Translate this page into:
Exogenous citric acid improves growth and yield by concerted modulation of antioxidant defense system in brinjal (Solanum melongena L.) under salt-stress
⁎Corresponding author. naila.ali@imbb.uol.edu.pk (Naila Ali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Brinjal is sensitive to salinity, a common factor responsible for reducing its biomass and yield components. Recent research has demonstrated that citric acid/citrates can provide abiotic stress resistance in plants. In this study, Brinjal variety Pusakranti was treated with four concentrations of CA at 0, 100, 200, and 300 ppm applied foliarly under two levels of salt stress (0 mM and 60 mM NaCl) during spring 2021 with four replication per treatment. Salt stress reduced plant growth and yield attributes, pigments as well as metabolites in plants. Antioxidant enzyme activities of the plant increased compared to the non-stressed plants. While 300 ppm CA concentrations oenhanced the shoot and root fresh biomass (75 % and 71.8 %) and dry biomass (82.5 % and 40.7 %), while 200 ppm CA application increased the fruits count by 50 %, fruit diameter by 49 % and leaf photosynthetic pigments by 61 % compared to only salt-stressed plants. Similarly, CA application enhanced the antioxidants both enzymatic and non-enzymatic such as catalase by 42 %, peroxidase by 66 %, superoxide dismutase by 44 %, polyphenol oxidase by 50 % and Glutathione peroxidase by 37 % compared to only salt stressed plants. Furthermore, 300 ppm CA application also promoted the content of primary metabolites such as total protein content by 75 % and total free amino acids by 32 % as well as improvement in secondary metabolites such as total phenols by 31 % and flavonoids by 96 % compared to only salt treated plants. Overall, the above described results suggested that the foliarly applied CA(200 ppm) is a proficient approach which effectively counteract salt stress in brinjal by improving plant biomass, pigments, primary and secondary metabolites as well as modulating the the antioxidant defense system of the studied plant.
Keywords
Citric acid
Biochemical
Antioxidants
Polyphenol oxidase
Salinity stress
Yield attributes
- CA
-
Citric Acid
- ROS
-
Reactive Oxygen Species
- CRD
-
Completely Randomized Design
- TPC
-
Total Phenolic Contents
- CAT
-
Catalase
- POD
-
PerOxiDase
- SOD
-
SuperOxide Dismutase
- GPX
-
Glutathione PerOxidase
- PPO
-
PolyPhenol Oxidase
Abbreviations
1 Introduction
Brinjal (Solanum melongena L.) of family Solanaceae has been originated in Indo-china domain, it is grown all over the world's tropical and subtropical regions (Rehman et al., 2019). In Pakistan, brinjal is grown over an area of 8,427 ha producing 84,255 tonnes annualy (Anonymous, 2019). Sindh and Baluchistan provinces of Pakistan follow Punjab in terms of area and production of this crop (Javed et al., 2017). Brinjal is Pakistan's most commonly used vegetable because of its unique flavor and high nutritional value. Its fruit is rich in vitamins, minerals and low-fat food. The brinjal fruit contains phenolic compounds, the most important being the chlorogenic acid which is beneficial for heart health and its bark has nasunin anthocyanin, which is causative agent of its specific black color. Nasunin is known as a powerful antioxidant and acts to combat free radicals and ultimately help to protect cell membranes.
Salinity, a global destructive environmental stress resulting in a notorious decrease in crop productivity. Globaly, 50 % of the agricultural land is disturbed by saltiness (Ahanger et al., 2020). Saline stress drastically decrease the availability of water by membrane instability, altering mineral distribution causing changes in turgor pressure, reduction in pigment synthesis, changes in gaseous exchange attributes, and subsequently decresead the crop yield (Ahanger et al., 2020; Kaya et al., 2020). Under the condition of stress different Reactive Oxygen Species (ROS) including hydroxyl, superoxides and hydrogen peroxide radicals produced which influences the damaging effects in the plants. In response to these ROS, plants activates stress resilience approaches including ion compartmentation, different antioxidant activites, and osmolytes production (Alam et al., 2019;). Moreover, manifestation of salt in soil exhibited the upregulation of antioxidant enzymes, osmolytes and metabolites including proline, flavonoids, glycine and other carbohydrates in plants (Ahanger et al., 2020; Kaya et al., 2020).
Brinjal plants are glycophytes and showed reduction in growth and productivity when exposed to salt stress as they are moderately sensitive to salinity as compared to other plants of the same family (Brenes et al., 2020a, 2020b). Brinjal is sensitive to salinity, a common factor responsible for reducing its biomass and yield components (Brenes et al., 2020a, 2020b). By these factors, understanding of intensity of salt amelioration in plants is important in increasing the yield of vegetable crops suplemented with saline solution. Organic acids expressed a substantial part in prolonging the physiological state of crops. The foliar application of citric acid (citrate or CA) has been developed as a practical method to enhance the plant resistance abil ity to conservational stresses and thus withstand crop yield. The (CA) is a six-carbon organic acid having a central role in the Krebs cycle. CA application can enhance the salt bearing abilily in plants and eventually improve the production (Mallhi et al., 2020). Plants have been displayed an improved tolerance under saline environment when supplemented with antioxidant solution (Ahmad et al., 2019; Kohli et al., 2019). Applying CA also modulates an antioxidant defense system, increase high photosynthetic pigment content affecting secondary metabolites enhancing plant productivity grown in stressed soil (Arif et al., 2021). CA treatment promoted essential oil components such as monoterpene hydrocarbons and oxygenated sesquiterpenes of Melissa officinalis grown in stress (salt) (Ahmed and Talaat, 2017). A study revealed that citrine fertilizers as a foliar application (0.3 %) improve maize plants' growth, yield, and chemical constituents (Mohamed and Yazal, 2019). Previous studies have revealed that the exogenous CA application can mitigates oxidative stress of plant by increasing the activity of antioxidant defense systems (Mallhi et al., 2020). There is non sufficient reports regarding the impacts of citric acid on the morphological, biochemical and physiological properties of brinjal plants in saline conditions has been put forward.
The functional characterization of the plants foliarly treated with citric acid especially the vegetables in salt stressed soil is not well described. Therefore, assessing the importance of citric acid in the brinjal plant's salt tolerance could be a valuable solution to mediate saline stress and increase the rate of crop production. It is postulated that appropriate doses of citric acid may enhance brinjal responses to saline stress by reducing the production of ROS thus, enhancing antioxidant enzymatic activities, ultimately stabilizing the plasma membrane. For that reason, the present study was designed to observe the physiological and biochemical effects of citric acid under salt-stressed brinjal plants reporting and an improved stress tolerance ability of the plant and mitigating the damages of plasma membrane caused by salinity via the synthesis of metabolites, osmoprotectants, and enzymatic as well as non-enzymatic antioxidants.
2 Methodology
A pot trial was executed to analyze the influence of citric acid (CA) on morphological and biochemical attributes in brinjal (Solanum melongena) under salt stress. The investigation was conceded in the field area of the University of Lahore, and lab work was performed in the General Botany Research Lab, Department of Institute of Molecular Biology and Biotechnology (IMBB), University of Lahore (UOL), Pakistan. The brinjal seeds of variety Puskranti were obtained from local market of Lahore, Pakistan and sown in pots containing 5 Kg of loam-sand-compost soil (w/w) in the ratio of 3:1:1. Seeds were disinfected by dipping in 5 % solution of sodium hypochlorite for 5 min before sowing and then rinsed meticulously thrice with distilled water. The pots were kept in a field under natural sun light and seasonal temperature conditions. The experiment was performed in a completely randomized design (CRD). Four replicates were taken for each treatment and it took 4.5 months from sowing of seeds to harvesting.
2.1 Application of citric acid and salinity
Two-week-old brinjal plants (immature plants are more sensitive to bear the abiotic stresses) were irrigated with a salt (NaCl) solution of 60 mM concentration (selected on the basis of previous study, Semiz and Suarez, 2019; Samy and Abd, 2012). The control group was not supplemented with salt solution (0 mM). Different concentrations of CA (0, 100, 200, and 300 ppm) were foliar applied to the plants at the onset of new leaves after salt stress. The CA was applied twice to maximize the effect of CA at an interval of 10 days after two weeks of salt stress. In 0 ppm CA treatment, distilled water was sprayed. The plants were harvested at the onset of fruiting, and data were analyzed. Different growth parameters were recorded. Plant samples were preserved in 0.2 M phosphate buffer solution comprising monobasic(NaH2PO4) and dibasic(Na2HPO4) solution after 30 days of citric acid application for different physiological and biochemical parameters. Leaf pigments were estimated from fresh plant material at the time of harvesting.
2.2 Evaluation of growth and yield attributes
Plants at maturity were excised avoiding root damages and leaves count per plant was noted. The graph paper (millimeter) method was used for estimation of leaf area. The length of root and shoot(cm) was measured. Fresh plant of each treatment from each pot was weighed on a digital weigh balance after that the plants were let to be oven dried at 50 °C for 72 h to compare the dry and fresh biomass of each plant. The fruits count were taken into account, then fruit volume was measured by the water displacement method (Costa et al., 2016), while, the fruit diameter was taken by a vernier caliper.
2.3 Determination of photosynthetic pigments
According to Davis (1976), the pigment including chlorophyll contents and carotenoids contents were measured using a UV/V spectrophotometer (HALO SB-10, Camlab, UK), and the formula provided by Arnon was used to compute the pigment concentrations (Arnon, 1949). The chlorophyll a and b, tota chlorophyll, and carotenoid were calculated. The results were represented as mg/g Fresh Weight (FW).
2.4 Determination of primary metabolites
Using the Bradford method (Bradford, 1976), the total amount of water-soluble proteins was calculated, and the at 595 nm absorbance, the values were measured spectrophotometerically. Bovine Serum Albumin(BSA) was considered as standard to calculate the amount of soluble proteins (BSA). Using Hamilton and Van Slyke's approach, the Total Free Aminoacids (TFA) were calculated (Hamilton and Slyke, 1943) considering a calibration curve (L-serine), and the findings were reported as mg/g FW.
2.5 Determination of specialized metabolites
Total Phenolic Contents (TPC) were calculated by the Folin-Ciocaltue method, as reported by Julkunen Titto (1985). The standard curve of Gallic Acid was used to express TPC as µg Gallic Acid Equivalent per g Dry Weight (µg GAE g−1 DW). Total Flavonoid Contents (TFC) were calculated by the method given by Pekal and Pyrzynska (2014). The standard calibration curve of quercetin was used for its determination, and the contents were expressed as µg Quercetin Equivalent per g Dry Weight (µg QE g−1 DW).
2.6 Determination of antioxidant activities
The activities of CATalase (CAT), PerOxiDase (POD), SuperOxide Dismutase (SOD), Glutathione PerOxidase (GPX), and PolyPhenol Oxidase (PPO) were measured alculated from frozen (-70 °C) leaf material. All the enzymatic activities in the extracts in this study were expressed in Units/mg of Protein. Catalase activity is the measurement of the rate of conversion in which hydrogen per oxide molecules are broken into water molecule and liberating an oxygen molecule (Chance and Maehly, 1955) while, in POD activity peroxidantion of hydrogen peroxide is being measures using guaicol as electron donor (Kuroda et al., 1990). The protocol of Dhindsa et al. (1981) was followed to find out the SOD activity. GPX analysis were performed by the procedure of Cichoski et al. (2012) while, PPO activity was checked using the protocol of Mayer et al. (1965).
2.7 Statistical analysis
The research was designed in a CRD arrangement. The Statistix (8.1), computer-based software, was performed to compute the analysis of the variance (ANOVA) of the data. The Tukey’s HSD test was used to compare means at p ≤ 0.05. The significances of treatments means were also compared by providing standard error values and alphabetical letterings. Same alphabetical letters in the treatments were considered statisitically non-significant to each other at 5 % probability.
3 Results
3.1 Impact effect of CA on growth attributes
Salinity significantly reduced the growth characteristics of brinjal plants (Tables 1a and b). In salinity stress decreased the number of leaves by 14 %, the size of the leaves by 13 %, the length of the shoots and roots by 14 % and 27 %, respectively, and the weight of the shoots and roots by 21 % rather than control plants. In a similar manner, the dry weight of the salt-stressed plants was decreased than that of the control plants without salt stress by 13 % and 15 %, respectively. Citric acid applied topically had triggering effects on growth metrics in both control and stressed environments. Citric acid given topically at a concentration of 300 ppm displayed a more favourable effect on growth characteristics (such as leaf number, leaf area, plant length, fresh and dry biomass, etc.) than individual salt treatment or even in non-stressed conditions. In appraisal to control plants without citric acid application, this treatment considerably increased the leaves count, leaf area, shoot and root length, shoot and root fresh and dry biomass under saline conditions by 75 %, 67 %, 72 %, 56 %, 75 %, 71 %, 82 %, and 40 %, and under on-saline conditions by 85 %, 81 %, 70 %, 59 %, 59 %, 63 %, 87 %, and 50 %. However, in the case of shoot fresh weight, the interactive effect of salinity with the application of citric acid was not significant in brinjal plants (Tables 1a and b).
Salinity
Foliar citric acid
Number of leaves (n)
Leaf area (cm2)
Shoot length (cm)
Shoot fresh weight (g)
Shoot dry weight (g)
0 mM
0 ppm
12.0 ± 0.81ef
11.0 ± 0.81e
10.2 ± 0.22e
16.5 ± 0.57d
3.32 ± 0.12f
100 ppm
16.5 ± 0.57 cd
15.0 ± 0.81c
12.5 ± 0.18c
20.0 ± 0.81c
4.82 ± 0.09d
200 ppm
20.0 ± 0.81b
17.5 ± 0.57b
15.1 ± 0.09b
22.5 ± 0.57b
5.62 ± 0.15b
300 ppm
22.2 ± 0.95a
20.0 ± 0.81a
17.2 ± 0.17a
26.5 ± 0.51a
6.25 ± 0.10a
60 mM
0 ppm
10.2 ± 0.95f
9.5 ± 0.22e
7.5 ± 0.45f
12.0 ± 0.81f
2.87 ± 0.05 g
100 ppm
13 ± 0.81e
13.0 ± 0.52d
9.5 ± 0.57e
14.5 ± 0.35e
3.52 ± 0.17f
200 ppm
15.5 ± 0.57d
11.1 ± 0.81e
11.2 ± 0.51d
18.0 ± 0.81d
4.51 ± 0.08e
300 ppm
18 ± 0.81c
16.0 ± 0.81bc
13.0 ± 0.18c
21.0 ± 0.81bc
5.24 ± 0.02c
ANOVA
Salinity
***
***
***
***
***
Citric acid
***
***
***
***
***
Salinity*citric
**
***
***
Ns
***
Salinity (mM)
Foliar application of citric acid (ppm)
Root length (cm)
Root fresh weight (g)
Root dry weight (g)
0 mM
0 ppm
6.75 ± 0.12f
4.50 ± 0.18e
1.82 ± 0.09d
100 ppm
8.15 ± 0.05d
5.45 ± 0.12d
2.35 ± 0.05c
200 ppm
9.71 ± 0.14b
6.67 ± 0.18b
2.56 ± 0.03b
300 ppm
10.75 ± 0.20a
7.35 ± 0.12a
2.75 ± 0.02a
60 mM
0 ppm
5.75 ± 0.12 g
3.55 ± 0.12f
1.54 ± 0.04e
100 ppm
5.25 ± 0.12 h
3.27 ± 0.12f
1.45 ± 0.05e
200 ppm
7.25 ± 0.12e
4.65 ± 0.12e
1.77 ± 0.05d
300 ppm
9.01 ± 0.18c
6.10 ± 0.08c
2.17 ± 0.09c
ANOVA
Salinity
***
***
***
Citric acid
***
***
***
Salinity*citric
***
***
***
3.2 Effect of CA on yield attributes
The yield characteristics of brinjal plants (fruit number, fruit volume and diameter, and fresh and dry biomass of fruit) were expressively reduced under salt stress (60 mM). In compared to control plants, the number, volume, diameter, fresh weight, and dry weight of the fruits reduced by 20 %, 14 %, 14 %, and 18 %, respectively, under stress. However, contrary to the control plants without citric acid application, the 200 ppm citric acid foliar application increased fruit counts by 50 % and 73 % and fruit volume by 43 % and 56 % in both stressed and non-stressed environments, respectively. In comparison to salt stress alone without citric acid supplementation, the fruit diameter and fresh and dry weight rose after foliar application of citric acid at 200 ppm concentration by 49 %, 70 %, and 66 %, respectively. Similar to this, under non-saline conditions, exogenously applied citric acid at 300 ppm increased the fruit diameter by 58 %, fruit fresh weight by 83 %, and fruit dry weight by 81 % compared to control plants without citric acid application. However, the interaction between salinity and foliar citric acid spray had no discernible impact on the fruit count (Table. 1c). Means of four replicates ± standard errors. Variations in alphabets designate significance among treatments *, **, and *** indicated significance at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, respectively, ns stands for non-significant difference.
Salinity (mM)
Foliar application of citric acid (ppm)
Fruit number (n)
Fruit volume (cmc)
Fruit diameter (cm)
Fruit fresh weight (g)
Fruit dry weight (g)
0 mM
0 ppm
3.7 ± 0.09 cd
352.5 ± 17.07e
2.9 ± 0.14de
17.7 ± 1.25e
1.9 ± 0.01 g
100 ppm
5.0 ± 0.08abc
445.0 ± 12.90c
3.7 ± 0.12b
24.0 ± 0.81c
2.7 ± 0.02c
200 ppm
6.5 ± 0.05a
550.0 ± 18.25a
4.3 ± 0.21a
29.0 ± 0.81b
3.2 ± 0.02b
300 ppm
5.7 ± 0.05ab
500.0 ± 16.32b
4.6 ± 0.21a
32.5 ± 1.29a
3.5 ± 0.01a
60 mM
0 ppm
3.0 ± 0.08d
300.0 ± 16.32f
2.4 ± 0.26e
14.5 ± 1.29f
1.6 ± 0.08 h
100 ppm
4.0 ± 0.08bcd
372.5 ± 17.07e
3.2 ± 0.21 cd
21.0 ± 0.81d
2.2 ± 0.02f
200 ppm
5.0 ± 0.08abc
430.0 ± 8.16 cd
3.7 ± 0.21b
24.7 ± 0.95c
2.6 ± 0.03d
300 ppm
4.5 ± 0.05bcd
410.0 ± 2.58d
3.4 ± 0.08bc
23.0 ± 0.81 cd
2.4 ± 0.01e
ANOVA
Salinity
***
***
***
***
***
Citric acid
***
***
***
***
***
Salinity*citric
ns
***
**
***
***
3.3 Increase of leaf pigments by CA application
Salt stress significantly influences the leaf pigments, including chlorophyll a, b, total chlorophyll contents, and carotenoids of brinjal. A significant decrease was exhibited in chlorophyll a by 15 %, chlorophyll b by 15 %, total chlorophyll by 10 %, and carotenoid contents by 11 % under saline conditions over control plants. Exogenously applied CA at 200 ppm under salt stressed condition improved the chlorophyll a by 54 %, Chlorophyll b by 65 %, total chlorophyll by 61 % and carotenoids by 27 %, while, under control conditions, foliar application of CA increased the chlorophyll a, b total and carotenoids by 59 %, 69 %, 72 % by and 36 %, respectively, over control plants without the application of citric acid (Fig. 1a-d).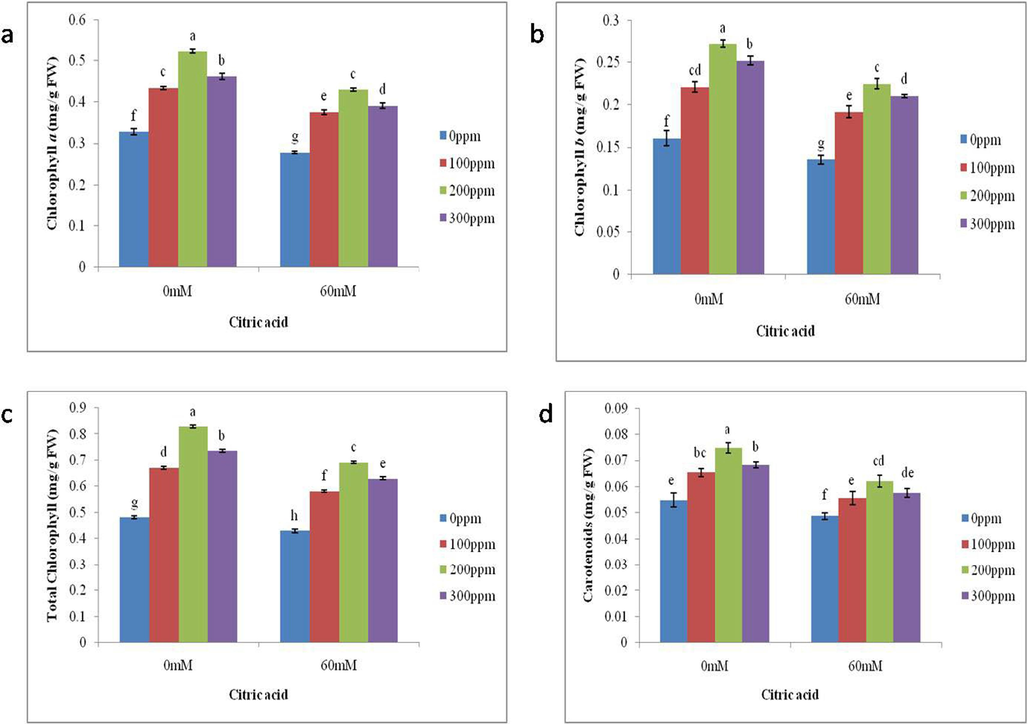
Impact of foliar application of differential CA concentrations on the (a) chlorophyll a, (b) chlorophyll b, (c) total chlorophyll, and (d) carotenoids of brinjal grown in salt stressed conditions. Letters on the bars signify different statistical means (p ≤ 0.05).
3.4 Determination of biochemical attributes under CA application
A significant amount of reduction (8 %) in Total Free Aminoacids was recorded in specimens grown in saline soil as compared to non-saline soil. However, a 36 % increase in total soluble protein content was observed under stressed conditions in contrast to control plants. While the foliar spray of CA increased the total free aminoacids and soluble protein content. The exogenously applied citric acid (300 ppm) increased the total soluble proteins by 75 % and total free amino acids by 32 % under salt-stressed condition, while, 50 % and 53 % increase in total solube proteins and total free amino acids were observed by the foliar application of 200 ppm CA under non-stressed conditions, respectively, as compared to without citric acid (Fig. 2a-b).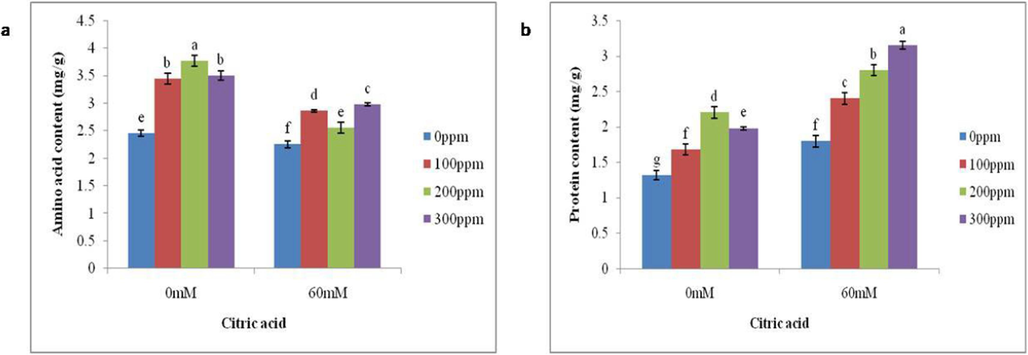
Impact of foliar application of differential CA concentrations on the (a) Amino acid and (b) protein content of brinjal grown in salt stressed conditions. Letters on the bars signify different statistical means (p ≤ 0.05).
Salt stress significantly increased the synthesis of phenolics and flavonoids. It causes a 31 % increase in total phenolics and a 96 % increase in total flavonoids under salt stress conditions over control plants. Moreover, exogenously applied citric acid at 200 ppm further promoted total phenolics up to 85 % and total flavonoids by 1.3-fold under saline condition. The concentration of 300 ppm CA under non saline environment increased the total phenolics by 82 % and total flavonoids by twofold (Fig. 3a-b).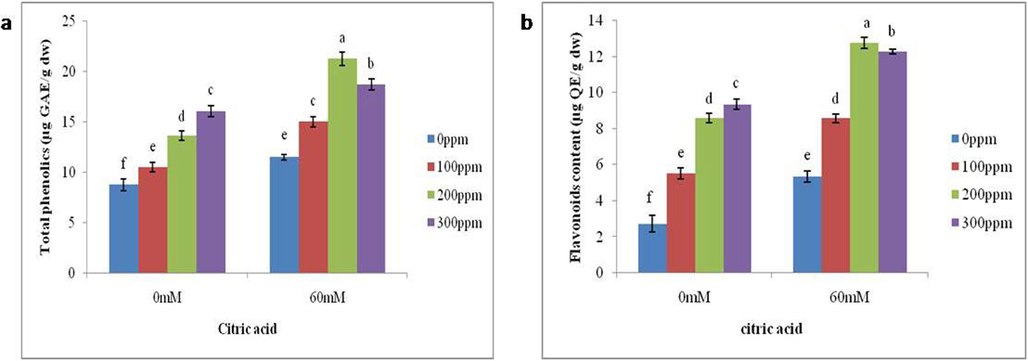
Impact of foliar application of differential CA concentrations on the (a) total phenolics and (b) flavonoid content of brinjal grown in salt stressed conditions. Letters on the bars signify different statistical means (p ≤ 0.05).
3.5 Determination of antioxidants
The effect of citric acid on antioxidant modulation, including CAT, POD, SOD, GPX, and POD, was measured under stressed environment. Salt stress increases the activities of CAT by 42 %, POD by 66 %, SOD by 44 %, GPX by 37 %, and PPO by 50 % than in non-saline conditions. Foliar application of citric acid causes more increases in antioxidant activities under salt-stressed as well as non-stressed conditions. Specifically, the foliar application of CA (300 ppm) increased the activities of CAT by 68 %, GPX by 1.6 fold PPO by 99 % and POD by 1.2 fold, but for SOD application of 200 ppm showed maximum value (2fold) than the plants without CA supplementation grown under saline condition. The plants grown under non saline environement and treated with 300 ppm CA showed an increase of 18 %, 20 % and 75 % in CAT, GPX and PPO as compared to the plants with no application of CA under non-saline environment. The POD and SOD activity was improved by 34 % and 43 %, respectively, in the plants grown under non-saline condition by treated with 200 ppm CA (Fig. 4a-e).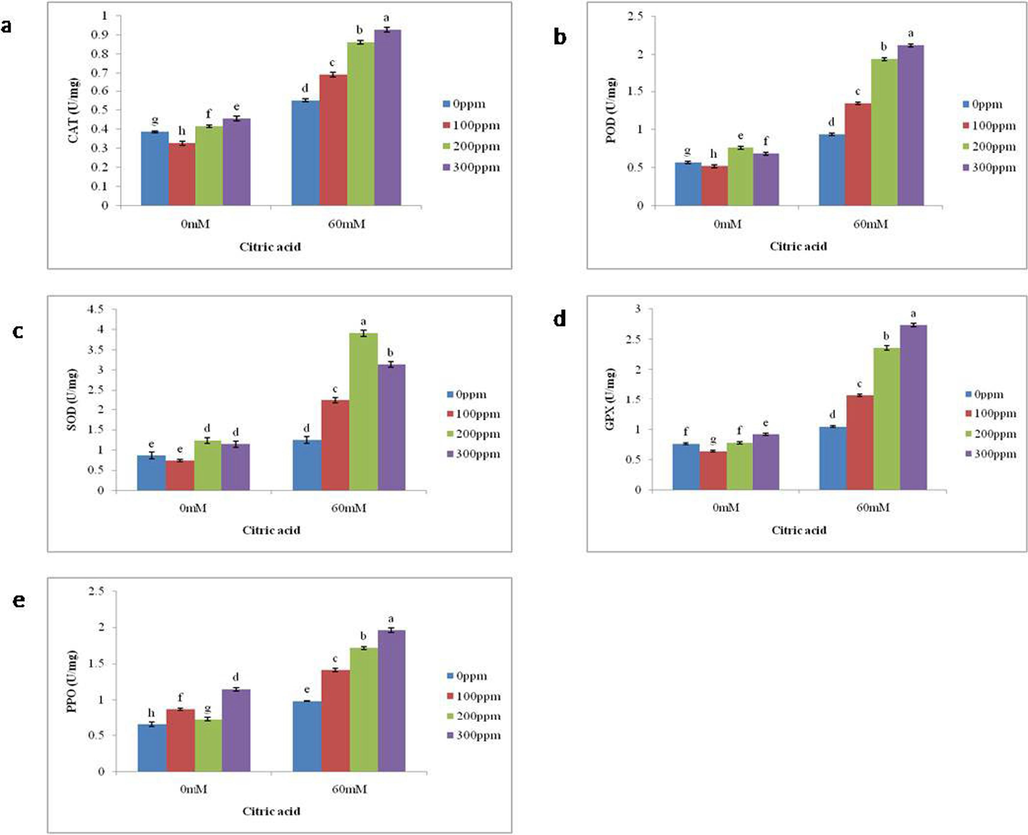
Impact of foliar application of differential CA concentrations on the (a) catalase, (b) peroxidase, (c) superoxide dismutase, (d) glutathione peroxidase, and (e) polyphenol oxidase activity of brinjal grown in salt stressed conditions. Letters on the bars signify different statistical means (p ≤ 0.05) (All enzymatic antioxidants are expressed as U/mg Protein).
4 Discussion
Plants often bare unfavorable environmental conditions, such as abiotic stresses, which intensely limit the yields of crop species. Salt stress has been proved a severe issue for plant growth, reducing crop yield and production, worldwide. The strategy of foliarly applying citric acid is widely used all over the world and is essential for promoting sustainable agriculture. Citric acid, an important component of Krebs cycle for the production of compounds involved in the synthesis of amino acids and fatty acids. The cirtic acid cycle is very important for the production of ATP in providing precursors for various metabolic pathways. Salt stress affects the enzymatic activites involved in respiration thus disturbing the cycle. While, CA converted to 5 or 4-carbon compounds by splitting hydrogen atoms and cycle again converted to oxaloacetic acid (Wang et al., 2017). So, goal of the recent study was to determine how salinity-stressed brinjal plants' morphological, physiological, and biochemical contents were affected by the exogenous application of citric acid.
A damaging abiotic stress called salt stress restricts plant growth showing negative influence on a plant's morphological, biochemical, and physiological characteristics. Primary problems of plants to stress include stunted growth of roots and shoots, nutritional imbalance, sluggish rate of germination, closure of stomata, reduction in seedling growth, and deterioration of photosynthetic activity (Ahanger et al., 2020). Salinity considerably reduced the growth (18–27 %) and yield parameters(18–20 %), according to our study. These findings agree with accounts on a variety of plants from earlier times (Ahanger et al., 2020; Alam et al., 2020; Kaya et al., 2020). That could imply that plant tissues growing in a saline environment produced more proline. The decrease in cell water potential was also seen at the time, which in turn causes salt-stressed plants to develop more slowly and have less photosynthetic activity and plastid pigment. The results showed that when different concentrations of CA (100, 200, and 300 ppm) were applied topically to brinjal plants, 300 ppm of CA increased several growth parameters(40–80 %), including the number of leaves, the area of leaves, the height of the plant, and the weight of the plant, while 200 ppm of CA increased various yield attributes (70 %), including the number of fruits, their weight, diameter, and volume. According to Mohamed and Yazal (2019) who published comparable findings on maize plants, the conclusions of this study. The ability of CA to participate in nutrient absorption, water relations, stress signalling, and enhance photosynthesis and phytochelatin synthesis in plants may be responsible for improvements in biomass and plant growth. These actions ultimately have a positive influence on plant growth, yield, and quality of crop as well as on plant vegetative and reproductive development (Mallhi et al., 2020).
The primary components of light reactions in photosynthesis are photosynthetic pigments. The thylakoid membrane, the location where various types of photosynthetic pigments are deposited, is said to be damaged by practically all types of abiotic challenges, including salinity stress. To keep the photosynthetic system operating as it should, moderate salinity stress increases the production of total chlorophyll and carotenoid concentration (Hameed et al., 2021). Salinity considerably condensed the total chlorophyll (15 %) and carotenoid content (11 %) in the current study as compared to control plants. Our findings in this regard are consistent with earlier studies on different plants (Ahanger et al., 2020; Alam et al., 2020; Kaya et al., 2020). A comparison of means showed that the exogeniously applied concentrations (100,200, 300 ppm) of citric acid increased leaves' total chlorophyll content. Among them, 200 ppm CA resulted in significantly higher total chlorophyll (65 %) and chlorophyll a content of leaves in contrary to the control plants which follows the trend observed in chlorophyll b and carotenoid content where 200 ppm citric acid has a more positive effect under stressed or non-stressed conditions. Similar to another study reported by Arif et al. (2021) who found that a number of morphological and yield attributes, and physiological parameters (CHl a, Chl b, total chlorophyll, carotenoids and proline) were amplified by exogeneous treatment of CA in Gosspium barbadense, also indicated the better physiological parameters results in higher photosynthetic activity, reducing Reactive Oxygen Species, and also increase osmoregulation.
The current study also showed that salinity greatly influences the Total Free Aminoacids which were considerably decreased than that of control plants. Our findings are in accordance to the previous reports (Riaz et al., 2021) which indicated that salinity decreases the total free amino acids by increasing the formation of osmolytes such as proline, and GB in cluster bean which significantly drained the osmotic potential of the cell, maintaining the integrity of proteins and working as a molecular chaperone. Therefore, higher levels of osmolytes also serve as an antioxidant in their own right under saline conditions. However, a significant increasing trend was observed in total soluble protein content under salt stress conditions. In this regard, our results are in line with the findings of Aly et al. (2019) on the wheat cultivars and stated that these proteins could have an important part in signal transduction, antioxidative defense, anti-freezing, heat shock, metal binding, anti-pathogenesis and osmolyte synthesis, which is an important part in plant’s growth and physiological attributes. The current research also indicated that citric acid application at the rate of 300 ppm significantly improved the Total Soluble Proteins and free amino acids under salt stress conditions compared to control plants on which citric acid was not applied. Our results are in compliance with El- Beltagi et al. (2017) who foliarly sprayed citric acid on G. Barbadence and observed similar results, which showed that citric acid is deliberately taken as an important organic acids of respiration into the plant cell. Energey currency (ATP) of all the biochemical and physiological processes is provided by the citric acid cycle operated in mitochondria of the cell.
According to several studies, phenolics and flavonoids, non-enzymatic antioxidants with low molecular weights or secondary metabolites, exhibited various biochemical and molecular roles in plants, including maintaining redox-homeostasis, acting as signalling molecules, activating plant defence mechanisms, mediating auxin transport, and enhancing antioxidant free radical scavenging assays (Sirin and Aslam, 2019). According to the study's findings, salinity-stressed plants produced more total phenolics (30 %) and flavonoids (90 %) in their leaves than salinity-free control plants. In this regard, our findings are consistent with those of Kiani et al. (2021) who noted comparable outcomes in wheat varieties. However, the foliar application of citric acid at (200 ppm) under salt stress considerably boosted the total phenolics of leaves and flavonoids than the control plants. Related findings were found by Rangel et al. (2018) who reported that CA-treated wheat sprouts activated signal transduction pathways that resulted in a greater concentration of secondary metabolites. Another study in tea plants found that the CA supplementation stimulated the production of more proline and other metabolites in response to abiotic stressors (including phenolic compounds, flavonoids, tannins, and sugars) (Li et al., 2019). In stressful conditions, the hydrolysis and breakdown of cellular constituents in cell walls results in the buildup of phenolic compounds. Also, the use of CA decreased pH, which boosted the presence of flavonoids and anthocyanins in the plants (Kaur et al., 2017).
According to our findings, various antioxidants (CAT, POD, SOD, GPX, and PPO) were increased (50 %) by salt rather than control plants. These outcomes are in line with a number of earlier studies (Ahanger et al., 2020; Kaya et al., 2020) which noted comparable outcomes in various plants. In the current study, 300 ppm CA improved all antioxidant activities (CAT, POD, GPX, and PPO) under stressed plants rather than control plants among the various CA concentrations applied foliarly. In contrast to control plants, superoxide dismutase activity (SOD) was considerably higher at 200 ppm citric acid under salt stress. Accordingly, current results are in line with those of several earlier studies (Ahmad et al., 2017) which noted comparable results in sabdariffa, Beta vulgaris, and Oryza sativa, respectively, and came to the conclusion that CA supplementation is involved in ROS scavenging assays, enhancing antioxidant enzymes and secondary metabolites. During salt stress, antioxidants help to rummage ROS, prevent oxidative destruction to plants, and enhance cellular redox equilibrium. SOD defends plants from oxidative loss by transforming O2– (superoxide anion) to H2O2, whereas CAT and GPX directly reclaim ROS by converting H2O2 to water and oxygen (Kohli et al., 2019). Furthermore, peroxidase (POD) is known to shield cells from ROS by catalysing redox processes (Su et al., 2020). Inducing the oxidation of phenols to quinines, polyphenol oxidase (PPO) is widely present in plants and is thought to play an essential role in the plant's defence mechanism against environmental challenges (Akhtar and Mahmoo, 2017).
5 Conclusion
The goal of the experiment was to find out how citric acid helped brinjals to cope with salt stress. The amount of total free amino acids, photosynthetic pigments, and growth and yield characteristics are all dramatically decreased by salt stress, although total soluble proteins and secondary metabolites, such as phenolics and flavonoids, are increased. Moreover, plants produced under salt stress have three times more enzymatic antioxidant activity than control plants. Supplemental citric acid (200 and 300 ppm) markedly improved growth and yield-related traits, chlorophyll pigments, and antioxidant potential. The beneficial effects of exogenous citric acid treatment in brinjal under salt stress may have been seen in free radical scavenging tests, preservation of membrane stability, improved root functioning, and stimulation of antioxidant production. Based on these findings, commercial formulations for boosting the development and production of brinjal crops cultivated under stress could do well to consider citric acid doses of 200 and 300 ppm. To learn more about its impacts on metabolic and molecular pathways, more research is needed.
Funding
This work was supported by the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia (RSP-2023R350).
7 Author’s contributions
The research conceptualization was a collaborative effort involving all authors. NA, RR, and ZN were primarily responsible for drafting the experimental design, while NA and RR conducted the experiments. ZN and LW were involved in data analysis and validation. AA and PK contributed to the language editing of the manuscript. All authors participated in reviewing and approving the final version of the manuscript before its submission.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R350) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules. 2020;10:42-46.
- [Google Scholar]
- Environmental impacts of some organic extracts on Sugar beet yield under saline-sodic soil conditions. J. Soil Sci. Agric. Eng.. 2017;8:821-827.
- [Google Scholar]
- Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot.. 2019;161:1-3.
- [Google Scholar]
- Citric acid affects Melissa officinalis, essential oil under saline soil. Asian J. Crop Sci.. 2017;9:40-49.
- [Google Scholar]
- Response of rice polyphenol oxidase promoter to drought and salt stress. Pak. J. Bot.. 2017;49:21-23.
- [Google Scholar]
- 24-epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules. 2019;9:640-646.
- [Google Scholar]
- Effects of gamma irradiation and salt stress on amino acids and protein fractions of two Egyptian bread wheat (Triticum aestivum L.) cultivars. Bangladesh J. Bot.. 2019;48(4):1175-1184.
- [Google Scholar]
- Anonymous, 2019. Fruit vegetables and condiments statistics of Pakistan 2017-18, Economic Wing, Ministry of National Food Security and Research, Government of Pakistan. Islamabad.
- Citric acid – mediated abiotic stress tolerance in plants. Int. J. Mol. Sci.. 2021;22
- [Google Scholar]
- Copper enzymes in isolated chloroplasts polyphenal oxidase in Beta vulgaris. Plant Physiol.. 1949;24:1-15.
- [Google Scholar]
- A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Compartive studies on the physiological and biochemical responses to salt stress of brinjal (Solanum melongena and its rootstock Solanum torvum. Agriculture. 2020;10:1-20.
- [Google Scholar]
- Physiological and biochemical responses to salt stress in cultivated eggplant (Solanum melongena L.) and in S. insanum L., a Close Wild Relative. Agronomy.. 2020;10(5):651.
- [Google Scholar]
- Investigation of glutathione peroxidase activity in chicken meat under different experimental conditions. Food Sci. Technol.. 2012;32:661-667.
- [Google Scholar]
- Measurement of volume of macaw palm fruit using traditional and the digital Moiré techniques. Revista Brasileira De Engenharia Agrícola e Ambiental.. 2016;20:152-157.
- [Google Scholar]
- Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot.. 1981;32:93-101.
- [Google Scholar]
- Effect of salicylic acid and potassium citrate on cotton plant under salt stress. Fresen. Environ. Bull.. 2017;26:1091-1100.
- [Google Scholar]
- Effects of salinity stress on chloroplast structure and function. Cells.. 2021;10:1-22.
- [Google Scholar]
- The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem.. 1943;150:231-250.
- [Google Scholar]
- Management of brinjal shoot and fruit borer (Leucinodes orbonalis guenee) by integrating different non- chemical approaches. Pak. J. Agric. Sci.. 2017;54:65-70.
- [Google Scholar]
- Phenolic constituents in the leaves of Northern Willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem.. 1985;83:213-217.
- [Google Scholar]
- Castasterone and citric acid treatment restores photosynthetic attributes in Brassica Juncea under Cd toxicity. Ecotoxicol. Environ. Saf.. 2017;145:466-475.
- [Google Scholar]
- Integrative roles of nitric oxide and hydrogen sulfde in melatonin induced tolerance of pepper (Capsicum annuum L.) plants to iron defciency and salt stress alone or in combination. Physiol Plant.. 2020;168:256-277.
- [Google Scholar]
- Polyphenols, Flavonoids, and antioxidant activity involved in salt tolerance in Wheat, Aegilops cylindrical and their Amphidiploids. Frontiers of Plant Science.. 2021;12:1-13.
- [Google Scholar]
- Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants.. 2019;8
- [Google Scholar]
- Changes in chloroplast peroxidase activities in relation to chlorophyll loss in barley leaf segments. Physiol. Plant.. 1990;80:560.
- [Google Scholar]
- Citric acid enhanced dissolution of polyphenols during soaking of different teas. J. Food Biochem.. 2019;43
- [Google Scholar]
- Citric acid assisted phytoremediation of chromium through sunflower plants irrigated with tannery wastewater. Plants.. 2020;9:380.
- [Google Scholar]
- Assay of Catechol Oxidase: a critical comparison of methods. Phytochemistry. 1965;5:783-789.
- [Google Scholar]
- The application of citric acid in combination with some micronutrients increases the growth, productivity, and a few chemical constituents of maize plants. Int. Lett. Nat. Sci.. 2019;76:86-97.
- [Google Scholar]
- Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods. 2014;7:1776-1782.
- [Google Scholar]
- The effect of citric acid on the phenolic compounds, flavonoids and antioxidant capacity of wheat sprouts. Revista Facultad Ciencias Agrarias Universidad Nacional De Cuyo.. 2018;50:119-127.
- [Google Scholar]
- Growth and yield response of different brinjal cultivars to irrigation deficit conditions. J. Horticultural Sci. Technol.. 2019;2:78-84.
- [Google Scholar]
- Choline chloride mediates salinity tolerance in cluster bean (Cyamopsis tetragonoloba L.) by improving growth, oxidative defense, and secondary metabolism. Int. J. 2021:1-19.
- [Google Scholar]
- Alleviation of salt stress in eggplant (Solanum melongena L.) by plant-growth-promoting rhizobacteria. Commun. Soil Sci. Plant Analysis. 2012;43: 9:1303-1315.
- [Google Scholar]
- Impact of grafting, salinity and irrigation water composition on eggplant fruit yield and ion relations. Sci. Rep.. 2019;9:19373.
- [Google Scholar]
- Determination of antioxidant capacity, phenolic acid composition and antiproliferative effect associated with phenylalanine ammonia lyase (PAL) activity in some plants naturally growing under salt stress. Med. Chem. Res.. 2019;28:229-238.
- [Google Scholar]
- A member of wheat class lll peroxidase gene family, TaPRX-2A enhanced the tolerance of salt stress. BMC Plant Biol.. 2020;20(392):391-1315.
- [Google Scholar]
- The important role of the citric acid cycle in plants. Genomics Appl. Biol.. 2017;8(4):25-29.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103012.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







