Translate this page into:
Pyrazolo[3,4-b]pyridin-3(2H)-one derivatives: Synthesis and their investigation of mosquito larvicidal activity
⁎Corresponding author. idhayadhulla@nmc.ac.in (Akbar Idhayadhulla) a.idhayadhulla@gmail.com (Akbar Idhayadhulla)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, nitrogen-nitrogen bonds containing compounds such as hydrazine derivatives are examined. There are relatively few hydrazine molecules in nature, but some have been isolated from plants, marine organisms and microorganisms. Thus, hydrazine molecules are widely used in the manufacture of synthetic catalysts, agriculture chemicals, pesticides, and also cause irreversible pollution to air, water, and soil. Hydrazine compounds were evaluated via larvicidal profile in a stagnant water system. According to the above observation, new 1H-pyrazolo[3,4-b]pyridin-3(2H)-one derivatives can be synthesized via catalyst free green chemistry approach. A range of FT-IR spectroscopic measures, 1H and 13C NMR, as well as mass spectra, were used to characterize the compounds (2a-e). The compound 2e was more potent (95,6% mortality, LD50: 20.1 µg/ mL) against Culex quinquefasciatus than natural pyrazolidine derivatives. Therefore, the objective of this study, prospered with a few of the pyrzolopyridine hydrazine models, which are demonstrated to be low toxic environmentally safe and high potential larvicidal profile.
Keywords
Hydrazine
Pyrazolo[3,4-b]pyridin
Environmental toxicity
Larvicidal activity
1 Introduction
Environmental pollution, energy shortage and global warming pose serious challenges to sustainable development. Energy sources that are clean, renewable, and sustainable are imperative in solving these problems. Hydrazine is a chemical precursor to several pharmaceuticals and pesticides. Hydrazine is often converted to heterocyclic rings such as pyrazoles and pyridazines for these applications. In addition to insecticides, miticides, nematicides, fungicides, antiviral agents, and attractants, hydrazine compounds can also be used as herbicides and plant growth regulators.
Hydrazine organic matter: Organic matter increased the degradation rate of hydrazine (Eimoori et al., 2020). Alkali and neutral conditions make hydrazine solutions unstable, but in strongly acidic or oxygen-free conditions, they are relatively stable (Moliner and Street, 1989). In the hydrosphere, hydrazine decomposes primarily through biodegradation and autoxidation. Aqueous biodegradation is driven primarily by bacterial abundance in the water column (Jingqiu et al., 1994). Nitrogen gas is produced by autooxidation when four electrons are oxidized (Slonim and Gisclard, 1979). At this time, the effectiveness of auto-oxidation is determined by the following factors: copper ion concentration, mineral content, pH, and the content of organic matter in the water (Jingqiu et al., 1994).
Hydralazine is primarily degraded by oxidation in water, but biodegradation can also occur (Choudhary and Hansen, 1998). Hydralazine half-life is affected by aquatic conditions, and alkaline solutions and metal ions promote its degradation. Temperature, water hardness, organic matter concentration, and dissolved oxygen concentration are factors affecting degradation (Atkinson and Carter, 1984). As shown by the degradation rate of hydrazine in waters with diverse characteristics, this is evident. Water dissolved in a polluted source of hydrazine degraded by nearly 66% in 2 h, while water chlorinated for potable use degraded by roughly 90% after 1 day and almost completely no reduction at 4 days (Slonim and Gisclard, 1979). It is attributed to the low organic matter and hardness of the water that the potable water decays slower (Choudhary and Hansen, 1998). Hydrazine in pond water has been reported to have a half-life of 8.3 days. Femur samples have been extracted and enzymatic with hydrazine. It is known that the bones contain functional groups of carbonate (CO32−) and phosphate (PO43−), as evidenced by their characteristic bands.
Natural hydrazine: This review will focus on the studies of natural products containing the pyrazole nucleus and exploring and highlighting their potential since pyrazole nucleus is very important in medicinal chemistry (Vinod et al., 2013). Currently pyrazole-containing natural products include: withasomnine, pyrazofurin, formycin, oxoformycin, nostocine, and fluviol nostocine were produced by N2-fixing freshwater cyanobacteria, as shown in Fig. 1 (Hirata et al., 2004).![Larvicidal interaction of natural pyrazole and design target of pyrazolo[3,4-b]pyridine derivatives.](/content/185/2022/34/2/img/10.1016_j.jksus.2021.101767-fig1.png)
Larvicidal interaction of natural pyrazole and design target of pyrazolo[3,4-b]pyridine derivatives.
Sustainability of hydrazine: The reaction of hydrazine with NH3 (pathway 2) is thermodynamically favorable. The decomposition reaction is also influenced by the catalyst applied and conditions (such as temperature and pressure) (Agusta et al., 2010). Hydrogen production is reduced due to the formation of ammonia during hydrazine decomposition (Dai et al., 2017). Hydrazine is a dangerous and toxic compound, containing carcinogenic, poisonous, hazardous, cyanogenic, and nephrotoxic properties (Guo et al., 2010). Hydrazine hydrates are used in a variety of applications, including fungicides, regulators in plant growth, dyes and photographic chemicals, pharmaceuticals, agrochemicals, rocket fuel, spacecraft fuel, and explosives (Gholiv and Azadbakht, 2011). Hydrazine toxicity can cause irritation of the eyes and nose, loss of short-term vision, fainting, vomiting, respiratory edema, liver dysfunction, and unconsciousness (Casella et al., 1997). A skin absorption of hydrazine can lead to burning and impair blood flow (Salimi et al., 2008).
Mosquito larvicidal: Every year, mosquitoes transmit diseases such as malaria, filariasis, dengue fever, and yellow fever, which cause millions of deaths (James, 1992). Mosquito larvae are the vector of these diseases, and therefore the only way to prevent their transmission. A larvicidal chemical is typically applied continuously, such as organophosphates and insect growth regulators (Yang et al., 2002). Repetition of spraying these chemicals may cause non target populations to become resistant to them (Cheng et al., 2008). The Culex quinquefasciatus species is particularly responsible for vector-spread diseases. Larvicides are insecticides that kill larvae of insects. Methoprene inhibits larvae from developing significantly after pupa stage. The Culex quinquefasciatus mosquito vector is common to both urban and rural areas (Alvarez et al., 2006). Botanical insecticide studies have been conducted in recent years to come up with alternatives to synthetic insecticides (Scott, et al., 2003).
They are biodegradable, natural, low-toxicity, and biodegradable, and can be used as insecticide, larvicide, antifeedants, repellents, discouragement agents, and growth inhibitors (Isman, 2006). We cannot continue to use synthetic insecticides that cause environmental problems in soil, water, and air, and contaminated animals and food. 1H-pyrrolo[3,4-b]pyridin-5(2H)-one : Pyrazo[3,4-b]pyridin-3-ones are fused heterocyclic molecules that have antispasmodic (Paronikyan et al., 2001), cytotoxic (Manpadi et al., 2007) properties, and inhibit hormone-sensitive lipases as well.
Pyrazo[3,4-b]pyridines play an important role in pharmaceutical research, particularly as antimicrobials, antimalarials, antivirals, antiproliferative, anticoagulant, hypotensive, and antiarrhythmics (Foks et al., 2005). Pyrazo[3,4-b]pyridines have been reported to be condensed with a type of microwave-assisted synthesis (Dyadyuchenko and Dmitrieva, 2020).
There is a broad spectrum of biological activity in the 1H-pyrazolo[3,4-b]pyridine framework (Fig. 1) (Beutner et al., 2009). In the past decade, studies have demonstrated that 1H-pyrazolo[3,4-b]pyridines possess antiviral properties, antitumor (Straub et al., 2002), anti-inflammatory (Tucker et al., 2008), and antimicrobial (Ye et al., 2009). Besides herbicides and fungicides, pyrazoles are also used as agrochemicals (Giornal et al., 2013). Mosquitoes are controlled primarily by chemical insecticides (Bowman et al., 2016), due to their inherently toxic properties and non-target effects, they are difficult to use (Jayaraj et al., 2016).
A variety of larval active targets have been published previously, however chemical insecticides pose greater challenges due to resistance development and disruption of the natural biological control system (Yang et al., 2013). In order to overcome these problems, we have developed pyrrolidine derivatives as new mosquito larvae inhibitors through green technologies.
2 Materials and methods
Chemicals were bought from Sigma-Aldrich Chemicals. Shimadzu 8201pc records FT IR (4000–400 cm−1). Recordings of 1H and 13C NMR via BRUKER- (300 MHz & 75 MHz, respectively) in DMSO‑d6. Fluorescence indicators were used for purity checking by thin layer chromatography.
2.1 Procedure for synthesizing compound 1a-1e
A reaction mixture, pyrazolidine-3,5-dione (0.01 mmol, 1.0 mg), and phenylhydrazine (0.01 mmol, 1.08 mg) were mixed and heat with 100 °C to gives 1a compound 5-(2-phenylhydrazinyl)-1H-pyrazol-3(2H)-one with 98% of yield. The above same method followed from compounds (1b-e). The above procedure was followed for compounds (1b-e) as well.
2.2 Procedure for synthesizing compounds 2a-2e
The compound 1a (0.01 mol, 1.90 mg) was reacted with cinnamaldehyde (0.01 mol, 1.32 mg) in ethanol medium with reflux 10 hr at RT. After that mixture was filtered, solid material was obtained, then washed with distilled water for soiled materials. By using column chromatography (Ethyl acetate 4: hexane 6), the final solid material has been separated. The above procedure was followed for compounds (2b-e) as well.
2.3 7-benzyl-6-phenyl-6,7-dihydro-1H-pyrazolo[3,4-b]pyridin-3(2H)-one (2a)
Yellow solid; yield 87%; mw: 303.36; Solubility in water: 4.23 g/L (25 °C); mp: 198 °C; IR (cm−1): 3358 (NH), 3174 (Ar-H), 2724 (Ph-CHstr), 1625 (C⚌O); 1H NMR (300 MHz): δ 9.34 (1H, s, NH), 7.48 (1H, s, NH), 7.33 (2H, t, J = 6.23, Ph), 7.22(1H, t, J = 6.23 Hz, Ph), 7.21(2H, d, J = 6.21 Hz, Ph), 7.33 (t, 2H), 7.24(t, 1H, J = 6.23 Hz), 7.21(d, 2H, J = 6.21 Hz), 6.25 (d, 1H, J = 6.21 Hz), 6.17 (d, 1H, J = 6.21 Hz), 4.59 (1H, s, CH-Ph), 3.81 (s, 2H); 13C NMR (300 MHz): 163.7, 167.1, 119.4, 116.4, 84.4, 138.1, 128.5, 127.9, 127.0, 136.1, 128.5, 127.9, 127.0, 60.5, 50.4 (1C, –CH2); EI-MS (m/z): 304.14 (M+,21%); Elemental analysis: Calcd. For (C19H17N3O): C, 75.23; H, 5.65; N, 13.85%; Found: C, 75.20; H, 5.66; N, 13.84%.
2.4 7-(benzylideneamino)-6-phenyl-6,7-dihydro-1H-pyrazolo[3,4-b]pyridin-3(2H)-one (2b)
Yellow solid; yield 89% ; mw: 316.36; Solubility in water: 3.87 g/L (25 °C); mp: 158 °C; IR (cm−1): 3354 (NH), 3170 (Ar-H), 2720 (Ph-CHstr), 1621 (C⚌O), 1540 ; 1H NMR (300 MHz): δ 9.32 (s, 1H, NH), 8.52(s, 1H), 7.46 (s, 1H, NH), 7.33 (2H, t, J = 6.23), 7.26 (t, 1H, J = 6.23 Hz), 7.23 (d, 2H, J = 6.21 Hz), 7.83 (t, 2H, J = 6.23 Hz), 7.54 (m, 3H), 6.25 (d, 1H, J = 6.21 Hz), 6.17 (d, 1H, J = 6.21 Hz), 4.59 (1H, s, CH-Ph); 13C NMR (300 MHz, DMSO‑d6): 163.3, 164.1, 146.9, 119.1, 116.2, 88.4, 143.5, 128.1, 126.2, 125.1, 133.5, 131.2, 129.8, 128.1, 63.2 (1C, –CH-Ph); EI-MS (m/z): 317.14 (M+,21%); Elemental analysis: Calcd. For (C19H16N4O): C, 72.13; H, 5.10; N, 17.71%; Found: C, 72.10; H, 5.12; N, 17.70%.
2.4.1 6-phenyl-7-((3-phenylallylidene)amino)-6,7-dihydro-1H-pyrazolo[3,4-b]pyri din −3-(2H)-one (2c)
Yellow solid; yield 91% ; mw: 342.39; Solubility in water: 3.98 g/L (25 °C); mp: 184 °C; IR (cm−1): 3356 (NH), 3172 (Ar-H), 2722 (Ph-CHstr), 1623 (C⚌O), 1542; 1H NMR (300 MHz): δ 9.30 (s, 1H), 7.50 (s, 1H, –CH = N), 7.42(s, 1H), 7.33 (t, 2H, J = 6.23), 7.26 (t, 1H, J = 6.23 Hz), 7.23 (d, 2H, J = 6.21 Hz, Ph), 7.60 (2H, t, J = 6.23 Hz), 7.60 (2H, d, J = 6.21 Hz), 7.33 (1H, t, J = 6.23 Hz, Ar), 7.22 (s, 1H), 6.81 (d, 1H, J = 6.21 Hz, =CH), 6.25 (d, 1H, J = 6.21 Hz), 6.17 (d, 1H, J = 6.21 Hz), 4.56 (s, 1H); 13C NMR (300 MHz): 163.7, 137.2, 164.1, 134.1, 126.3, 119.9, 116.0, 88.4, 142.9, 129.1, 127.1, 126.8, 135.2, 127.9, 128.4, 127.2, 63.3; EI-MS (m/z): 343.15 (M+,24%); Elemental analysis: Calcd. For (C21H18N4O): C, 73.67; H, 5.30; N, 16.36%; Found: C, 73.65; H, 5.28; N, 16.38%.
2.4.2 7-((3,7-dimethylocta-2,6-dien-1-ylidene)amino)-6-phenyl-6,7-dihydro-1H-pyra zolo [3,4-b]pyridin-3(2H)-one (2d)
Yellow solid; yield 95%; mw: 362.47; Solubility in water: 2.58 g/L (25 °C); mp: 166 °C; IR (cm−1): 3360, 3176 (Ar-H), 2726 (Ph-CHstr), 1627, 1546; 1H NMR (300 MHz): δ 9.26(s, 1H), 7.42(s, 1H, –CH⚌N), 7.38(s, 1H), 7.33 (t, 2H, J = 6.23), 7.26 (t, 1H, J = 6.23 Hz), 7.23 (d, 2H, J = 6.21 Hz), 6.25 (d, 1H, J = 6.22 Hz), 6.15(1H, d, J = 6.22 Hz), 5.20 (1H, d, J = 6.21 Hz), 4.81 (1H, d, J = 6.21 Hz, =CH), 4.59 (s, 1H), 2.0 (4H, m, –CH2), 1.80 (s, 6H), 1.70 (s, 3H); 13C NMR (300 MHz, DMSO‑d6): 163.1, 137.2, 164.1, 150.8, 132.6, 124.1, 122.4, 119.0, 116.2, 88.4, 143.3, 127.1, 126.1, 125.2, 63.0, 38.9, 25.9, 25.1, 18.9, 17.9; EI-MS (m/z): 363.21 (M+,25%); Elemental analysis: Calcd. For (C22H26N4O): C, 72.90; H, 7.23; N, 15.46%; Found: C, 72.92; H, 7.21; N, 15.45%.
2.4.3 7-((3-methylbut-2-en-1-ylidene)amino)-6-phenyl-6,7-dihydro-1H-pyrazolo[3,4-b]pyridine-3(2H)-one (2e)
Yellow solid; yield 88%; mw: 294.35; Solubility in water: 4.21 g/L (25 °C); mp: 178 °C; IR (cm−1): 3361, 3177, 2727, 1628, 1547 ; 1H NMR (300 MHz): δ 9.24 (s, 1H), 7.50 (1H, s, –CH⚌N), 7.38 (s, 1H, NH), 7.33 (t, 2H, J = 6.23), 7.24(t, 1H, J = 6.23 Hz), 7.23 (d, 2H, J = 6.21 Hz), 6.25 (1H, d, J = 6.21 Hz), 6.15 (1H, d, J = 6.21 Hz), 4.80 (1H, s), 4.56 (s, 1H, CH-Ph), 2.15 (3H, s), 1.93(3H, s); 13C NMR (300 MHz): 163.5, 137.2, 164.1, 151.1, 123.2, 119.2, 116.0, 88.4, 142.9, 128.4, 125.8, 125.8, 63.9, 26.8, 20.8; EI-MS (m/z): 295.15 (M+,20%); Elemental analysis: Calcd. For (C17H18N4O): C, 69.37; H, 6.16; N, 19.03%; Found: C, 69.35; H, 6.14; N, 19.05%.
2.5 Biological activities
2.5.1 Larvicidal activity
Culex quinquefasciatus eggs were collected from the drainage water. A method described previously (Abdel-Fattah Mostafa et al., 2019) was used to deviate test compounds at concentrations of 10, 25, 50, and 100 µg/mL. Cotton buds were used to remove excess water from larvae collected with a pasteur pipette. By calculating the ratio of dead to live larvae, the compounds were calculated to cause mortality in larvae (%). Probit analysis was used to calculate the LD50 values.
The number of larvae still alive after 24 h was counted. Statistically, the results had to be repeated three times.
3 Results
The grindstone method was used to synthesize the pyrazolo[3,4-b]pyridin-3(2H)-one derivatives using ethanol medium. The reaction is a [3 + 2] cycloaddition with an 85–92 % yield. Scheme 1 shows that synthetic route outline for pyrazolo[3,4-b]pyridin-3(2H)-one derivatives (1a-e, 2a-2e). As a result of column chromatography, the final product was characterized by FT-IR, 1H NMR, 13C NMR, and mass spectrometry. A reaction mixture, pyrazolidine-3,5-dione reacted with phenylhydrazine was heat with 100 °C to gives 1a compoumd 5-(2-phenyl hydrazinyl) −1H-pyrazol-3(2H)-one with 98% of yield. The compound 1a was treated with cinnamaldehyde in EtOH medium with reflux 10hr at RT to give compound 2a. After that mixture was filtered, solid material was obtained, then washed with distilled water for soild materials. From column chromatography (Ethyl acetate4:hexane6), the final solid material was separated. Compounds 1b-1e and 2b-2e were prepared using the same method. The mothod of preapration easy way for syntheed all reamining compounds and yield such as 91, 89, 90, 92 % respectivily. The compoumd 2a-e where chaterized by FT-IR, the strching frequincy for –NH-CO–, NH, –CH–, and Ar-CHstr corresponding to 1621–1621 cm−1, 3354–3361 cm−1, 2727–2720 cm−1 and 3170–3177 cm−1 respectivily. The 1H NMR spectarl inticated that –CO-NH, NH, and –CH– chemical shift values correspoding of 7.38–7.48, 9.28–9.34, 4.56–4.59 ppm respectivily. 13C NMR spectra of carbon group presence such as –C⚌O-, –HC⚌CH–, and –CH corresponding to 163.1–163.7, 119.0–119.9, 116.0–116.4, and 60.5–63.9 ppm respectivity.![Synthedic route of pyrazolo[3,4-b]pyridin-3(2H)-one derivatives (1a-e, 2a-2e).](/content/185/2022/34/2/img/10.1016_j.jksus.2021.101767-fig2.png)
Synthedic route of pyrazolo[3,4-b]pyridin-3(2H)-one derivatives (1a-e, 2a-2e).
The larvicital activity value shows that Table 1, as an indication of the result of compound compared with natural larvicidal compounds and pyrazolidine-3,5-dione, the compound 1a has very low activity and no effect in 50 µg/mL concentration.
Compounds
Mortality (%)
LD50
25 µg/mL
50 µg/mL
100 µg/mL
(µg/mL)a
pyrazolidine-3,5-dione
05.2 ± 0.3
–
–
>100
1a
09.14 ± 0.1
12.14 ± 0.1
12.14 ± 0.1
>100
1b
08.14 ± 0.1
18.16 ± 0.7
35.11 ± 0.4
>100
1c
0.0 ± 0.0
14.28 ± 0.7
30.74 ± 0.2
>100
1d
0.0 ± 0.1
12.14 ± 0.1
23.09 ± 0.3
>100
1e
11.14 ± 0.1
22.10 ± 0.3
43.04 ± 0.2
>100
2a
44.1 ± 0.1
63.3 ± 0.3
82.2 ± 0.6
31.3
2b
42.2 ± 0.3
69.1 ± 0.1
88.6 ± 0.2
29.9
2c
46.3 ± 0.1
72.3 ± 0.2
92.3 ± 0.2
23.5
2d
49.1 ± 0.1
82.2 ± 0.3
100 ± 0.4
15.4
2e
47.3 ± 0.2
76.5 ± 0.5
95.6 ± 0.3
20.1
The compound 1a-1e were obtained LD50: >100 µg/mL, it shows that very low active even 100 µg/mL also not reached 50% activity, whereas the compound containing pyrazolo [3,4-b]pyridin-3(2H)-one 2a-2e has significant of activity, based on the data for compound 2a, 82.2% showed activity at 100 µg/mL and LD50 was 31.3 µg/mL. With the compound 2b, 88.6% of the activity was at 100 µg/mL and the LD50 was 29.9 µg/mL and the compound 2b shows that 88.6% of activity reached 100 µg/mL with LD50: 29.9 µg/mL. For compound 2c, 92.3% of activity reached 100 µg/mL with an LD50 of 23.5 g/mL, and for compound 2d, 100% reached 100 µg/mL with an LD50 of 15.4 µg/mL. Compound 2e displays 95.6% on the activity scale with LD50 of 20.1 µg/mL. The compounds were all highly active when compared with environmentally benign compounds.
4 Discussion
Fig. 2 shows mortality between natural and synthetic hydrazide at 100 µg/mL concentration analysis. Natural products such as withasomnino, pyrazofurin, formycin B, nostocine A, Fluviol A, were observed at 12 – 33 % of activity and low toxicity in the water system, whereas larvicidal also had very low activity. Withasomnine (pyrazole alkaloid), first isolated from withania somnifera Dun, the root bark of an Indian medicinal plant, shows 10% mortality in larvicidal screening (Schroter et al., 1966); which larvicidal activity ranges 22% mortality in stagnant water.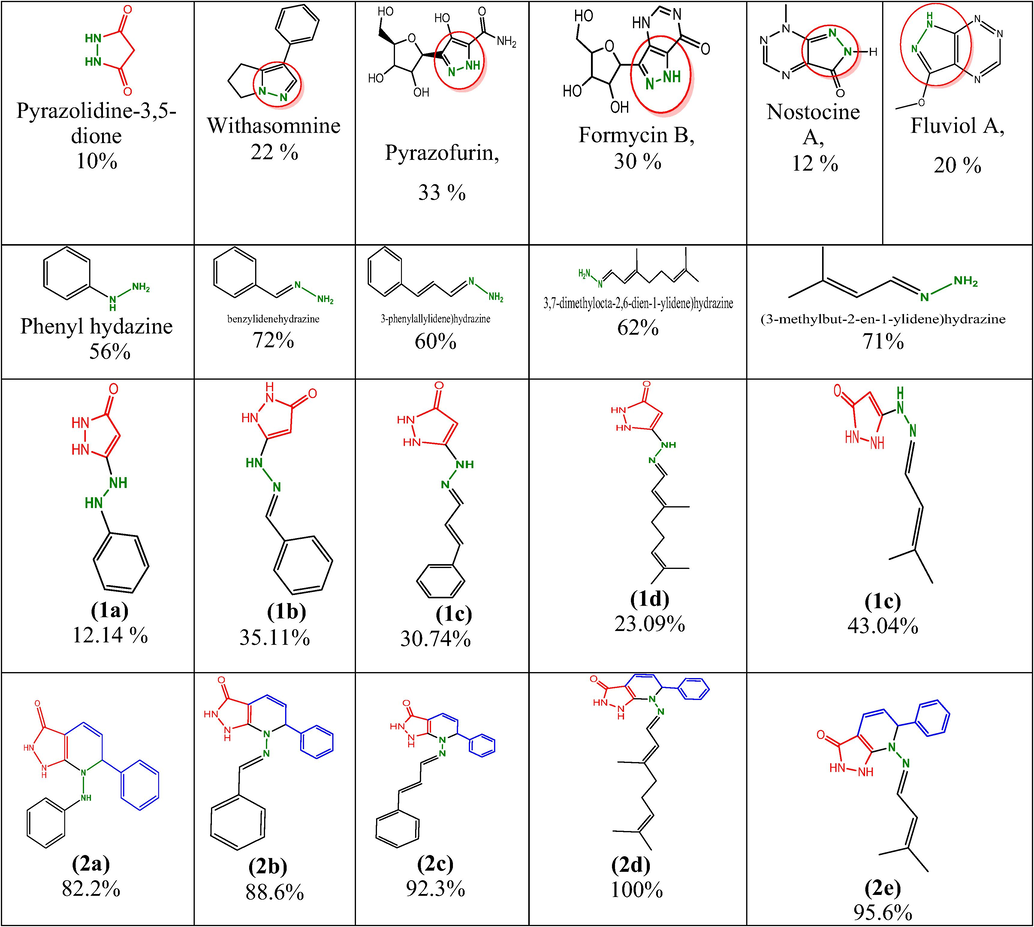
Analyaia mortality butween natural and synthedic hydarzide at 100 µg/mL.
Pyrazofurin (Pyrazomycin) is a nucleoside analogue found in Streptomyces candidus, and is related to ribavirin (Canonico et al., 1982) which obtained 30% of mortality against larvicidal activity in stagnant water samples. The compound formycin B (FB) is also an effective inhibitor of bacterial (Escherichia coli) enzymes because it is a moderate inhibitor of mammalian purine nucleoside phosphorylase (PNP) (Bzowska et al., 1992), which performed larvicidal activity 30% mortality in stagnant water systems. The secondary metabolites of cyanobacteria (Patterson et al., 1994) are useful for agriculture or medicine. While the uses of cyanobacterial metabolites in the natural environment haven't been studied extensively, some of them act as toxins or alkylating agents (Pflungmacher, 2002). In a study by Kelly (Kelly et al., 2006), the structures of 2 pigments of this group were clearly delineated, namely nostocine A and fluviol A, with larval mortality profiles of 12–20%, respectively. An evaluation of the ovicidal, larvicidal, and repellent properties of Cinnamomum verum (CV) extracts against mosquito vectors of Culex quinquefasciatus and also cinnamaldehyde showed that 83.53% of larvicidal activity (Nakasen et al., 2021). The citral showed (33.50%) marked larvicidal activity against Cx. pipiens quinquefasciatus (Yang et al., 2005). Based on above report, which is compared with phenylhydrazine 56% mortality reached but very high risk in environmental toxicity whereas phenyl hydrazine connected with pyrazol-3(2H)-one, which obtained low mortality same time toxicity range also reduced, as a requirement only target for mosquito larvae same time that need low toxic compounds, so that we design the pyridine moiety connected with pyazolidne, the preparation of pyrazolidine conned with pyridine via green chemistry approach for synthesis of cinnamaldehyde reacted with 5-(2-phenyl hydrazinyl)-1H-pyrazol-3(2H)-one to give high yield (87%) for compound 2a, which reached morality range 82.2% whereas very low toxicity in water system. As same way remaining compounds 2b also reached 88.6 % of activity when compared with 5-(2-benzylidenehydrazinyl)-1H-pyrazol-3(2H)-one 35% of and also benzylidenehydrazine reached 72% of mortality. The compounds 2c also reached 92.3 % of activity when compared with 5-((2-((3-phenyl allylidene)hydrazinyl)-1H-pyrazol-3(2H)-one 35% of and also 3-phenylallylidene)hydrazine reached 92.3% of mortality. The compound 2d also reached 100% of activity when compared with 5-((2-(3,7-dimethy locta-2,6-dien-1-ylidene)hydrazinyl)-1H-pyrazol-3(2H)-one 23% of and also 3,7-dimethylocta-2,6-dien-1-ylidene)hydrazine 62% reached 92.3% of mortality. The compound 2e also reached 95.6% of activity when compared with 5-(2-(3-methylbut-2-en-1–ylidene)hydrazinyl)-1H-pyrazol-3(2H)-one (43.04%) of and also (3-methylbut-2-en-1-yli dene)hydrazine hydrazine (71%) reached for mortality.
5 Conclusions
This study identified the most effective and easily prepared 1H-pyrazolo[3,4-b]pyridin-3(2H)-one derivatives using the [3 + 2] cycloaddition grindstone method, with high yields. It was investigated whether these compounds could be used as larvicides against Culex quinquefasciatus. Based on the screening of 10 compounds (1a-e), and (2a-e), compound 2e was found to be most active against Culex quinquefasciatus (95% mortality rate, LD50 = 12.09 µg/mL). The results of this study indicate that compound 2e has the best larvicidal activity and that the compounds described here could be used to develop eco-friendly pesticides and biopharmaceuticals.
Funding
This research received no external funding.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work through Research Group no. RG-21-09-87.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of novel benzopyran- connected pyrimidine and pyrazole derivatives via a green method using Cu(II)-tyrosinase enzyme catalyst as potential larvicidal, antifeedant activities. RSC Adv.. 2019;9:25533-25543.
- [Google Scholar]
- Resistance to Temephos in populations of Aedes aegypti (Diptera: Culicidae) of the west of Venezuela. Rev. Colomb. Entomol.. 2006;32:172-175.
- [Google Scholar]
- Kinetics and mechanisms of the gas-phase reactions of ozone with organic compounds under atmospheric conditions. Chem. Rev.. 1984;84:437-470.
- [Google Scholar]
- Expedient synthesis of 3- alkoxymethyl- and 3-aminomethyl-pyrazolo[3,4-b]pyridines. J. Org. Chem.. 2009;74:789-794.
- [Google Scholar]
- Is dengue vector control deficient in effectiveness or evidence? systematic review and metaanalysis. PLoS Negl. Trop. Dis.. 2016;10(1):e0004551-e4624.
- [Google Scholar]
- Formycins A and B and some analogues: selective inhibitors of bacterial (Escherichia coli) purine nucleoside phosphorylase. Biochim. Biophys. Acta.. 1992;17:239-247.
- [Google Scholar]
- Human health perspective on environmental exposure to hydrazines: a review. Chemosphere. 1998;37(5):801-843.
- [Google Scholar]
- Catalytic oxidation and flow detection of hydrazine compounds at a nafion/ruthenium(III) chemically modified electrod. Analytica Chim. Acta.. 1997;354:333.
- [Google Scholar]
- Larvicidal activity of tectoquinone isolated from red heartwood type Cryptomeria japonica against two mosquito species. Bioresour. Technol.. 2008;99:3617-3622.
- [Google Scholar]
- Antiviral efficacy of pyrazofurin against selected RNA viruses. Antiviral Res.. 1982;2(6):331-337.
- [Google Scholar]
- Contribution of Azolla filiculoides to hydrazine elimination from water. Wetlands Ecol Manage. 2020;28:439-444.
- [Google Scholar]
- Kinetics of catalytic decomposition of hydrous hydrazine over CeO2-supported Ni–Pt nano catalysts. Int. J. Hydrogen Energy.. 2017;42:5684-5693.
- [Google Scholar]
- Microwave-assisted synthesis of pyrazolo[3,4- b]pyridine derivatives (microreview) Chem. Heterocycl. Comp.. 2020;56:1414-1416.
- [Google Scholar]
- Synthesis and antibacterial activity of 1H- pyrazolo[3,4-b]pyrazine and -pyridine derivatives. Il Farmaco. 2005;60:513.
- [Google Scholar]
- Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles Phy. Chem. C.. 2010;114:21390.
- [Google Scholar]
- A novel hydrazine electrochemical sensor based on a zirconium hexacyanoferrate film-bimetallic Au–Pt inorganic–organic hybrid nanocomposite onto glassy carbon-modified electrode. Electrochim. Acta.. 2011;56:10044.
- [Google Scholar]
- Synthesis of diversely fluorinated pyrazoles as novel active agrochemical ingredients. J. Fluorine Chem.. 2013;152:2-11.
- [Google Scholar]
- Generation of reactive oxygen species undergoing redox cycle of nostocine A: a cytotoxic violet pigment produced by fresh water cyanobacterium nostoc spongiaeforme. J. Biotechnol.. 2004;110:29-35.
- [Google Scholar]
- Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol.. 2006;51:45-66.
- [Google Scholar]
- On degradation regulation of hydrazine hydrate in wastewater. Water Treat.. 1994;9:299-304.
- [Google Scholar]
- Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol.. 2016;9:90-100.
- [Google Scholar]
- Mosquito molecular genetics: the hands that feed bite back. Science. 1992;257:37-38.
- [Google Scholar]
- Synthesis of the pyrazolo[4,3-e][1,2,4]triazine family of natural products: nostocine A, fluviol A, and pseudoiodinine. J. Am. Chem. Soc.. 2006;128:5646-5647.
- [Google Scholar]
- Three-component synthesis and anticancer evaluation of polycyclic indenopyridines lead to the discovery of a novel indenoheterocycle with potent apoptosis inducing properties. Org. Biomol. Chem.. 2007;5:3865.
- [Google Scholar]
- Decompostion of hydrazine in aqueous solutions. J. Environ. Qual.. 1989;18:483-487.
- [Google Scholar]
- io efficacy of Cinnamaldehyde from Cinnamomum verum essential oil against Culex quinquefasciatus (Diptera: Culicidae) J. Ento. Acaro. Res.. 2021;53:9400.
- [Google Scholar]
- Synthesis and anticonvulsant activity of pyrazolo[3,4-b]pyrano(thiopyrano)[4,3-d]pyridine and pyrazolo[3,4- c]isoquinoline derivatives. Pharm. Chem. J.. 2001;35:8-10.
- [Google Scholar]
- Bioactive natural products from blue- green algae. J. Appl. Phycol.. 1994;6:151-157.
- [Google Scholar]
- Posssible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosysterms. Environ. Toxical.. 2002;17:407-413.
- [Google Scholar]
- Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta. 2008;75:147.
- [Google Scholar]
- Botanical insecticides for controlling agricultural pests: piperamides and the Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae) Arch. Insect Biochem.. 2003;54:212-225.
- [Google Scholar]
- Metabolites of orally active NO-independent pyrazolopyridine stimulators of soluble guanylate cyclase. Bioorg. Med. Chem.. 2002;10:1711-1717.
- [Google Scholar]
- Hydrazine degradation in aquatic systems. Bull Environ. Contam. Toxicol.. 1979;16:301-309.
- [Google Scholar]
- Discovery of 3-{5-[(6-amino-1H- pyrazolo[3,4-b]pyridine-3-yl) methoxy]-2- chlorophenoxy}-5-chlorobenzonitrile (MK-4965): a potent, orally bioavailable HIV-1 non- nucleoside reverse transcriptase inhibitor with improved potency against key mutant viruses. J. Med. Chem.. 2008;51:6503-6511.
- [Google Scholar]
- Pyrazole containing natural products: synthetic preview and biological significance. Euro. J. Med. Che.. 2013;69:735-753.
- [Google Scholar]
- Constituents of volatile compounds derived from Melaleuca alternifolia leaf oil and acaricidal toxicities against house dust mites. J. Korean Soc. App. Biol. Chem.. 2013;56:91-94.
- [Google Scholar]
- Piperidine amide extracted from Piper long um L. fruit shows activity against Aedes aegypti mosquito larvae. J. Agric. Food. Chem.. 2002;50:3765-3767.
- [Google Scholar]
- Adulticidal Activity of Five Essential Oils against Culex pipiens quinquefasciatus. J. Pestic. Sci.. 2005;30(2):84-89.
- [Google Scholar]
- Synthesis and evalua, tion of novel 7-azaindazolyl-indolyl-maleimide derivatives as antitumor agents and protein kinase C inhibitors. Bioorg. Med. Chem.. 2009;17:4763.
- [Google Scholar]







