Translate this page into:
Impact of Cinnamomum verum against different Escherichia coli strains isolated from drinking water sources of rural areas in Riyadh, Saudi Arabia
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The present study was aimed to evaluate the antimicrobial activity of aqueous, methanolic and essential oil extracts of Cinnamomum verum (cinnamon) against two waterborne pathogenic strains isolated from bottled, tap and wells’ water from the rural areas of Riyadh, Saudi Arabia.
Methods
The water samples were drawn from different sources and two strains of Escherichia coli were isolated, purified and confirmed using the MALI-TOF-MS technique. The C. verum extraction was carried out using different solvents and essential oil by hydrodistillation according to the standard methods. The antimicrobial potential was evaluated using a well-diffusion assay and percentage inhibition was calculated.
Results
The two isolates were identified as Escherichia coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB by MALDI-TOF-MS. Aqueous, methanolic and oil extracts of cinnamon were tested for their inhibitory activity against the selected strains using the well diffusion method. E. coli strains were more sensitive towards the essential oil extract with the inhibitory zone of 4.5–5.2 cm rather than the aqueous and methanolic extracts. Analysis of cinnamon essential oil by GCMS showed the presence of Cinnamic acid, Cinnamaldehyde and 3-Allyl-6-methoxyphenol in the essential oil.
Conclusion
The results indicated the possible potential of Cinnamomum verum essential oil and extracts in the management of E. coli from different water sources. Possibly, the bioactive compounds including cinnamic acid derivatives may responsible for the bioactivities of the plant.
Keywords
Cinnamon oil
Antimicrobial activity
MALDI-TOF-MS
GCMS spectrometry
Well diffusion method
Saudi Arabia
1 Introduction
Waterborne disease is a universal burden that is determined to cause more than two million deaths per year, including diarrhoea, gastrointestinal diseases and systemic illnesses (WHO, 2015; Bitton, 2014). Causes of waterborne diseases take place by ingestion of microbes, airborne transmission or direct contact with viruses, bacteria, protozoa and helminths found in contaminated water (Leclerc et al., 2002). Unfortunately, more than 800 million people are under the risk of drinking polluted water and they do not even have access to clean and drinkable water (Ramírez-Castillo et al., 2015). As reported by (WHO, 2008), enteric pathogens have developed a gradual increase in their antimicrobial resistance which is characterized as a worldwide major concern. Also, as in the report of WHO in 2015, the decision-makers have noted that by improving the water quality; we could save millions of people around the world. The improvement of bacterial resistance to currently available antibiotics is indeed needed to search for new antibacterial agents. Gram-negative bacteria such as E. coli is present in the human intestine and causes infantile diarrhoea, lower urinary tract infection, coleocystis or septicemia (Surbatovic et al., 2015).
Cinnamon species bark is considered as one of the most important species used all over the world for traditional medicine (Błaszczyk, et al., 2021). It belongs to kingdom Plantae, Division: Magnoliophyta and belongs to the Lauraceae family, representing 82.5% of Cinnamon bark total composition (Rao and Gan, 2014; Paliwal et al., 2018). Cinnamon consists of unique components including cinnamaldehyde, cinnamic acid, cinnamate and essential fatty acids such as Eugenol, L-borneol, L-bornyl acetate –Enerolidol, Terpinolene which give its spicy taste and fragrance (Wariyapperuma et al., 2020). The major components of Cinnamon essential oil are trans-cinnamaldehyde or cinnamaldehyde (Zhang et al., 2016). One of the applications of cinnamon in the food industry, its addition to chewing gum as a bad breath removal because of its refreshing effects, (Rao and Gan, 2014). Cinnamon is used as a coagulant as it prevents bleeding and also, it increases blood circulation (Mahmoodnia et al., 2017). Cinnamon has also been used as tooth powder to treat toothaches- dental problems, oral microbiota (Zouheyr et al., 2014). Also, they respond to free radicals and alleviates metabolic diseases of humans and other animals due to the presence of Eugenol and linalool oils (Mohamed et al., 2020). Cinnamon acts as an anti-inflammatory agent due to the presence of flavonoid compounds such as gossypin, gnaphalin, hesperidin, hibifolin and oroxindin which suppress the expression of inducible nitric oxide synthesis (Cho et al., 2013). Using this mechanism, Cinnamon is considered as a nitric oxide producer in the central nervous system which decreases the lipopolysaccharide-induced tumor necrosis in the serum (Han and Parker, 2017).
Cinnamon oils are well known for their inhibitory potential activity against many pathogenic bacteria such as Pseudomonas aeruginosa, E. coli and staphylococcus aureaus, and also against fungal pathogens such as Candida albicans, Torulopsis utilis, Saccharomyces cerevisiae and Schizosaccharomyces pombe (Narayanankutty et al. 2021a; Baker and Grant, 2018; Abd El-Hack, et al., 2020). It is revealed that essential oil from Cinnamon is more effective than its aqueous extract as an antibacterial agent (Parthasarathy and Thombare, 2013; Syafiq et al., 2021) as it exerted potent inhibitory effects against E. coli and S. aureus with a diameter of inhibition zone values of 19.2 and 28.7 mm, respectively. Liaqat et al. (2017) stated that Cinnamon has an inhibition effect against wide Gram-negative and Gram-positive bacteria to reach 12.17–29.5 mm suggesting a high antibacterial activity. Thus, we herein aimed to detect the inhibitory activity of cinnamon oil extract against the growth of pathogenic E. coli strains.
2 Materials and methods
2.1 Microorganisms and media
Two isolates were isolated from drinking water bottled, tap and wells’ water from rural areas in Riyadh, Saudi Arabia; namely Escherichia coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB. Media used: all media were prepared as described by (APHA, 2005). Violet red bile lactose (VRBL) Agar was prepared for E. coli sp. isolation. It has the following composition (g/l): Lactose 10.0, Peptone 7.0, Yeast extract 3.0, Sodium chloride 5.0, Bile salts mixture 1.5, Neutral red 0.03, Crystal violet 0.002, Agar 15.000 with pH adjusted to 7.4 ± 0.2 at 25 °C. For isolate maintenance, Nutrient agar medium (g/l) was prepared. It has the following composition: meat extract 3, peptone 5, pH 7. For testing the inhibitory activities of cinnamon essential oil, Muller and Hinton agar with a composition of (g/l) beef, dehydrated infusion 30.0, casein hydrolysate 17, Starch 1.5, agar 15.0 with pH adjusted to 7.2 ± 0.1 at 25 °C was prepared.
2.2 Preparation of cinnamon extracts
-
Aqueous Extract: Ten Gram of dried and crushed cinnamon barks were soaked in 100 ml of sterilized distilled water for 6 h. at 50 °C. filtration was done every two hours using eight layered muslin cloth then centrifuged at 10000 rpm for 15 min. The supernatant was then collected and concentrated through evaporation at 40 °C to the final volume. The aqueous extract was sterilized by filtration. For aqueous extract maintenance, it was stored at 4 °C in airtight bottles for further studies. (Parekh et al., 2005).
-
Solvent extract: Ten grams of dried and crushed cinnamon barks was added to 100 ml of methanol solvent 90 % (v/v) then, kept on a rotatory shaker at 120 rpm for 24◦C (Lab-Line Orbital Shaker- USA Lab Equipment) for 24 h. filtration and maintenance were carried out as previously described by (Parekh et al., 2005).
-
Essential oil of cinnamon: The cinnamon essential oil was prepared by extraction of aromatic oil unit. The extraction was done by hydro distillation process following the methods of Narayanankutty et al., (2021b).
2.3 Standard inoculum
The standard inoculum was prepared by inoculating 5 ml of peptone water by 3–5 single colonies of the selected isolate and incubated at 37 °C for 24 h. Thereafter, the Optical density (O.D.) of the grown culture was adjusted to 0.06–0.8 using the spectrophotometer at 625 nm which is equivalent to (14x106 CFU/ml) (Ebrahim et al., 2018).
2.4 Inhibitory effect of Cinnamomum verum extracts against E. Coli sp. Using well diffusion test
Inhibitory activity of cinnamon (aqueous, methanolic and oil) extracts was tested individually using well-diffusion method as recommended by (NCCLS, 1993). Muller Hinton agar medium was poured into petri dishes and inoculated with 1 ml of E. coli isolates (14X106 CFU/ml) using the spreading technique. Agar wells were made using a sterilized 7 mm corkborer and filled with 100 µl of the tested cinnamon essential oil. Incubation of petri dishes was done at 37 °C for 24 h. All experiments were carried out in triplicates. The cinnamon inhibitory activity was expressed as the inhibition zone diameter’s means (NCCLS, 1993; Narayanankutty et al 2021c).
2.5 Effect of different concentrations of cinnamon essential oil against E. Coli sp. By well diffusion method
Serial concentrations of cinnamon oil were prepared by emulsifying (30–40–50–60–70–80 % v/v) in 2 % of tween 80. These concentrations of cinnamon oil were tested for their inhibitory activity against E. coli isolate sp. The inoculum was prepared by inoculating the nutrient broth by 3–5 colonies of E. coli and incubated for 24 h. at 37 °C. Inhibitory activity was determined as described above. All experiments were carried out in triplicates and the cinnamon inhibitory activity was expressed as the inhibition zone diameter's mean (Mahdi et al., 2018).
2.6 GCMS analysis of essential oil of Cinnamomum verum
A Hewlett Packard gas chromatograph (HP6890) connected to a VG Analytical 70250S mass spectrometer with an HP5MS capillary column (30 m × 0.25 mm, film thickness 0.25 µm). In this system, helium is used as the carrier gas and the flow rate is 1 ml/min. The oven temperature range is adjusted from 50° C in 5 min to 280° C, and the oven temperature increase program is 40° C / 5 min, and finally, the temperature is kept isothermal for 5 min. Inject 1 μl of cinnamon oil sample in split mode. GC–MS detection was performed using a 70-eV ionization energy electron ionization system. A scan rate of 0.6 s (cycle time: 0.2 s) was applied, covering a mass range from 35 to 600 amu. The identification of the essential oil compounds was based on the comparison of retention time and mass spectra of the homolog’s series of (C4-C28) with data generated under identical experimental conditions (Adams, 2017).
2.7 Identification of pathogenic waterborne isolates by MALDI-TOF MS
MALDI-TOF-MS was used to identify the E. coli isolates by picking up a single colony of the selected isolate and transferring it directly using wood backs on MALDI target plate at ambient temperature (25 °C) until dry. Overlaying of samples was done by adding 1 µl of Bruker HCCA solution to all MALDI plates and leaving them until drying.
2.8 Statistical analysis
The determination coefficient (R2) was calculated according to Microsoft office Excel 2016 package.
3 Results
3.1 Isolation and identification of E. Coli isolates by MALDI-TOF MS
VRBL agar medium was prepared to isolate E. coli isolates from drinking water in the rural areas in Riyadh, Saudi Arabia, which represent 35 % of the bacterial waterborne microorganisms. The medium was inoculated using 1 ml of water sample then incubated at 37 °C for 24 h. Single colonies with lactose-fermenting appearance (pink colonies) were picked up and sub-cultured into nutrient agar for maintenance. The results indicate the high score of the two selected E. coli isolates ranged from 2.41 and 2.46 identified as E. coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB (Table 1).
Isolate no.
Isolate source
Score Value
Suggested strains
3A
Bottled and tap water
2.41
E. coli MB 11,464 1 CHB
40C
wells’ water
2.46
E. coli DSM 1103 QC DSM
3.2 Inhibitory effect of Cinnamomum verum against E. Coli strains
Different (Aqueous, methanolic and oil) cinnamon extracts were prepared and tested for their inhibitory activity against E. coli strains using the well-diffusion method. The current results show that the oil extract of cinnamon inhibited the growth of E. coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB rather than the aqueous and methanolic extracts that indicates the high sensitivity of E. coli strains towards the oil extract with inhibition zone 4.5 cm and 5.2 cm for E. coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB (Figs. 1 & 2).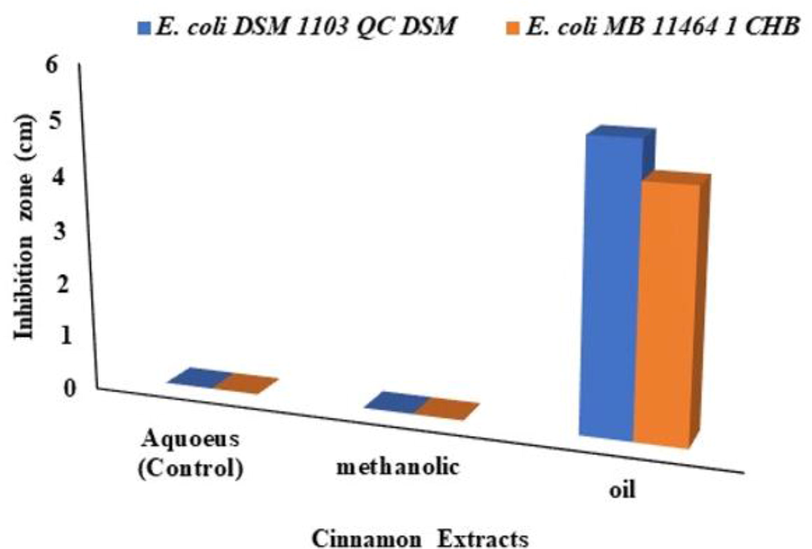
Antimicrobial activity of different cinnamon extracts against E. coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB strains.
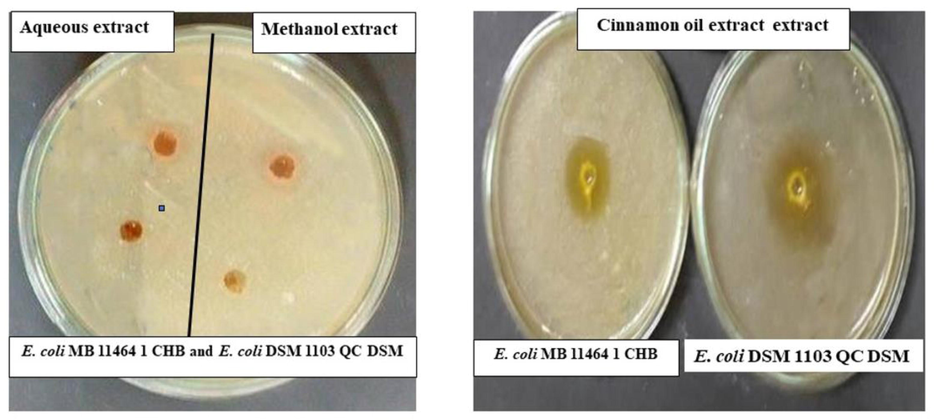
Antimicrobial activity of different cinnamon extracts against E. coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB strains using well diffusion method.
3.3 Effect of different concentrations of cinnamon essential oil against E. Coli DSM 1103 QC DSM and E. Coli MB 11,464 1 CHB strains
Different concentrations of cinnamon oil extracts were prepared from 30 to 80% (v/v) and tested against E. coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB strains. The results show that oil concentration of 80% (v/v) had the highest inhibitory activity on E. coli strains followed by the 70% and 60% (v/v) concentrations, respectively (Fig. 3). Determination coefficient of R2 = 0.9449 and 0.9667.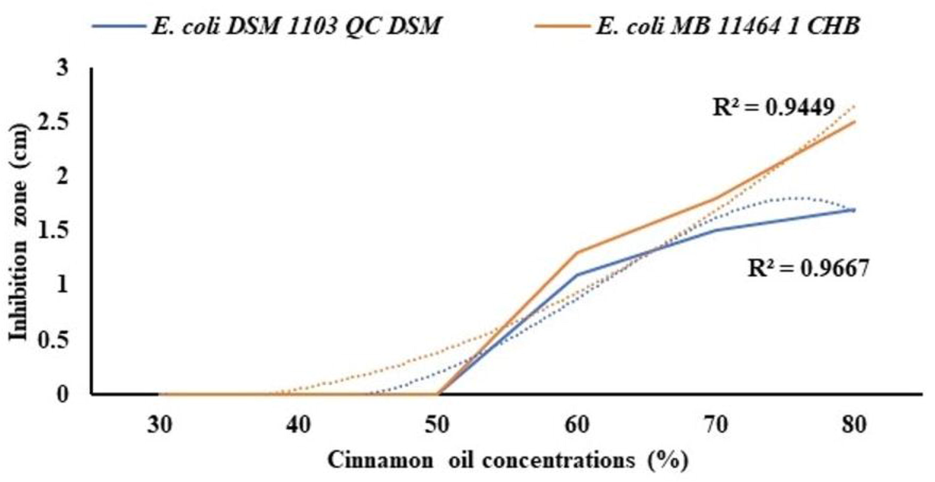
Different concentrations of cinnamon oil extract against E. coli DSM 1103 QC DSM and E. coli MB 11,464 1 CHB strains.
3.4 Chemical components of cinnamon essential oil extract
The chemical composition of cinnamon essential oil was done using GCMS analysis (Table 2). The major constituents include Propylene glycol, Linalool, Cinnamaldehyde, 3-Allyl-6-methoxyphenol, Methyl cis-cinnamate, Ethyl- cinnamate, Cinnamic acid, phenethyl ester, and Benzyl cinnamate. Cinnamaldehyde was found to have the highest percentage (24.42%) followed by cinnamic acid (20.93 %) and 3-Allyl-6-methoxypheno (m-eugenol) (18.55%).
Sample
Retention time
%
Compound name
1
4.7443
7.94
Propylene Glycol
2
8.0698
1.05
Linalool
3
10.0979
24.42
Cinnamaldehyde
4
10.7863
18.55
3-Allyl-6-methoxyphenol
5
11.051
4.42
Methyl cis-cinnamate
6
11.7341
1.04
Ethyl cinnamate
7
12.4331
21.03
Cinnamic acid, phenethyl ester
8
12.4967
20.93
Cinnamic acid, phenethyl ester
9
16.4205
0.57
Benzyl cinnamate
4 Discussion
Waterborne disease is a worldwide burden that is causing the death of more than two million people per year. A lot of waterborne associated disease like diarrhea, gastrointestinal diseases and systemic illnesses are invading many regions around the world (WHO, 2015). Unfortunately, more than 800 million people are at risk of waterborne diseases (Ramírez-Castillo et al., 2015). Cinnamon composition of Propylene Glycol, Linalool, Cinnamaldehyde, 3-Allyl-6-methoxyphenol, Methyl cis-cinnamate, Ethyl- cinnamate, Cinnamic acid, phenethyl ester, and Benzyl was similarly to El Atki et al., (2019) and Wong et al., (2014) who found that cinnamic acid, phenethyl ester are found to have the highest component of cinnamon with a ratio of 45%. Previous studies have also indicated the higher composition of cinnamic acid derivatives in the Cinnamomum essential oil (Narayanankutty et al., 2021a). Moreover, the presence of antimicrobial agent, cinnamic acid exhibits different antimicrobial activities at its higher concentrations. Also, Becerril et al., 2012 found that cinnamon essential oils inhibited the growth of Staphylococcus aureus. Adams et al., 2019 found that cinnamon essential oil has a great effect on combating E. coli growth with an inhibition zone of 5.1 mm when oil is applied with a concentration of 2%.
Our results contributed to the presence of the antimicrobial agent, Cinnamic acid which exhibits different antimicrobial activities at its high concentrations as reported by El Atki et al., (2019). The determination coefficient of R2 = 0.9449 and 0.9667 confirmed that the cinnamon oil extract inhibited the E. coli growth significantly. Our results about the ability of cinnamon essential oils to inhibit the growth of Staphylococcus aureus were similarly to Becerril et al., 2012. Cinnamaldehyde was found to have the highest percentage (24.42%) and followed by cinnamic acid (20.93 %) and 3-Allyl-6-methoxypheno (m-eugenol) (18.55%). Similar results were found by Wong et al., (2014) who stated that cinnamic acid, phenethyl ester is found to have the highest component of cinnamon with a ratio of 45%.
In conclusion, the inhibitory activity of cinnamon oil extracts showed a high sensitivity activity against E. coli isolated from drinking water in the rural areas in Riyadh, Saudi Arabia rather than the aqueous and methanolic extractions. The results open the door for more researches on the biological ways of treatment of water and encourage the local scientists to participate in such an important field.
5 Conclusion
The study concludes that Cinnamon derived molecules are important antibacterial agents and are capable of limiting the population of E. coli. Hence, the plant may be further used for the isolation of bioactive compounds and also can be employed for preventing water-borne bacterial diseases.
Acknowledgements
The author would like to thank the Deanship of Scientific Research, Majmaah University, Saudi Arabia, for funding this work under Project No: R-2021-286.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics. 2020;9(5):210.
- [CrossRef] [Google Scholar]
- Adams, R.P., 2017. Identification of essential oil components by gas chromatography/mass spectrometry. 5th ed. Carol Stream, IL: Allured Publ. Corp, pp. 2-7.
- Adams, V.R., Johnson, A.K., Santos, F.B., Santos Jr, A.A., 2019. The Effect of Cinnamon Essential Oil on Escherichia coli. The FASEB Journal, 33(S1), lb286-lb286.
- Standard Methods for the Examination of Water and Wastewaters (21st ed.). DC: Washington; 2005.
- Cinnamon & Cinnamon Oil Profile. In: New York State Integrated Pest Management (IPM Program). Geneva NY: Cornell University Library; 2018. p. :2-16.
- [Google Scholar]
- Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne pathogens and disease. 2012;9(8):699-705.
- [Google Scholar]
- Microbiology of Drinking Water Production and Distribution (1st ed.). Hoboken, NJ, USA: John Wiley & Sons Inc; 2014. p. :312.
- Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food and chemical toxicology. 2013;58:355-361.
- [Google Scholar]
- Impact of Allium sativum against Enterobacter sp. As water borne pathogenic bacteria isolated from River Nile. Arab Universities Journal of. Agricultural Sciences. 2018;26(Special issue (2D)):2525-2531.
- [Google Scholar]
- Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. Journal of advanced pharmaceutical technology & research. 2019;10(2):63.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of Cinnamon (Cinnamomum zeylanicum) bark essential oil in a human skin disease model. Phytotherapy Research. 2017;31(7):1034-1038.
- [Google Scholar]
- Anti-Bio gram of the most common dietary additives (Spices) against common problematic organisms. Annals of Punjab Medical College (APMC). 2017;11(4):276-282.
- [Google Scholar]
- Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 2002;28(4):371-409.
- [Google Scholar]
- A comparative evaluation of antimicrobial activity of the ethanolic extract of Cinnamomum zeylanicum and NaOCl against oral pathogens and against swabs taken from nonvital teeth-An in vitro study. Int. J. Pharm. Tech. Res.. 2018;10:39-47.
- [Google Scholar]
- Ameliorative impact of cinnamon against high blood pressure; an updated review. Journal of Renal Injury Prevention. 2017;6(3):171-176.
- [Google Scholar]
- Cinnamon bark as antibacterial agent: A mini-review. GSC Biological and Pharmaceutical Sciences. 2020;10(1):103-108.
- [Google Scholar]
- Chemical Composition of Cinnamomum verum Leaf and Flower Essential Oils and Analysis of Their Antibacterial, Insecticidal, and Larvicidal Properties. Molecules. 2021;26(20):6303.
- [CrossRef] [Google Scholar]
- Mango ginger (Curcuma amada Roxb.) rhizome essential oils as source of environmental friendly biocides: Comparison of the chemical composition, antibacterial, insecticidal and larvicidal properties of essential oils extracted by different methods. Environmental Research. 2021;202:111718.
- [CrossRef] [Google Scholar]
- Analysis of the chemical composition of root essential oil from Indian sarsaparilla (Hemidesmus indicus) Saudi Journal of Biological Sciences. 2021;28(12):7248-7252.
- [CrossRef] [Google Scholar]
- NCCLS [National Committee for Clinical Laboratory Standards], 1993. Performance Standards for Antimicrobial Disc Susceptibility Testing. Approved Standard NCCLS Publication M2-A5, Villanova, PA, USA.
- Efficacy of Aqueous and Methanol Extracts of Some Medicinal Plants for Potential Antibacterial Activity. Turk J. Bio.. 2005;29:203-210.
- [Google Scholar]
- Evaluation of antimicrobial activity of Azadirachta indica, Syzygium aromaticum and Cinnamomum zeyalnicum against oral microflora. Asian Journal of Experimental Sciences. 2013;27(2):13-16.
- [Google Scholar]
- Phytochemical analysis, phytochemical activity and antibacterial effects of Cinnamon zeylanicum (dalchini) extracts. Int J Eng Sci Res Technol. 2018;7:162-170.
- [Google Scholar]
- Waterborne pathogens: detection methods and challenges. Pathogens. 2015;4(2):307-334.
- [Google Scholar]
- Cinnamon: a multifaceted medicinal plant. Evidence-Based Complementary and Alternative Medicine. 2014;2014:1-12.
- [Google Scholar]
- Cytokine profile in severe Gram-positive and Gram-negative abdominal sepsis. Scientific Reports. 2015;5(11355):1-12.
- [Google Scholar]
- Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. Journal of Materials Research and Technology. 2021;11:144-157.
- [Google Scholar]
- In vitro anti-diabetic effects and phytochemical profiling of novel varieties of Cinnamomum zeylanicum (L.) extracts. Biochem. Biophys. Mol. Biol.. 2020;8:1-10.
- [Google Scholar]
- World Health Organization (WHO), 2015. Water Sanitation and Health. Available online: http://www.who.int/water_sanitation_health/diseases (assessed on 17 February 2015).
- World Health Organization (WHO), 2008. Guidelines for Drinking-water Quality. Second Addendum to 3rd Edition, Genva, Switzerland. http://www.who.int.water_sanitation_health.
- Extraction of essential oil from cinnamon (Cinnamomum zeylanicum) Orient. J. Chem.. 2014;30(1):37-47.
- [Google Scholar]
- Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282-289.
- [Google Scholar]
- Effect of essential oil of Cinnamomum zeylanicum on some pathogenic bacteria. African Journal of Microbiology Research. 2014;8(10):1026-1031.
- [Google Scholar]







