidative stress in male Wistar rats and capsaicin protects its toxicity. Bull. Environ. Pharmacol. Sci.. 2016;5:5-11.
- [Google Scholar]
- Apoptosis and cell cycle aberrations in epithelial odontogenic lesions: an evidence by the expression of p53, Bcl-2 and Bax. Med. Oral Patol. Oral Cir. Bucal.. 2018;23:e120.
- [Google Scholar]
- Leukemia therapy by flavonoids: Future and involved mechanisms. J.cell. Physiol.. 2019;234:8203-8220.
- [Google Scholar]
- Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: throwing up alarms of wide spread health risk of exposure. Int. J. Mol. Sci.. 2020;21:1410.
- [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- Differential effects of indoxyl sulfate and inorganic phospShate in a murine cerebral endothelial cell line (bEnd. 3) Toxins. 2014;6:1742-1760.
- [Google Scholar]
- Characterization of patients with biopsy-proven non-alcoholic fatty liver disease and normal aminotransferase levels. J. Gastrointestin. Liver Dis.. 2019;28:427-431.
- [Google Scholar]
- Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health. 2020;17:1509.
- [Google Scholar]
- A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157: H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron.. 2019;124:143-149.
- [Google Scholar]







