Translate this page into:
Translocation and survival of trunk injected Beauveria bassiana (Hypocreales: Cordycipitaceae) in healthy date palm trees
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
The date palm, Phoenix dactylifera L., is an ancient and valuable tree that provides food and other products. The date palm trees are attacked by several pests, including the red palm weevil, which is devastating to date palm plantations. Knowledge of the functionality of entomopathogenic fungi, including Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae) species, in the tree trunk is critical for controlling date palm weevil and other pests. The goal of this study was to assess the movement of the entomopathogenic fungus, B. bassiana, within the date palm tree.
Methods
Beauveria bassiana (BbSA-4) mixed with food colors was trunk-injected using a balloon injector into healthy date palm plants. Trunks were cut into one-meter logs 2, 20, and 86 days post-injection. Each log was further dissected into four quarters to examine the present of the fungus. The appearance of food colors and the detection of fungal spores at different heights from the point of injection revealed the translocation of B. bassiana within the trunk. The samples were taken from several locations where food color could be visible, and the distance traveled by the fungal spores was measured.
Results
The injected palm tissue samples were cultured on PDA media in the laboratory, and the present of fungal spores was confirmed. B. bassiana (BbSA-4) was found to be surviving in all treated date palm trees. The survival rate of isolate BbSA-4 averaged 70.5%, 34.9%, and 13.9% from dissected trunks examined at 2, 20, and 86 days, respectively, after injection. Isolate BbSA-4 was more apparent in the trunk after spiral injected than bottom injection. The findings revealed that using an entomopathogenic fungus as an endophytic to supplement IPM programs could be beneficial.
Keywords
Entomopathogenic fungus
Germination
Trunk treatment
Trunk injection
1 Introduction
Crop protection is critical for the survival of humanity, and the scarcity of food resources and the increasing world population make it even more sensitive. The insect and disease management of crops or fruit trees is challenging for growers (Bottrell and Schoenly, 2018). Crop protection against pests varies with the target crop and the nature of the problem. Insect pests might attack the woody parts (Held and Pickens, 2020, Selikhovkin et al., 2020) or foliage of the plant (Held, 2020). Use of toxic chemicals is one of the most common methods of pest control, with concerns about environmental contamination issues. To address these issues, many agricultural ecosystems across the world use potentially harmful pesticides, which in turn pollute the environment and leave chemical residues in food and water (Lescovak and Petrovic, 2023), and promote insect resistance (Wakil et al., 2018) and microbial resistance (Miller et al., 2022, Abdusalam et al., 2023). Therefore, the use of entomopathogenic fungi has been emphasized as an environmentally friendly alternative to chemical control (Vega, 2018, Qayyum et al., 2020).
In the context of pest control for perennial trees such as date palms, it is crucial to consider the movement of entomopathogenic fungi within the tree and their ability to generate poisons for the purpose of eliminating concealed pests residing within the tree trunk. Therefore, studies have been conducted to investigate various entomopathogenic fungi for their potential to be plant endophytic (Jaber and Ownley, 2018, Vega, 2018). Endophytes, such as fungi or bacteria, can colonize inside host tissues without affecting their host (Hyde and Soytong, 2008). Most entomopathogenic fungi that kill insects, on the other hand, can't live in the tissues of growing plants (Bamisile et al., 2023). Endophyte fungi are present in the vascular tissue of plants (Yan et al., 2015). Endophyte fungi germination in plant tissues begins with penetration and colonization. The endophyte fungi germinative tubes-haped hype penetrated the plant cell wall and colonized plant tissues (endoderm, pericycle, xylem, and phloem) (Yan et al., 2019, Patel et al., 2021). It is feasible for the endophyte fungi to propagate systematically once they have entered plant tissues (Oliveira et al., 2013). Endophyte fungi can promote plant fitness and growth, either directly or indirectly (Baron and Rigobelo, 2022).
Entomopathogenic fungi colonization inside the plant has benefits for countering insect pests (Bing and Lewis, 1992, Gurulingappa et al., 2010, Arab and El-Deeb, 2012, Muvea et al., 2014), plant diseases (Arab and El-Deeb, 2012), and enhancing plant development (Elena et al., 2011, Russo et al., 2015, Jaber and Enkerli, 2016, Jaber and Enkerli, 2017). Entomopathogenic fungi such as Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae), Metarhizium anisopliae (Metsch.) (Hypocreales: Clavicipitaceae), and Lecanicillium lecanii (Zimm.) (Hypocreales: Cordycipitaceae) have been shown to colonize plant tissue endophytically (Vidal and Jaber, 2015). B. bassiana germinated within the parenchymal cell after being injected into a palm leaf (Gómez-Vidal et al., 2006). It is an endophyte fungus used in maize (Bing and Lewis 1992), coffee (Posada et al., 2007), cotton (Griffin, 2007), and banana (Akello et al., 2008). Therefore, as an endophyte, it may protect plants from insects and plant pathogens (Parsa et al., 2013).
Entomopathogenic fungi, including B. bassiana, have been reported to be effective against an insect pest, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Dryophthoridae) (Sutanto et al., 2021, Sutanto et al., 2023, Sutanto et al., 2023). As a biocontrol pest management tool against insects on trees, field crops, and stored agricultural products, B. bassiana has been shown to be effective in the past (Vega and Kaya, 2012, Alagesan et al., 2019, Ahmed and Freed, 2021, Sutanto et al., 2022, Sutanto et al., 2023, Wakil et al., 2023). Studies investigated a number of ways to introduce B. bassiana to plants, such as root immersion (Tefera and Vidal, 2009), stem injection (Posada et al., 2007), foliar spray (Quesada-Moraga et al., 2006, Posada et al., 2007, Tefera and Vidal, 2009), Posada et al., 2007, Quesada-Moraga et al., 2006), and soil drenches (Posada et al., 2007, Tefera and Vidal, 2009).
The entomopathogenic fungi needs to be able to live inside the date palm tree in order to attack pests. Finding out how long the pathogen remains viable in the palm tree would be helpful to its effectiveness in the field, particularly in harsh environmental conditions. The main purpose of our study was to assess the survival and movement of B. bassiana conidia inside the date palm tree following trunk injections, thus being able to act as a potential biocontrol agent against date palm pests and diseases.
2 Materials and methods
2.1 Entomopathogenic fungus source
An adult red palm weevil (RPW), Rhynchophorus ferrugineus cadavers covered with a powdery white fungus coded as BbSA-4, originally collected from the Al-Qatif area of Saudi Arabia (N: 26.34437°; E: 43.69217°), were obtained from the Saudi Arabia Ministry of Environment, Water, and Agriculture in 2017. The isolated fungus was grown on a potato dextrose agar (PDA) medium and purified.
2.2 Fungus isolation, purification, and identification
The fungal isolation procedure was conducted in a laboratory of the Plant Protection Department, in the College of Food and Agriculture Sciences at King Saud University in Riyadh, Kingdom of Saudi Arabia. Fungus-infected RPW adult cadavers were ‘‘surface’’ sterilized for 3 min with 1% sodium hypochlorite. Subsequently, following three rinses in distilled water, allow the cadavers get a normal dried on filter paper. The cadaver was dissected into small fragments, which were subsequently dehydrated on filter paper. These dried fragments were then put onto potato dextrose agar (PDA) placed in a Petri dish (Scharlau, Microbiology 01–483, Eur. Pharm). The Petri dishes were kept at 25 ± 2 °C and 85 ± 5% relative humidity for 7 days in an incubator (Orbital Incubator, Stuart, SI 500) (Sahayaraj and Namasivayam, 2008). A hyphal tip technique was used for fungal purification (Gutierrez-Coarite et al., 2018). After the purification process, the fast germinating fungus isolates were screened. Light microscopy (Carl Zeiss, Axiostar plus, HBO 50, Göttingen, German) was used to examine the morphological character of the isolates. All fungus isolates were identified using the fungus identification key, which relied on the morphology of the fungus' conidia and hyphae (Humber, 2012). The purified isolate was identified using molecular identification.
2.3 Molecular identification of fungi
2.3.1 Extraction of DNA and Amplification by polymerase chain reaction
The DNA from B. bassiana was extracted by lysing the fungus with the extraction buffer and heating it to 94 °C for 60 min. After that, alcohol precipitation was used to separate the DNA from the rest of the supernatant. Amplification by polymerase chain reaction was utilized to determine what kind of fungus was present. In this molecular identification, a general primer of the internal transcribed spacer region, like ITS5 Forwards (5′ CTTGGTCATTTAGAGGAAGTAA 3′) and ITS4 Reverse (3′ TCCTCCGCTTATTGATATGC 5′) (Shanker et al., 2023), was used to amplify a ribosomal DNA fragment from the fungus.
2.3.2 DNA sequencing of fungal isolates
The PCR products of B. bassiana were sent to the Korean company Macrogen, Inc. to obtain the sequenced data. The use of the Basic Local Alignment Search Tool (BLAST) verified the fungal sequence identification. The B. bassiana sequences were submitted to NCBI (OQ630462).
2.4 Mass production of Beauveria bassiana
Beauveria basiana isolate was grown in large quantities on autoclaved wheat (Latifian et al., 2013). To achieve germination, small amounts of fungus isolate grown primarily with PDA were thoroughly mixed in the wheat and incubated for 15 days at 28 ± 1 °C. When the fungal growth was complete, the wheat medium was dried in an incubator for one day and then ground. The fungus conidia were measured using a hematocytometer, and the final concentration was 1x109 conidia/mL.
2.5 Field experiments
2.5.1 Selection of date palm trees
Twenty-four healthy date palm trees of the same age (8–10 yr old) were selected for the experiments at Al Jubaylah farm (24.921918 “N, 46.436469 ”E) Riyadh Region, in the Kingdom of Saudi Arabia.
2.5.2 Preparation of a fungus and color marker suspension
Balloon injector requirements tools were prepared. Then, each fungus suspension was placed in the special balloon (Ynject, Fertinyect, S.L., Spain) by using a 20-ml special syringe (Henke-Sass, Wolf, Tutlingen, Germany). Thirty-two liters of fungus suspension with a concentration of 1x109 conidia/ml were prepared. This suspension was divided into four containers, each with eight liters of fungus suspension. To track the movement of the fungus in the treated palm trees, four different synthetic food colors, red, brown, orange, and green; were mixed with fungus suspension. In total, 232 ml of each color was added to a container. Each balloon injector contained 300 ml of fungus suspension and color. The procedure for the preparation of a fungus and color suspension is shown in Fig. 1. Similarly, distilled water and food colour were used for the control treatment.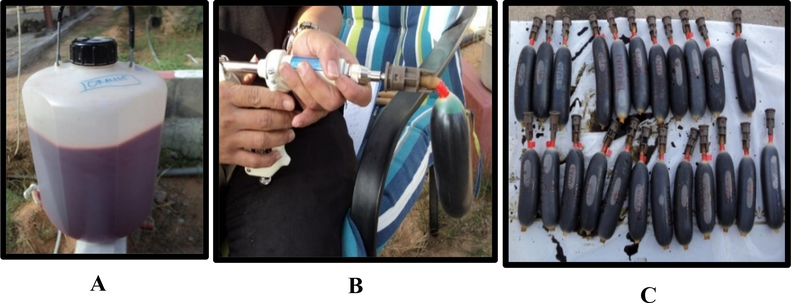
Prepared of fungus and color suspension: eight liters of fungus suspension (A); filling fungus suspension into the balloon using a special balloon injector (B); filled balloons with a fungus and color suspension (C).
2.5.3 Injection of the fungus suspension
Each palm tree designated for treatment was injected with a 1200 ml fungus suspension, using four balloon injections each containing 300 ml suspension of fungus and color mix (Fig. 2). Eighteen date palm trees were injected with fungus and color suspension, and six were used as a control and injected only with colored water (H2O). In total, twenty-four palm trees were injected with four different colors, with eight trees replicated for each time interval. Eight trees were cut and investigated after each interval of exposure. Each tree represents a single replicate. In each tree all four colors were injected either with fungus or only colored water as a control. The control tree data was only used to compare the fungal survival rate. In this experiment, two injection methods (spiral and bottom) were used. Date palm trees were labeled and treated with standardized mixed fungus suspension, injected with green, brown, red, and orange colors in the east, north, west, and south directions. The study injected trees using bottom, spiral, and control methods, with injection points ranging from 25 cm to 150 cm above ground level, and used water mixed with food colors instead of fungus suspension in the control treatment.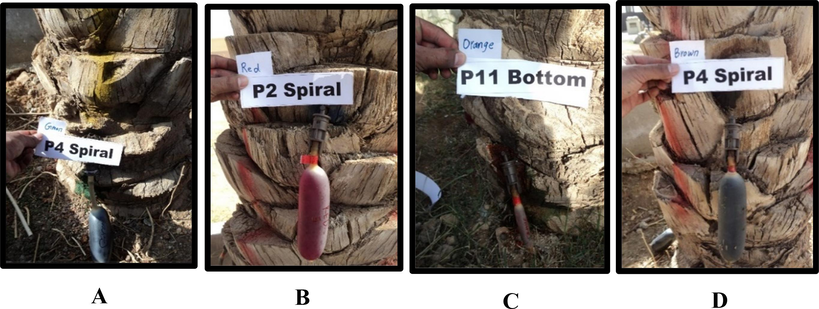
Injection of the fungus suspension into date palm trees: fungus suspension mixed with a green color (A); fungus suspension mixed with a red color (B); fungus suspension mixed with an orange color (C); fungus suspension mixed with a brown color (D).
2.6 Data collection
Trunk tissue samples were collected to locate fungal movement. After 2, 20, and 86 days of injection, each tree was cut down from the base and was further dissected to examine final movement and viability of the fungus. The tree was cut longitudinally into 1-meter lengths (logs) (X, Y, and Z). Each 1-meter log was then divided into two equal halves and then four quarters for more extensive inside inspections. Two data collection observations were considered. The first observation was for the purpose of collecting date palm tissue samples. Tissue samples were obtained from portions of the logs showing color movements at three different points: bottom, middle, and top. These tissues were scraped off using a sterile knife and placed straight on a potato dextrose agar medium plate. If the color movement was visible at the top of the first log in the bottom injection procedure, the 2nd 1-meter log was also cut. If there were no signs of color at the top of the first log, the examination of the bottom injection method was restricted only to that first log. For the spiral injection method, it was mandatory to cut two logs. If color movement was observed at the top of 2nd log then we proceeded to cut the third log. All the logs were cut in a standardized way as mentioned earlier. The samples were placed in a big plastic container (Al-Gothi plastic, 392/150 L, d: 45 cm; h: 75 cm) with a blue ice chest (Rubbermaid-Blue Ice Brand Weekender Pack, 7″x1.63″x6.75) for cooling. To determine if the fungus inoculum might persist in tissues, these samples were cultured on PDA media in the lab. Samples were incubated at 28 ± 2 °C and 85 ± 1% RH for seven days. During the incubation period, the samples were analyzed to measure the diameter of fungal growth, and the viability of the fungus was verified through microscopic observation.
2.7 Statistical analysis
The B. bassiana percentage survival rate was calculated and converted to angular values (arcsine) before statistical analysis (Little and Hills 1978). A one-way ANOVA was performed on the transformed percentages. Duncan multiple range test (p < 0.05) was used to differentiate the fungal survival rate means. The LSD test (p < 0.05) was used to determine the mean distance travelled by the fungus from tissue samples taken at various trunk heights. Analysis was done with SAS 9.4 (SAS 2004).
3 Results
After different intervals of post treatment, the palm trunks were cut and the present of different colors inside the palm trunk at different heights was noticed and measured (Fig. 3).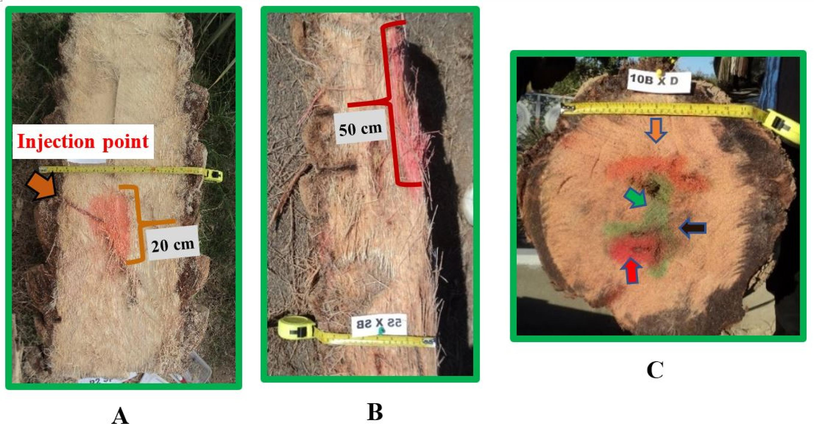
Date palm tree logs: half (A), quarter (B), and base (C) complete 1-meter log showing the present of colors after cutting.
The distance traveled by different colors was not significant with respect to the post treatment interval. However, the distance traveled differed significantly for each color with a decreasing trend over time (Table 1). The green color was detected at a maximum height of 74 cm from the point of injection after two days of post treatment. Whereas, brown color traveled the least distance of 34 cm after two days (Table 1). The color present was observed inside the trunk even after 20 and 86 days of injection but at shorter distance as compared to the distance traveled by the color after two days.
Colors
Time intervals
Color movement [cm]
F Value
Pr > F
Brown
2 days
34 ± 6.4a
5.88
0.0050
20 days
27.5 ± 1.1a
86 days
14.5 ± 2.5b
Green
2 days
74.3 ± 2.1a
33.93
0.0001
20 days
29 ± 7.2b
86 days
24.3 ± 3.1b
Orange
2 days
55.7 ± 9.5a
8.99
0.0005
20 days
23.8 ± 5b
86 days
12.3 ± 4b
Red
2 days
54.3 ± 9.4a
9.65
0.0003
20 days
34.1 ± 4b
86 days
16.5 ± 2.3c
Means followed by the same letters within a color do not differ significantly (P < 0.05). There were six replicates for each color at each time interval.
Furthermore, when comparing the spiral injection method to the bottom injection, the presence of BbSA-4 isolate in date palm trunk tissues was more distant in the spiral injection method. The BbSA-4 isolate was identified at distances of 64.4 cm, 31.8 cm, and 23.3 cm in the spiral injection method and 49.5 cm, 18.8 cm, and 17 cm in the bottom injection approach after 2, 20, and 86 days of treatment (Table 2).
Time interval
Injection method
Sample collection height [cm]
F Value
Pr > F
2 days
Spiral
64.4 ± 5.6a
3.42
0.06
Bottom
49.5 ± 5.7a
20 days
Spiral
31.8 ± 2.3a
7
0.01
Bottom
18.8 ± 4.3b
86 days
Spiral
23.3 ± 1.6a
3.65
0.06
Bottom
17 ± 2.8a
Means followed by the same letters do not differ significantly between the two methods within a time interval (P < 0.05). There were six replicates at each time interval.
Fig. 4, represents the germination of BbSA-4 isolate growth from the collected samples. There was a significant difference in the survival rate of BbSA-4 isolate among three time points when trunk tissue samples were collected and cultivated on PDA media under laboratory conditions.
Germination of BbSA-4 isolate from date palm trunk tissues samples.
The survivals of BbSA-4 isolate decreased with increasing time intervals when cuttings were performed and samples were collected (F: 24.7; df: 2, 15; P: < 0.0001). The highest survival rate-70.5% samples showing the growth of BbSA-4 isolate was observed in the samples collected and cultivated at 2 days after injection, followed by 34.9% samples at 20 days and 13.9% at 86 days (Table 3).
Treatment
Time Intervals
2 days
20 days
86 days
B. bassiana (BbSA-4)
70.5 ± 6.7a
34.9 ± 3.4b
13.9 ± 6.4c
Water (H2O)
0 ± 0a
0 ± 0a
0 ± 0a
Means followed by the same letters row wise do not differ significantly (P < 0.05). There were six replicates for BbSA-4 isolate at each time interval and two replicates for control (water).
4 Discussion
The current study is unique in that it examines fungus survival and mobility within a monocot tree trunk. There are only a few similar studies in the scientific literature. We used trunk injections to deliver BbSA-4 isolate inside the date palm trunk and observed its movement and survival. The findings indicate that BbSA-4 isolate moved, possibly by the movement of fungus spores or through the formation of fungus hyphae on plant tissue. The synthetic food colors used to detect the fungal movement in date palm tissues are different in their chemical compositions. The particular information about their movement in the plant tissues is not yet studied; however, the studies showed that all of the synthetic colors are soluble in water (Silva et al., 2022).
This is, to the best of our knowledge, the first report of fungus colonization and movement in a date palm trunk. Previously, the movement of B. bassiana and other fungi colonization and movement has been reported in date palm petiole after 30 days of inoculation (Gómez-Vidal et al., 2006). Similarly, the upward movement of the entomopathogenic fungus B. bassiana in corn plants has been observed, confirming the upward movement of the fungus within plant tissues (Bing and Lewis 1992, Wagner and Lewis 2000). The colonization and movement of the fungus depend on the inoculation method; for example, if the fungus is inoculated with soil drenched, it will colonize only the roots (Posada et al., 2007, Parsa et al., 2013, Greenfield et al., 2016). There are other studies that prove the movement of B. bassiana from roots or seeds to the aerial sections of the plant after soil or seed treatment (Biswas et al., 2012, Yerukala et al., 2021). The banana seedlings were inoculated with B. bassiana, and the presence of B. bassiana inoculum was reported in different parts of banana plants when dissected and cultivated on growth media (Akello et al., 2008). Also, Parsa et al., (2013) indicate the movement of B. bassiana to the leaves, roots, and stems after soil drench treatment. However, movement within the plant is likely dependent on plant species (Yerukala et al., 2022).
Endophyte fungus survival inside the plant is determined by a variety of factors, including the environment, plant health, and bacterial and fungus inhabitants within the host plant, which may not allow entomopathogenic fungus to colonize in plant tissues or survive for long periods of time (Schulz et al., 2015). Various fungus inoculum survival rates have been reported for various trees and fields, showing 30 days in live palm petioles and up to three months in jute plants (Gómez-Vidal et al., 2006, Biswas et al., 2012). Similar results have been documented in field settings in sorghum plants when B. bassiana and two other fungi were sprayed as foliar treatments, and the fungus inoculums lasted up to 30 days (Mantzoukas et al., 2015).
The current study discovered the variable distance traveled by the fungus spores within the injected trunk. The movement of the fungus hyphae within the tree trunk may vary due to a variety of factors. In date palm, B. bassiana can colonize endophytically in parenchyma and vascular tissue, particularly in the petiole (Gómez-Vidal et al., 2006). Whereas, in plants like maize, most studies have reported upward movement inside plant tissues, potentially in parenchyma and mesophyll tissues, in conjunction with plant photosynthesis, in xylem channels, and in air spaces between parenchyma (Bing and Lewis, 1992, Wagner and Lewis, 2000, Griffin, 2007). All of these studies support our findings of fungus translocation to varying lengths in the tree trunk after several days of post-injection.
5 Conclusions
The 13.9% survival of the fungus B. bassiana isolate inside the date palm tree were detected until 86 days of the post injection. The maximum distance traveled by the fungus was recorded at 74 cm in height in the case of the green color. Synthetic food colors can be used to track how far the fungus inoculum has spread through a tree's vascular system after it has been injected. The findings reveal that the fungus isolate that survived inside the date palm trees may possess characteristics that would be helpful to its long-term viability in the field, particularly in harsh environmental conditions.
Disclosure of funding
Present study was funded by the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia through project number (RSPD2024R721).
Acknowledgements
The authors would like to thanks the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for financial support of the present work through project number (RSPD2024R721).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Exploring antibiotic resistance with chemical tools. Healthcare (Basel).. 2023;11(13):1946.
- [Google Scholar]
- Virulence of Beauveria bassiana Balsamo to red palm weevil, Rhynchophorus ferrugineus (Olivier)(Coleoptera: Curculionidae) Egypt. J. Biol. Pest Control.. 2021;31(1):1-4.
- [Google Scholar]
- Akello, J., T. Dubois, C. Gold, et al., 2008. Beauveria bassiana. Balsamo (Vuillemin) as an endophyte to control the Banana weevil, Cosmopolites sordidus.
- An assessment of biological control of the banana pseudostem weevil Odoiporus longicollis (Olivier) by entomopathogenic fungi Beauveria bassiana. Biocatal. Agric. Biotechnol.. 2019;20:101262
- [Google Scholar]
- The use of endophyte Beauveria bassiana for bio-protection of date palm seedlings against red palm weevil and Rhizoctonia root-rot disease. Scientific J. King Faisal Univ. (Basic and Applied Sciences).. 2012;13(2):1433.
- [Google Scholar]
- Endophytic insect pathogenic fungi-host plant-herbivore mutualism: elucidating the mechanisms involved in the tripartite interactions. World J. Microbiol. Biotechnol.. 2023;39(12):326.
- [Google Scholar]
- Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology. 2022;13(1):39-55.
- [Google Scholar]
- Endophytic Beauveria bassiana (Balsamo) Vuillemin in corn: the influence of the plant growth stage and Ostrinia nubilalis (Hübner) Biocontrol Sci. Tech.. 1992;2(1):39-47.
- [Google Scholar]
- Establishment of the fungal entomopathogen Beauveria bassiana as a season long endophyte in jute (Corchorus olitorius) and its rapid detection using SCAR marker. BioControl. 2012;57(4):565-571.
- [Google Scholar]
- Integrated pest management for resource limited farmers: challenges for achieving ecological, social, and economic sustainability. J. Agric. Sci.. 2018;156(3):408-426.
- [Google Scholar]
- Metarhizium anisopliae (Metschnikoff) Sorokin promotes growth and has endophytic activity in tomato plants. Adv. Biol. Res.. 2011;5(1):22-27.
- [Google Scholar]
- Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron. 2006;37(7):624-632.
- [Google Scholar]
- Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biol. Control. 2016;95:40-48.
- [Google Scholar]
- Griffin, M. R., 2007. Beauveria bassiana, a cotton endophyte with biocontrol activity against seedling disease.
- Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control. 2010;55(1):34-41.
- [Google Scholar]
- Entomopathogenic fungi as mortality factors of macadamia felted coccid, Eriococcus ironsidei. Hemiptera: Eriococcidae; 2018. in Hawaii
- Borer pests of woody ornamental plants. ExtensionAlabama and Auburn University; 2020.
- Held, D. W., 2020. Chapter 7-Insect and mites attacking woody and herbaceous plants. Urban Lanscape Entomology. 135-164.
- Identification of entomopathogenic fungi. Manual Techniques Invertebrate Pathol. 2012:151-187.
- [Google Scholar]
- Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control. 2016;103:187-195.
- [Google Scholar]
- Fungal entomopathogens as endophytes: can they promote plant growth? Biocontrol Sci. Tech.. 2017;27(1):28-41.
- [Google Scholar]
- Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control. 2018;116:36-45.
- [Google Scholar]
- Mass production of entomopathogenic fungi Beauveria bassiana (Balsamo) by using agricultural products based on liquid-solid diphasic method for date palm pest control. Int. J. Agric. Crop Sci.. 2013;5(19):2337.
- [Google Scholar]
- Pesticide use and degradation strategis: Food safety, challenges and perspectives. Foods.. 2023;12(2709)
- [Google Scholar]
- Agricultural experimentation design and analysis. John Wiley & Sons; 1978.
- Effects of three endophytic entomopathogens on sweet sorghum and on the larvae of the stalk borer Sesamia nonagrioides. Entomol. Exp. Appl.. 2015;154(1):78-87.
- [Google Scholar]
- Antimicrobial use and resistance in plant agriculture: a one health perspective. Agriculture. 2022;12(289):1-27.
- [Google Scholar]
- Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS One. 2014;9(9):e108242.
- [Google Scholar]
- Endophytic microbial diversity in coffee cherries of Coffea arabica from southeastern Brazil. Can. J. Microbiol.. 2013;59(4):221-230.
- [Google Scholar]
- Establishing fungal entomopathogens as endophytes: towards endophytic biological control. JoVE (Journal of Visualized Experiments).. 2013;74:e50360.
- [Google Scholar]
- Patel, P., S. Kumar, A. Modi, et al., 2021. Deciphering fungal endophytes combating abiotic stresses in crop plants (cereals and vegetables). Microbial Management of Plant Stresses, Elsevier: 131-147.
- Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) Mycol. Res.. 2007;111(6):748-757.
- [Google Scholar]
- Integration of entomopathogenic fungi and eco-friendly insecticides for management of red palm weevil, Rhynchophorus ferrugineus (Olivier) Saudi J. Biol. Sci.. 2020;27(7):1811-1817.
- [Google Scholar]
- Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia. 2006;161(5):323.
- [Google Scholar]
- Endophytic colonisation of tobacco, corn, wheat and soybeans by the fungal entomopathogen Beauveria bassiana (Ascomycota, Hypocreales) Biocontrol Sci. Tech.. 2015;25(4):475-480.
- [Google Scholar]
- Mass production of EPF using agricultural products and by products. Afr. J. Biotechnol. 2008:1907-1910.
- [Google Scholar]
- SAS, 2004. SAS/STAT® 9.1. users guide. SAS Institute, Cary, NC. USA.
- Fungal endophytes are involved in multiple balanced antagonisms. Curr. Sci. 2015:39-45.
- [Google Scholar]
- Invasions of insectpests and fungal pathogens of woody plants into northwestern part of European Russia. Earth Sci.. 2020;65(2)
- [Google Scholar]
- Isolation, molecular characterization of indigenous Metarhizium anisopliae (Metchnikoff) isolate, using ITS-5.8 s rDNA region, and its efficacy against the Helicoverpa armigera (Hubner)(Lepidoptera: Noctuidae). Egyptian Journal of Biological. Pest Control. 2023;33(1):23.
- [Google Scholar]
- Food colour additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods.. 2022;11(3):379.
- [Google Scholar]
- Pathogenicity of local and exotic entomopathogenic fungi isolates against different life stages of red palm weevil (Rhynchophorus ferrugineus) PLoS One. 2021;16(7):e0255029.
- [Google Scholar]
- Persistency of indigenous and exotic entomopathogenic fungi isolates under ultraviolet B (UV-B) irradiation to enhance field application efficacy and obtain sustainable control of the red palm weevil. Insects.. 2022;13(1):103.
- [Google Scholar]
- Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl. 2009;54:663-669.
- [Google Scholar]
- The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia. 2018;110(1):4-30.
- [Google Scholar]
- Insect pathology. Academic press; 2012.
- Entomopathogenic fungi as endophytes: plant–endophyte–herbivore interactions and prospects for use in biological control. Curr. Sci. 2015:46-54.
- [Google Scholar]
- Colonization of corn, Zea mays, by the entomopathogenic fungus Beauveria bassiana. Appl. Environ. Microbiol.. 2000;66(8):3468-3473.
- [Google Scholar]
- Resistance to commonly used insecticides and phosphine fumigant in red palm weevil, Rhynchophorus ferrugineus (Olivier) in Pakistan. PLoS One. 2018;13(7):1-11.
- [Google Scholar]
- Entomopathogenic Fungus and Enhanced Diatomaceous Earth: The Sustainable Lethal Combination against Tribolium castaneum. Sustainability.. 2023;15(5):4403.
- [Google Scholar]
- Do endophytic fungi grow through their hosts systemically? Fungal Ecol.. 2015;13:53-59.
- [Google Scholar]
- Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol.. 2019;103:3327-3340.
- [Google Scholar]
- Endophytic increases galling of ‘Rutgers’ tomato roots with. J. Nematol.. 2021;53(1):1-16.
- [Google Scholar]
- Colonization efficacy of the endophytic insect-pathogenic fungus, Beauveria bassiana, across the plant kingdom: a meta-analysis. Crit. Rev. Plant Sci.. 2022;41(4):241-270.
- [Google Scholar]







