Translate this page into:
Discovery of secondary metabolites from Avicennia marina to inhibit the anti-oxidant and anti-biofilm activities of biofilm forming bacteria
⁎Corresponding author at: State Key Laboratory of Biocontrol, Guangdong Provincial Key Laboratory of Plant Resources and Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, PR China. liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study is concentrated on screening of phytochemical and bioactive compounds from marine mangrove plant of Avicennia marina (A. marina). The plant extract was purified by soxhlet extraction process, and the available phytochemicals, bioactive compounds and other organic compounds were confirmed by LC-MS analysis. Subsequently, the antioxidant and DPPH scavenging activities of the A. marina extract was shown 79 % and 88 % at 500 µg/mL concentrations were observed respectively. Further, the antimicrobial efficacy of A. marina extract was shown 22 and 26 mm and 18 and 24 mm zones of inhibition against K. pneumoniae, A. baumannii, S. aureus and Enterococcus sp. at 500 µg/ml concentrations by agar well diffusion method. In addition, the liquid medium based bacterial inactivation was clearly observed with 78 %, 89 %, 81 % and 90 % at 500 µg/ml concentration. Furthermore, the biofilm inhibition concentration and exopolysaccharide degradation effect of A. marina extract indicated that the bacteria were completely arrested and the inhibition range of 92 %, 88 % 84 % and 90 % and 89 %, 94 %, 90 % and 86 % were observed respectively. Finally, the result evidences were clearly indicated that the mangrove plant A. marina was excellent antimicrobial inhibitor and also future drug discovery plant to fight against some important multi drug resistant pathogens.
Keywords
Mangrove environment
Phytochemical derivatives
Crude compound
Biological properties
Exopolysaccharides
1 Introduction
Mangrove ecosystems have complex nutritional conditions and produce diverse pharmaceutical molecules in microorganisms. Mangrove microbes can acquire the nutrients from revers process to grow and produce novel bioactive metabolites, thus driving geochemical cycles of elements. Also, it is produce important impact on the material cycle and energy flow in mangrove ecosystems (Tao et al., 2022). Generally, microbes are more sensitive to environmental conditions, and their community composition can be changed accordingly with the help of changes in the environment. In addition, the relationship between living materials and mangrove environment can be produce extraordinary biopharmaceutical products (Arulkumar et al., 2020). Mangrove plants are grown in difficult environmental nature halophytic plants which can grow in intertidal zones between lands and coastal tropical and also subtropical regions (Jing et al., 2022). Sometimes, mangrove plants are used as a traditional folk remedies in South and central Asia including India, China, Thailand, Indonesia, Malaysia and Singapore.
Previously several studies reported that the mangrove plants have efficient anti-bacterial, anti-cancer, anti-inflammatory, anti-viral and anti-diabetics activities. In particular, the genus Avicennia is the most important worldwide available plant having ability to produce new types of biopharmaceutical product. Previous reports are reported that the mangrove Avicennia marina has high content of tannin in their woods. Then, the bark of Avicennia marina is used in leather and dying industries. It has excellent healing properties having high phytochemicals contents including terpenoids, glucosides and naphthalene (Lalitha et al., 2021). Also, the rich phenol and flavonoid rich content of the A. marina was identified with bioactive compounds and antimicrobial properties (Sreekanth and Anupama, 2021).The leaf extract of mangrove plants have excellent phytochemical producing ability like alkaloids, steroids, saponins, tannins, terpenoids and anti-oxidant with scavenging activity (Vasanthakumar et al., 2019). Also, all these phytoderivatives are played important role in chemical compound production and also protect the plant through host defense mechanism under the conditions of biotic or abiotic stress (Shekhar Das, 2020). The rich availability of these derivatives is evidence to hold medicinal properties and use in remedial approach of health. The failure of existing drugs and synthetic drugs are the leading reason for concentrate in new kind of plant materials and their related chemical derivatives. It is also helped to find new chemical derivatives that improve the plant nature effectively. Importantly, 95 % of the synthetic drugs are more hazardous and it affect very dangerous side effect and it have chance to collapse the nature of drugs. Due to this defect, recent years researchers are focused natural product drug discovery from different sources. In particular, unaware place of the mangrove is the essential source to increase the drug discovery against various pathogens with low hazardous effect (Kandasamy et al., 2021).
Recent years, mangrove antioxidants are heightened more worldwide because of their positive involvement as health improved factors including cardiovascular diseases, cancer and other heart diseases. Many natural plants are the main source to produce free radical scavengers and they are contributed great attention worldwide. As same as mangrove plant A. marina is the considerable plant source finding naturally occurring antioxidant for use in food and medicinal industries to replace the synthetic antioxidants which decreased the side effects. Subsequently, more antioxidant compounds were reported from A. marina plant, notably 2, 3-tert-butyl-4-methoxyphenol, butylated hydroxyanisole and tert-butylhydoquinonone (Tian et al., 2020).
In addition, more researchers are concentrated mangrove plant research that it has extra ordinary activity against microbes, virus and larvicidal (Rajivgandhi et al., 2018). Based on these advantages, previous researchers are also performed and identified the anti-oxidant and phytochemical properties. In particular, the mangrove plant of A. marina and Rhizophora mucronata are the important plant in secondary metabolites production with greater efficiency (Titah et al., 2021). Sometimes, the lower pH is also responsible for improve the chemical nature of the plant due to the increase the addition of food supplement nature in salt marshes. High level of salt conditions may influence the stress responses in particular genes for production of increased efficiency of chemical compounds. Sometimes, it has the complete destroying ability against some important pathogens. It is prime approach to detect the efficiency of A. marina in different stress conditions like carbon, nitrogen, pH, temperature, NaCl and other sources (Besley and Birch, 2021).
The mangrove plant of A. marina is the dominant marine mangrove plant in India and has potential biological properties. The anti-oxidant rich A. marina extract has been already reported with anti-microbial, anti-biofilm and anti-cancer activities. In addition, more new compounds or derivatives or phytochemicals are heavily available in this plant. Based on the above said advantages, the present study is focused on marine mangrove plant A. marina, and their antioxidant and biological activities using various invitro assays.
2 Materials and methods
2.1 Chemical and glasswares
All the chemicals and glass wares of this study were purchased from Merk, India PVT Limited, Mumbai, India. The plastic bags, washing and extraction solutions were procured from Suresh Scientific & Co, Tiruchirappalli, Tamil Nadu, India. The phytochemical derivatives detection chemicals, anti-oxidant activity chemicals and other invitro assay of the media and chemicals were ordered from Ponmani & Co, Tiruchirappalli, Tamil Nadu, India.
2.2 Collection of samples
The healthy, fresh, uncontaminated A. marina plant leaf, stem, bark were collected from Muthupet Mangrove Region (Latitude 9°15′41.88″N, Longitude 79°04′05.81″E), Thiruvarur District, East Coast of Tamil Nadu, India. Initially tap water was used to remove the surface contaminants and followed by double distilled water. Next treat the samples with alcohol for removal of free floating contaminants which available on the plant surface. Then, the samples were air dry, and clean, neat samples were taken for further experiments. Finally, the samples were ground by using grinder and taken the powder for extraction process.
2.3 Active crude extract preparation
For crude extract, the powdered sample was mixed with ethanol solution in the ration of 1:10 was used. The samples were applied in the soxhlet apparatus with needed temperature until the color was turn into yellow, green to clear yellow within the time period of half day. The heated extraction process was cooled until the sample come original nature environment with settlement in bottom of the flask. For clear sample, the solvent was completely removed using rotary evaporator. The yield of the sample was identified based on the available formula of Tian et al. (2020). Then, the extract was separated was used for further work and stored in deep freezer.
2.4 Available phytochemical derivatives in A. marina
The available chemical derivative of A. marina extract was performed to LC-MS analysis. In LC-MS, the alkaloid, phenol, flavonoid, secondary metabolites, growth hormones, alcoholic derivatives, steroids, terpenoids, amino acid leads, and other available chemical compounds were screened and the screening of these chemical derivatives by LC-MS was followed by previously published article of Rajivgandhi et al. (2021). Shortly, column capacity of 30 m × 200 μm × 0.30 μm was chosen, it was available automatically in the LC-MS machine. Also, the machine was made by CP-Silicon 5 of chrome pack. Before start process, must check the temperature initial and extended, it should be maintained 60 °C−150 °C with 5–15 min respectively. In addition 150–300 °C of constant temperature was also fixed and the injector detector was attached in this place. Further, 30 cm/s of carrier gas with 1 mL/m of flow rate were set in linier velocity. Based on the peak evidences, the holding material of A. marina was clearly detected. The available materials were confirmed by retention time, occupied are and occupied percentages. The chemical derivatives and their peaks were further cross checked by NIST library which available in Bharathidasan University, Thiruchirappalli, Tamil Nadu, India.
2.5 Anti-oxidant properties of A. marina
The anti-oxidant properties present in the marine mangrove plant of A. marina was measured by invtitro sodium phosphate and ammonium molybdate assay followed by recently reported study of Loo et al. (2007). Briefly, the stock solution of A. marina extract (1 g) was taken in 10 mL of methanol solution. Aliquot 2 mL of the samples into separate test tube, as well as take 2 mL of sodium phosphate and ammonium molybdate combination in another tubes. Each tube was allowed to run the reaction for 5 min time interval. The second test tube containing samples were mixed together well and allowed to run the reaction for 5 min. Subsequently, 2 mL H2SO4 was added into the reaction mixture of the sample and maintain into the water bath at 95 °C for 40 min time interval. Following, 2 mL of prepared A. marina extract sample was added into the reaction mixture of the sample tube and run the process at 37 °C for 20 min. After reaching the time, the test tube was monitored to seen the anti-oxidant activity by visible observation and then followed to analyzed the 600 nm O.D value by spectrophotometer. The same set up of the procedure with ascorbic acid except A. marina extract was acted as a standard control, and the result was taken by spectrophotometer. Both the standard and tested values were carefully interpreted and measured the anti-oxidant activity level of the A. marina extract. Here, 1 mg of the ascorbic acid was equal to 1 g of the A. marina extract.
2.6 Calculation of free radical scavenging properties by DPPH assay
As per previous experiment of Rajivgandhi et al. (2020), the ascorbic acid was used as a standard for calculate the free radical scavenging activity of A. marina extract. At 50–300 µg/mL concentration of A. marina extract was separately filled by 100 mL of DPPH in single test tube. Whereas, each 5 mL of butylated hydroxyl toluene and DPPH were taken together in another test tube. Both the samples containing tubes were allowed to run the process until reach 45 min at 37 °C, and then calculated separately their anti-oxidant properties under 600 nm O.D value by spectrophotometer. In addition, 70 % ethanol was used as an experiment control. Finally, the test and control O.D values were interpreted each other and confirmed their anti-oxidant properties based on the bellowed formula,
2.7 Biological property of extract
Weather the antimicrobial ability of A. marina was increased or not was confirmed by using agar well diffusion method with help of earlier report of Dhayanithi et al. (2012). The 12 h old staled culture of biofilm forming K. pneumoniae, P. aeruginosa, S. aureus and Enterococcus sp. were spread on previously prepared cooled muller hinton agar plate. The clear wells of 6 mm distance were made into the agar surface and total of 5 were made. Then, the crude A. marina was taken mixed in methanol with different concentration added into the four well. Fifth well was filled by methanol for detect the originality of A. marina extract. All the procedure plate was put into incubator with 37 °C for 1 day. After 1 day, the zone formation around the well was calculated and interpreted with control for original result.
2.8 Confirmation of anti-bacterial property by minimum inhibition concentration assay
The minimum concentration of A. marina was inhibited the highest number of bacterial growth was suggested as minimum inhibition concentration assay (Jamal et al., 2021). It was suggested that the extract has excellent anti-bacterial property against tested pathogens. So, the 96-well plate method with quantification in spectrometer was used to detect the ability of A. marina extract. The methanol extract of A. marina was used as different concentration (25–250 µg/mL), and added into 96-well plate. The plate was already mixed with 100 µL of sterile broth with 10 µL of biofilm forming K. pneumoniae, P. aeruginosa, S. aureus and Enterococcus sp. in separate plates. After mix, all the plates were slightly by rotation for mixed thoroughly, and then maintained one day in 37 °C incubation. After incubation, the turbidity level of the wells were clearly monitored by named eye and went to taken spectrometric analysis using 540 nm O.D including control well also. Based on the turbidity level and O. D values were clearly noted and converted to result in percentage use control as a substrate. Finally, the inhibition ability of A. marina was calculated using bellowed formula,
2.9 Minimum biofilm inhibition concentration
The 24-well polystyrene plate was used to eradicate the biofilm cells using crude extract of A. marina at 50–300 µg/mL concentration followed by the recent report of Okla et al. (2021). 1 mL of 48 h matured g K. pneumoniae, P. aeruginosa, S. aureus and Enterococcus sp.cultures were inoculate into 24-well containing sterile freshly prepared tryptic soy broth. Subsequently, 50–300 µg/mL concentration of A. marina extract was treated into the respective wells. After, treated cells were discarded and washed initially by 10 % PBS to remove the non-adherent cells, followed by treated with 4 % crystal violet to stain the attached cells and allowed 10 min to complete fixation. After treatment of biofilm fixation, the crystal violet was removed and then washed by double distilled water. Five min later, the cells were further washed by 500 µL of 20 % glacial acetic acid and maintained at 37℃ for 24 h to wait for dissolve the biofilm cells. Finally, the stained cells were measured by 450 nm O.D values by microtitre reader. Absences of A. marina extract with medium containing pathogens were served as a control. Finally, the inhibition of biofilm formation was interpreted based on the control and treated values using bellowed formula,
2.10 Exopolysaccharide degradation in biofilm forming bacteria using A. marine extract
In biofilm production, the exopolysaccharide is the important virulence factor, which is used to detect the effective of biofilm formation. This experiment was conducted based on the previous report of Rajivgandhi et al. (2020). Some modified procedure of biofilm inhibition concentration treated selected pathogens were taken into centrifuged tube and centrifuged the samples at 7,500 rpm for 25 min at 4 °C. After the pellets were received and resuspended in sterile saline solution. Subsequently, the enzymatic treatment of 50 µL of pronase E (Sigma Aldrich, India) was added and vortexes the samples at 37 °C for 60 min. Following, 100 µL of 10 % trichloroacetic acid was added into the samples to precipitate the protein in an ice bath for 45 min time interval. Then, the samples were centrifuged at 7,500 rpm for 25 min at 4 °C and followed by added 10 mL of cold absolute alcohol drop wise on the side wall of the tubes. Then, monitored the samples to precipitate the polysaccharide quantities and maintained at −20 °C for 24 h. Next, the samples were centrifuged at 7, 500 rpm for 25 min to collect the polysaccharide and mixed in 1 mL of DD H2O. Then, 5 mL H2SO4 was used to digest the samples containing monosaccharides and then add 1 mL of 5 % phenol, the process were allowed to maintain in the 90 °C water bath 25 mi. Finally, the samples were cooled on ice and result of the exopolysaccharide degradation from biofilm cells were calculated using 460 nm O.D value by spectrophotometer (Shimadzu, Japan). Here, glucose was performed as a standard and water as blank. The degradation of exopolysaccharide in the treated samples result was interpreted with untreated control and percentages of inhibition was confirmed by bellowed formula
3 Result and discussion
3.1 Measurement of available anti-oxidant properties of a. Marina
Based on the NIST Wiley library of Bharathidasan University, Tiruchirappalli, tamil Nadu, India, totally 20 different peaks were clearly observed after analysis with LC-MS (Fig. 1). All these peaks were originally derived from A. marine and confirmed by previous reports of Ebrahem et al. (2020); Ananthavalli and Karpagam (2017); Jairaman et al. (2019), Tian et al. (2020); Vasanthakumar et al. (2019). Consecutively, all those peaks were interpreted based on the retention time, occupied are and occupied percentages and also cross checked by previously published articles. In addition, the observed result was exhibited the chemical derivatives of terpenoids, phenols, bioactive metabolites, organic compounds, alkaloids, hydrocarbons, flavonoids. In result, pinene, rubiadin, copaene, di-tert-butyl-2-hydroxybenzylidene-amino-2-indanol, Phenol, 2,4-bis(1,1-dimethylethyl), pyrollo derivatives, thujene, scopoletin, phthalate, 3-hydroxy-4-phenyl, di-tert-butyl-2-hydroxybenzylidene-amino-2-indanol, octadecenamide and adecenoic acid. These phytochemical derivatives, essential oils and bioactive compounds were clearly shown in the result. Also, the exhibited phyto chemical derivatives and compounds were reported previously in their A. marina study Moradi et al., 2020; Kathiresan et al., 2018. In addition, the excellent anti-bacterial properties of the A. marina secondary metabolites were also evidenced by Natwar et al. (2022). The differential environmental nature of marine mangrove environmental conditions such as pH, carbon, nitrogen, stress, NaCl and other sources were very important against pathogenic microbes (Babaei-Bondarti and Shahpiri, 2020). The statement was agreed by Ton et al., (2022) and previously, A. marina extract has excellent biological properties and standardized by LC-MS analysis for chemical derivatives identification. Recently, Karpagavinayagam and Vedhi (2019), also strongly suggested that the LC-MS based analysis of plant phyto compounds are used to future drug design and delivery against various infectious pathogenic microbes. Altogether, this A. marina mediated compound identification study was clearly stated that the extract has more phyto compounds and it was used to analyze the anti-pathogenic study against multi drug resistant bacteria.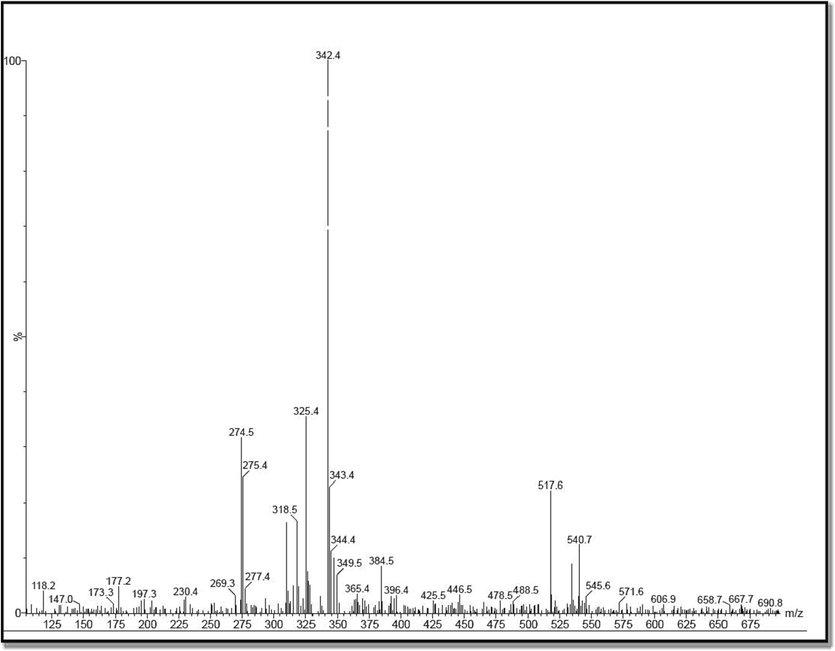
Identification of available phytochemical compounds from crude extract of Avicennia marina by LC-MS spectrum.
3.2 Total anti-oxidant and DPPH scavenging properties of a. Marina
The significant anti-oxidant and DPPH scavenging activities of the marine mangrove plant A. marina extract was effectively screened by the respective invitro experiments. Previously, Hassan et al. (2022) reported that the marine mangrove plant of A. marina has considerable anti-oxidant and DPPH scavenging activities. Similarly, Arulkumar et al. (2020) agreed the present result, where the extracted A. marina has excellent anti-oxidant and DPPH scavenging activities. In the current study, the total anti-oxidant activities present in the A. marina extract was very high antioxidant activities 79 % and 88 % after interpreted with standard control of ascorbic acid. Among the tested concentration, the result was shown excellent antioxidant activity at 500 µg/mL concentration (Fig. 2a). This concentration was very effective compared with recent reports of terrestrial plant extract Audah et al. (2022); Keerthi et al. (2022). Further, the antioxidant rich A. marina extract was indicated the excellent DPPH scavenging activity and it was confirmed by standard control result of ascorbic acid (Fig. 2b). When, the concentration of 500 µg/mL concentration, the DPPH scavenging activity in A. marina result was indicated that the A. marina is the excellent reservoir to produce anti-oxidant activities. As per obtained result, the total anti-oxidant producing effect of A. marina was 88 % and 78 % for DPPH scavenging activities, whereas, the 79 % and 70 % of the standard control of ascorbic acid were also shown for both the antioxidant and DPPH scavenging activities respectively. Both the result was indicated that the total anti-oxidant activity and DPPH scavenging activity results were interlinked within the result of A. marina extract. In addition, there were no vast differences between the concentrations against both the experiments results. Recently, carbon and nitrogen content rich mangrove A. marina produced excellent antioxidant activity and DPPH scavenging activities (Eswaraiah et al., 2020). As per previous results, the anti-oxidant rich plant extract has excellent biomedical properties against multi drug resistant pathogens and cancer cells. Subsequently, the current result study of A. marina extract research may influence the biological properties against various infections after perform the invitro anti-infectious experiments.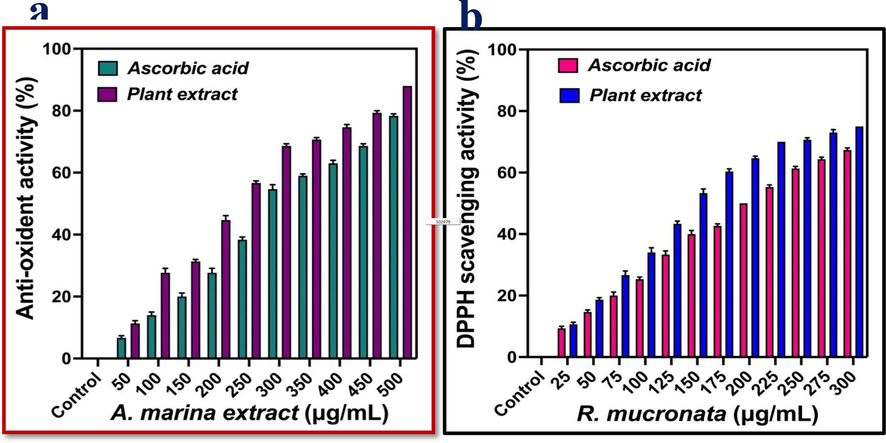
Anti-oxidant (a) and DPPH scavenging (b) properties of crude extract of Avicennia marina by various invitro assays.
3.3 Anti-bacterial activity study of A. marina
Based on the well diffusion, the result was clearly indicated that the A. marina extract has improved antibacterial activity against tested pathogens. When it performed against gram negative bacteria of K. pneumoniae, and P. aeruginosa, it was exhibited 22 and 26 mm zones were shown respectively at 250 µg/ml concentration (Fig. 3a, b). As same as against gram positive bacteria of S. aureus and Enterococcus sp. were shown 18 and 24 mm zones were observed respectively at 250 µg/ml concentrations (Fig. 3c, d). Subsequently, the minimum bactericidal result of minimum bactericidal concentration results were also confirmed from aliquot samples of A. marina extract in the muller hinton agar plates. As same as 250 µg/ml concentrations was shown no any bactericidal growth until 24 h were also observed for all the gram positive and gram negative bacteria. As per minimum inhibition concentration and minimum bactericidal concentration results were clearly suggested that the mangrove plant A. marina is excellent anti-bacterial agent against multi drug resistant bacteria. Also, the result evidences were conveyed to society, A. marina was excellent reservoir for anti-bacterial compounds against various gram positive and gram negative bacteria. The 250 µg/mL concentration is important concentration for plant mediated extract inhibit against pathogenic microbes. The various concentrations of A. marina extract against tested pathogens of gram positive and gram negative bacteria results were effectively drawn in Fig. 3e. Compared with earlier reports of marine mangrove A. marina and also other mangrove plants, this concentration and also inhibition zones were very usual. Researchers can be used frequently A. marina extract in future for drug discovery and also eradicate the multi-drug resistant effect in bacteria. May be, the nutritional and environmental factors such as carbon, nitrogen, phosphorous, NaCl, stress tolerance, temperature, growth hormones and pH may influence the extract nature and helped to improve the bioactivities against tested pathogenic microbes (Saenger and West, 2018). All the resulted evidences and previous reports were clearly supported to the A. marina extract and also it was most important plant in future drug discovery.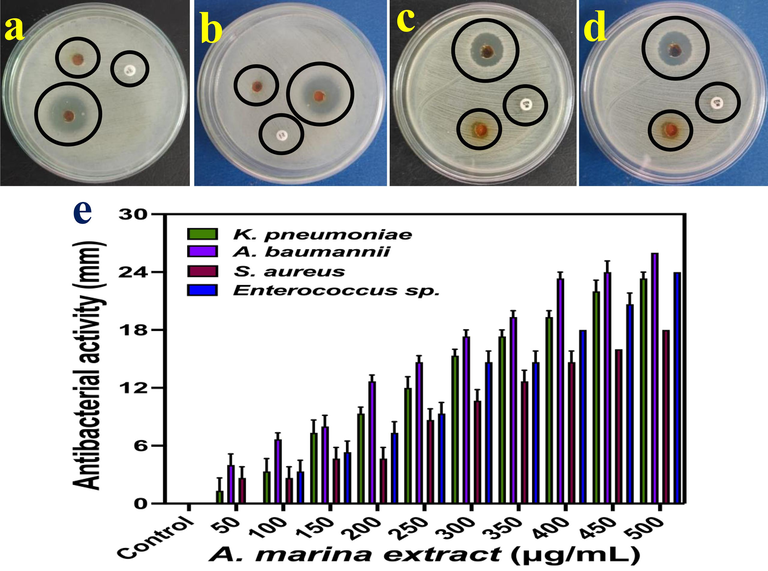
Anti-bacterial activity of Avicennia marina extract against K. pneumoniae (a), P. aeruginosa (b), S. aureus (c) and Enterococci sp. (d) and different concentration (e) by using agar well diffusion.
3.4 Confirmation of anti-bacterial property by minimum inhibition concentration assay
The well diffusion evidences of anti-bacterial activity was further cross checked and confirmed by minimum inhibition concentration assay using A. marina extract. Also their extract has excellent bioactivities against tested gram negative and gram positive bacteria at 500 µg/mL also cross checked. In result, after 24 h, more turbidity was seen in naked eye of 96-well plate and it started from 50 µg/mL concentration. After 12 h, the confluent layer formation was shown also clearly shown in 500 µg/mL concentrations. Both the results were conveyed important information, that the extract was inhibiting the bacteria at increasing concentration. In increasing concentration, the bacterial growth was decreased and death rate was very high in calculation. After made O.D values in spectrophotometer, the 78 %, 89 %, 81 % and 90 % of inhibition was observed at 500 µg/mL concentration against gram negative K. pneumoniae and P. aeruginosa (Fig. 4a) and gram positive S. aureus and Enterococcus sp. respectively. These results were suggested that the A. marina extract has anti-bacterial property in their nature and also has improved anti-bacterial activities. Subsequently, the minimum bactericidal concentration result of absence of microbial growth in the aliquot minimum inhibition concentration culture inoculated plates were drawn in 4b, c, d, e. Previously, other researchers reported that the A. marina damaged the bacterial structure at very high concentration only. So, minimum inhibition concentration result was agreed the anti-bacterial activity of well diffusion method, and it has excellent anti-bacterial property extract. The inhibition percentages were also very excellent, it may influence by unpredicted environmental parameters and stressed growth hormones emergence (Kathiresan et al., 2018). The phytochemical compounds may influenced by mangrove environmental factors and also plant growth and growth hormones are influenced by environmental parameters are very important in drug discovery nature (Shah et al., 2021).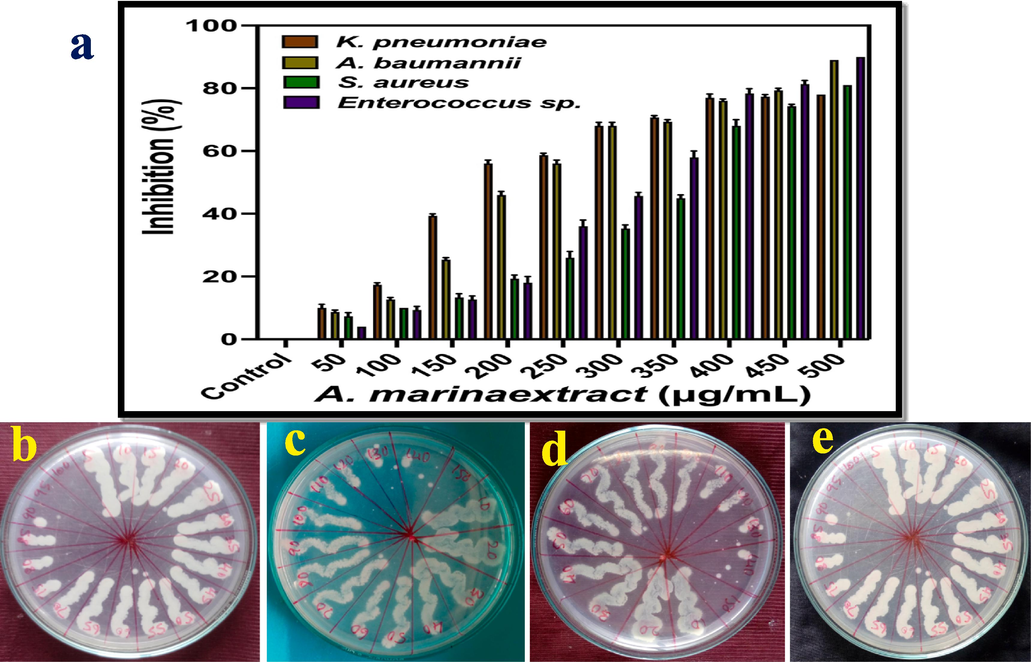
Minimum inhibition concentration (a) and minimum bactericidal concentration of Avicennia marina extract against K. pneumoniae (b), P. aeruginosa (c), S. aureus (d) and Enterococci sp. (e).
3.5 Minimum biofilm inhibition concentration
Inhibition of biofilm formation in the liquid culture format using 24-well plate in the presence of A. marina extract is the important experiment. In this current study of biofilm inhibition procedure, the modern crystal violet stain was used to detect the damaged growth of the bacterial cells using microtire plate analysis. In 24-well plate treatment, the entire architecture formed biofilm culture was destructed and sparse in and around the wells, and this is the prime factor to detect the initial detection of biofilm inhibition. After carful interpretation, the increasing concentration of the A. marina extract has been decrease the growth, and no growth cells were seen at 500 µg/mL concentration. At this concentration, the confluent layers were degraded in the surface of the liquid culture of 24-well plate. Instead, the control cell of mat structure on the surface of the liquid culture was indicated that the biofilm was formed. So, the interpretations of control and treatment evidences were indicated that the A. marina was disrupted the biofilm formation in K. pneumoniae, A. baumannii, S. aureus and Enterococci sp. Subsequently, the quantitative biofilm eradications at concentration dependent inhibition were shown at 500 µg/mL concentration. Also, the inhibition percentages of 92 %, 88 % 84 % and 90 % were effectively shown at 500 µg/mL concentrations. Also, the inhibition percentages of 52 %, 50 %, 54 % and 52 % was identified at the concentration of 300 µg/mL concentration (Fig. 5). Among the results, the biofilm inhibitions of this result were confirmed that the A. marina extract were indicated on the concentration dependent manner. Importantly, the bacterial pathogenicity was lost instead of antigenicity in this experiment, and it may confirm by without seeing absence of the mat formation when compared with mat formed control. Recently, Suvik et al. (2020) documented that the mat was indicated that the exopolysaccharide layer in and around the biofilm cells, it is the physical barrier to foreign particles including drugs and antibiotics. So, our A. marina was entered into the intracellular regions and disrupted the bacterial cellular layers. Finally, the current result was proved that the A. marina was effective anti-biofilm against multi drug resistant bacteria. Similar result was reported by recent researcher of Hassan et al. (2022), the biofilm inhibited A. marina is an effective drug candidate. Recently, - Eswaraiah et al. (2020) was agreed the statement of previous report and confirmed that the mangrove plant of A. marina is an excellent anti-biofilm plant.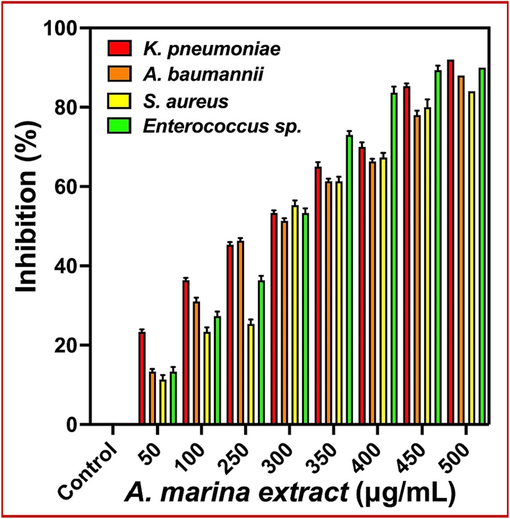
Minimum biofilm inhibition concentration of Avicennia marina extracts against multi drug resistant bacteria by 24-well polystyrene method.
3.6 Exopolysaccharide degradation in biofilm forming bacteria using A. marine extract
Exopolysaccharide is one of the important virulence factor in biofilm formation due to effective physical barrier. In addition, it is related to growing cells of the biofilm through supply of nutrients, stimulation of signaling molecules, production of budding and stimulation of cell cycle. In the current result, the complete exopolysaccharide based biofilm inhibition in A. marina treated 24-well was clearly shown at the concentration of 500 µg/mL concentration. When could see by visibly, the biofilm layer on the upper surface of the liquid culture in the 24-well plate was clearly shown in untreated control cells. Instead, the A. marina extract treated wells were shown clean and turbidity of the layers was shown, and also it was indicated, the biofilm cells were absent due to the influence of A. marina extract treatment. At the concentration of 500 µg/mL, the turbidity level was significantly decreased compared with previous concentration. As per the microtitre plate based lowest turbidity results were indicated 89 %, 94 %, 90 % and 86 % of biofilm inhibition was observed (Fig. 6). In addition, the inhibition results were conveyed, the exopolysaccharide was decreased and the virulence factors were completely arrested. As per similar statement of Janmanchi et al. (2017), the exopolysaccharide damage is the important factor for inhibition of biofilm formation in bacteria. Similar result was shown in the A. marina extract mediated nanoparticles when compared with other plant was shown effectively (Tian et al., 2020). Recently, Jha (2021); Parthiban et al. (2022), reported that the marine mangrove plant A. marina is the excellent anti-microbial and anti-biofilm inhibitor than any other plant.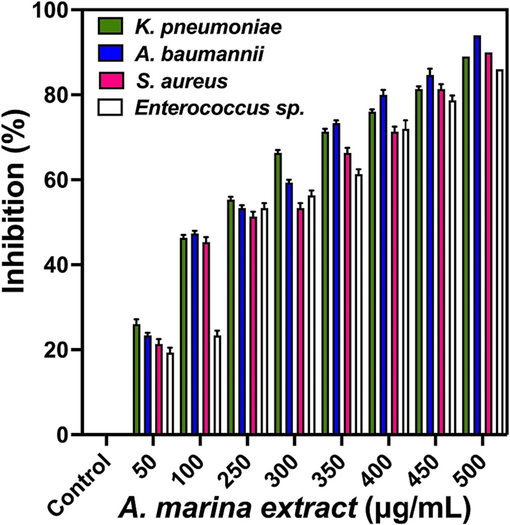
Exopolysaccharide degradation of Avicennia marina extracts against multi drug resistant bacteria by microtitre plate method.
4 Conclusion
Mangrove environment is an exceptional store factory of new secondary metabolites products, with structural and chemical characteristic generally not available in terrestrial bioactive metabolites. In addition, the unfavorable environmental nature and inter tidal changes were influenced microbes, plants and organisms to produce excellent bioactive metabolites. Based on the advantages, the current study was focused on the anti-oxidant and anti-biofilm rich mangrove plant A. marina was chosen to fight against multi drug resistant bacteria. Subsequently, the result of A. marina has excellent antioxidant and DPPH scavenging activities and confirmed by invitro assay. In addition, the available phyto compound derivatives, secondary metabolites and stress responded nutrients were shown excellent anti-biofilm effect. The well diffusion and minimum biofilm inhibition assay result was also suggested that the A. marina extract has excellent bio sources reservoir and it can be suitable alternative drug candidate plant for future drug discovery.
Acknowledgement
All the authors gratefully acknowledge the National Natural Science Foundation of China (Project Approval Numbers: 41950410573) and Postdoctoral Science Foundation of China (Project Approval Number: 2019M663213) for financial support for this work. G. Rajivgandhi and F. Quero acknowledge the financial support from ANID-FONDECYT (Chile) under the Postdoctoral Fellowship No. 3220019. The authors express their sincere appreciation to the Researchers Supporting Project Number (RSPD2023R679), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antibacterial activity and phytochemical content of A. marina collected from polluted and unpolluted site. J. Medicinal Plants Stud.. 2017;5:47-49.
- [Google Scholar]
- Antibacterial and invitro antioxidant potential of Indian mangroves. Biocatal. Agric. Biotechnol.. 2020;23:101491
- [Google Scholar]
- Indonesian Mangrove Sonneratia caseolaris leaves ethanol extract is a potential super antioxidant and anti methicillin-resistant Staphylococcus aureus drug. Molecules. 2022;27:8369.
- [Google Scholar]
- A metallothionein type 2 from A. marina binds to iron and mediates hydrogen peroxide balance by activation of enzyme catalase. Phytochemistry. 2020;176:112396
- [Google Scholar]
- Comparison of mangrove (A. marina) metal tissue concentrations to ambient sediment with an extensive range of contaminant levels in a highly-modified estuary (Sydney estuary, Australia) Marine Pollution Bull.. 2021;171:112680
- [Google Scholar]
- Isolation of antibacterials from the mangrove, A. marina and their activity against multi drug resistant Staphylococcus aureus. Asian Pacific J. Tropical Biomed.. 2012;2:S1892-S1895.
- [Google Scholar]
- Evaluation of carbon stock in the sediment of two mangrove species, A. marina and Rhizophora mucronata, growing in the Farasan Islands, Saudi Arabia. Oceanologia. 2020;62:200-213.
- [Google Scholar]
- Studies on phytochemical, antioxidant, antimicrobial analysis and separation of bioactive leads of leaf extract from the selected mangroves. J. King Saud Univ. – Sci.. 2020;32:842-847.
- [Google Scholar]
- Phytochemical composition of Avicennia marina leaf extract, its antioxidant, antimicrobial potentials and inhibitory properties on Pseudomonas fluorescens biofilm. Egypt. J. Aquat. Res.. 2022;48:29-35.
- [Google Scholar]
- Screening of phytochemical and antioxidant capacity of A. marina leaf extract from backwaters of muthukadu lake, Tamil nadu. IJRAR. 2019;6:1-10.
- [Google Scholar]
- Anti-biofilm activity of LC-MS based Solanum nigrum essential oils against multi drug resistant biofilm forming P. mirabilis. Saudi J. Biol. Sci.. 2021;28:302-309.
- [Google Scholar]
- Antituberculosis, antibacterial and antioxidant activities of Aegiceras corniculatum, a mangrove plant and effect of various extraction processes on its phytoconstituents and bioactivity. S. Afr. J. Bot.. 2017;113:421-427.
- [Google Scholar]
- Physicochemical properties, preliminary characterization, and assessment of potential bioactivities of polysaccharide purified from the leaves of A. marina. Biocatal. Agric. Biotechnol.. 2021;35
- [Google Scholar]
- Two new polyketides from endophytic fungus Pestalotiopsis sp. HQD-6 isolated from the Chinese mangrove plant Rhizophora mucronata. J. Asian Nat. Prod. Res.. 2022;24:52-58.
- [Google Scholar]
- Carbon sequestration and storage in planted mangrove stands of A. marina. Regional Stud. Marine Sci.. 2021;43:101701
- [Google Scholar]
- Green synthesis of iron oxide nanoparticles using A. marina flower extract. Vacuum. 2019;160:286-292.
- [Google Scholar]
- Biochemical markers for carbon sequestration in two mangrove species A. marina and Rhizophora mucronata. Beni-Suef Univ. J. Basic Appl. Sci.. 2018;7:733-739.
- [Google Scholar]
- Antibacterial and antioxidant activity of mangrove plant (Avicennia Marina) extract against some specific oral pathogens. Int. J. Health Sci.. 2022;6:1348-1355.
- [Google Scholar]
- Antibacterial and antioxidant potential of GC-MS analysis of crude ethyl acetate extract from the tropical mangrove plant Avicennia officinalis L. S. Afr. J. Bot.. 2021;142:149-155.
- [Google Scholar]
- Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculata. Food Chem.. 2007;104:300-307.
- [Google Scholar]
- Physico-chemical and functional characterization of polysaccharide purified from mangrove Rhizophora mucronata leaves having potent biological activity. S. Afr. J. Bot.. 2022;147:659-669.
- [Google Scholar]
- Antibacterial and antifungal activity of the extracts of different parts of Avicennia marina (Forssk.) Vierh. Plants. 2021;252:1-14.
- [Google Scholar]
- An integrative review on bioactive compounds from Indian mangroves for future drug discovery. S. Afr. J. Bot.. 2022;149:899-915.
- [Google Scholar]
- Antibiofilm activity of marine endophytic actinomycetes compound isolated from mangrove plant Rhizophora mucronata, Muthupet Mangrove Region, Tamil Nadu, India. J. Terr. Mar. Res.. 2018;2:1-7.
- [Google Scholar]
- anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumonia. Colloids Surf. A. 2020;600:124830
- [Google Scholar]
- Phytochemical screening and anti-oxidant activity of Sargassum wightii enhances the anti-bacterial activity against Pseudomonas aeruginosa. Saudi J. Biol. Sci.. 2021;28:1763-1769.
- [Google Scholar]
- Phenotypic variation of the mangrove species A. marina (Forssk.) Vierh from seven provenances around Australia. Aquat. Bot.. 2018;149:28-32.
- [Google Scholar]
- Antimicrobial and antioxidant chlorinated azaphilones from mangrove Diaporthe perseae sp. isolated from the stem of Chinese mangrove Pongamia pinnata. J. Asian Nat. Prod. Res.. 2021;23:1077-1084.
- [Google Scholar]
- Phytochemical profile and antibacterial activity of the mangrove plant avicennia officinalis L. Int. J. Curr. Res.. 2020;12:9973-9977.
- [Google Scholar]
- Genetic diversity of mangrove tree species A. marina in eco-geographic regions of Kerala coast, Southern India. Ecol. Genetics Genomics. 2021;20:100094
- [Google Scholar]
- Antibacterial and antioxidant activity of naphthofuranquinones from the twigs of tropical mangrove Avicennia officinalis. Nat. Prod. Res.. 2020;34:2403-2406.
- [Google Scholar]
- Microcosm study on cold adaptation and recovery of an exotic mangrove plant, Laguncularia Racemosa in China. Marine Environ. Res.. 2022;176:105611
- [Google Scholar]
- Anti-cancer activity of biosynthesized silver nanoparticles using A. marina against A549 lung cancer cells through ROS/mitochondrial damages. Saudi J. Biol. Sci.. 2020;27:3018-3024.
- [Google Scholar]
- Uptake of copper and chromium by A. marina and Avicennia alba at Wonorejo Estuary, East-coastal area of Surabaya, Indonesia. Regional Stud. Marine Sci.. 2021;47:101943
- [Google Scholar]
- The study on biological activity and molecular docking of secondary metabolites from Bacillus sp. isolated from the mangrove plant Rhizophora apiculata Blume, Regional Studies in Marine. Science. 2022;55:102583
- [Google Scholar]
- Phytochemical screening, GC-MS analysis and antibacterial evaluation of Ethanolic Leaves Extract of A. marina. J. Drug Delivery Therapeut.. 2019;9:145-150.
- [Google Scholar]







