Translate this page into:
Extraction and identification of fungal pigment from Penicillium europium using different spectral studies
⁎Corresponding authors at: Department of General Science, Ibn Sina National College for Medical sciences, Al Mahajar Street: 31906, Jeddah 21418, Saudi Arabia (S.M. Iqubal). i.ibrahimshaikh09@gmail.com (Ibrahim Ahmed Shaikh), shakeeliqubal@gmail.com (S.M. Shakeel Iqubal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
There is a growing demand for colourants of natural origin in the food, pharmaceutical, cosmetic and textile sectors. Previously, our group has screened a fungal species from forest soil, identified as Penicillium europium. The isolated fungus transformed the longifolene into various metabolites, of which 12 were isolated in pure form, with potential to be utilized in the perfumery industry. This study aimed to isolate and identify novel fungal pigments from Penicillium europium.
Methods
The current study showcases the extraction and identification of fungal pigment from Penicillium europium using different spectral studies. The strain was isolated from forest soil, Western Ghats, India, and was found to be capable of using longifolene as the sole carbon source. The yellowish pink coloured pigment-producing fungal strain was identified as Penicillium europium. Further, the pinkish pigment was extracted, purified, and using spectral studies like UV, IR, NMR and Mass, the structure of the pure pigment was identified.
Results
The pure pigment structure was analyzed and tentatively confirmed as 2-(1,5, dimethyl hexyl)-3,5-dimethyl-6-hydro-1,4-benzoquinone having the molecular formula C16H24O3. Toxicity study using LD50 on Albino rats revealed that the pigment had no toxic effect on rats.
Conclusion
Penicillium europium synthesized pigments could contribute to biotechnology and add value to the food, feed, and pharmaceutical industries. They can be used for various industrial applications, for example, as dyes for textile and non-textile substrates such as paper, leather, coatings and paints, in cosmetics, and food additives. Negative cytotoxicity result inferred that the pigment could be a potential replacement for hazardous synthetic dyes.
Keywords
Pigments
Penicillium europium
Benzoquinone
Biotransformation
1 Introduction
There is growing scrutiny over the detrimental impact of synthetic colourants on both the environment and consumers' health, which has shifted the focus towards natural colouring alternatives. Hence, international demand for natural pigments is quickly growing in the cosmetic, pharmaceutical, food, and textile industries. Natural colourants can be used for diverse industrial applications, such as dyes for fabric and non-fabric substrates such as paper, leather, coatings and paints, and as additives in food and cosmetics (Kalra et al., 2020).
Colour plays a vital role in the food production and processing sector, contributing to food's sensory attribute. It signifies the nutritional value, freshness, safety, and aesthetic value of food, directly influencing the coloured food product's market value. Natural colours are considered safe if they are non-toxic, non-allergic, biodegradable, and non-carcinogenic, thereby rendering no risk to the environment. Due to the lower risk advantage of natural colours and consumers' changing attitudes toward consuming natural products, there is an expanding interest in discovering new natural colours. The consumer demand for natural colours and their growth as a category is expected to increase by 7% annually. Natural food colours have varied food industry applications, with almost all major natural pigment classes being used in at least one food industry sector (Aberoumand, 2011). Micro-organisms derived pigments commonly used as food colorants include violacein, riboflavin, astaxanthin, canthaxanthin, phycocyanin, prodigiosin, beta-carotene, lycopene, and melanin (Sen et al., 2019).
All life on earth ultimately depends on light energy from the sun, converted to other forms of energy or biomass by plants and photosynthetic microorganisms. The electromagnetic spectrum includes the wavelength range humans recognize as visible light (380–750 nm) (Orna, 1984). When pigments are illuminated by visible light, part of the energy may be absorbed, and the emitted light will appear as colour. Pigments contain a chromophore, a colour-bearing group, which directly produces colour by light absorption or transfer energy to auxochromes which are colour increasers, giving characteristic colours. Various chemical classes of pigments occur in nature, of which the highest is produced by microorganisms (Gill and Steglich, 1987).
Microbial pigments can be classified into relatively few major biosynthetic and structural classes; the pyrroles and tetrapyrroles, tetraterpenoids or carotenoids, N- or O-heterocyclic pigments and metalloproteins. Some microbial pigments have important biological functions for the producing organisms, such as the production of energy and fixation of matter by bacteriochlorophylls and phycobilins in microbial photosynthesis. Whereas, in some organisms, the only known attribute of pigments is to give colour. Some microbial pigments such as the phenazines are secondary metabolites, and their natural functions are not well understood (Turner and Messenger, 1986). Most fungal pigments have economic importance, such as food, feed and drug colourants, or essential nutrients (Hendry and Haugton, 1996). Thus, the microbes producing pigments could contribute to biotechnology and add value to the food, feed, and pharmaceutical industries. Different types of fungi show the presence of different kinds of pigments like carotenoids (Britton, 1991), ubiquinones (Takizawa et al., 1992), anthraquinones (William et al., 1994; Brauers et al., 2000; Gill and Saubern Simon, 2000), xanthorin (Gill and Qureshi, 1992; Gill and Morgan, 1999), indigo, monascins, quinines, phenazines, violacein, flavins and melanins (Dufosse et al., 2014). Fungi and bacteria offer a tremendous resource in that they produce hundreds to thousands of various pigments, and it has been estimated that only 5–7% of fungi and 10–12% of bacteria have been isolated from nature (Demain, 1981). Many more natural pigments will likely be isolated from microorganisms. Microorganisms offer advantages over plants and animals for pigment production. Microorganisms have an exceedingly small size compared to animal and plant cells with high growth rates. Microbial cells provide excellent host environment for the expression of foreign genes. Certain mammalian and plant pigments, such as haemoglobin and nonmicrobial carotenoids, have been expressed in microorganisms (Kushwaha et al., 2014). Microbes will undoubtedly be more extensively utilized in the future for basic and applied studies of pigments.
This research objective deals with the study of pigment produced by the fungal strain that has been used for longifolene fermentation, characterization of purified pigment and toxicity studies.
2 Materials and methods
2.1 Chemicals
All the chemicals used in this study were of analytical grade and procured from different commercial firms like Sigma, Himedia, S.D fine, SRL and Aldrich Co. Byrde medium was used, which contained 0.696 g KH2PO4, 0.149 g KCl, 0.008 g ZnSO4·7H2O, 0.2 g MgSO4, 0.020 g FeSO4, 0.006 g MnSO4 and 1 g NH4NO3 per litre. pH was adjusted to 7.0 using 4 N sodium hydroxide solution.
2.2 Fungal strain
The strain was isolated from the soil collected from the forests of Western Ghats, South India, and was found to be capable of using longifolene as the sole carbon source. The yellowish pink coloured pigment-producing fungal strain was identified as Penicillium europium based on microscopical and morphological aspects (Koneman et al., 1997; Khan et al., 2021). The stock cultures were maintained on potato dextrose agar (PDA) slants.
2.3 Cultivation conditions
For pigment production, the strain was grown statically at 37 °C in 250 ml Erlenmeyer flasks containing 50 ml Byrde Medium (Vogel et al., 1989; Khan et al., 2021).
2.4 Preparation of inoculum
The fungus was subcultured, grown and maintained on PDA slants for 72 hrs at 37 °C. Each flask containing 50 ml Byrde media was inoculated with loopful of Penicillium europium culture using sterile inoculation needle. The pigment produced was extracted after ten days.
2.5 Pigment extraction, isolation and characterisation
On the tenth day after inoculation, the culture broth was filtered, and the filtrate was extracted with acetone. Different solvents were tried for separation of the pigment but acetone was found to be more reliable. The mixture was concentrated and was further subjected to neutral silica gel column chromatography to get pure pigment, eluted with acetone and methanol. This was subjected to thin layer chromatography (TLC) for checking the purity with benzene (Vogel et al., 1989; Khan et al., 2021). Isolated fungal pigment was further characterized based on physical methods like UV–vis (Cooksey et al., 1998), Infrared (IR), Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy and mass spectra (MAS SPEC using Electron Impact), for structural determination.
2.6 Acute toxicity study
Acute toxicity study was performed in accordance with guidelines of Organization for Economic Cooperation and Development (OECD-423) (OECD, 2002). Animals were fasted overnight before dosing. Different doses of pigment (5 mg/Kg to 5000 mg/Kg b.w.) were administered by oral route using an oral feeding cannula in a single dose. During the first 24 h after dosing, each animal was observed with more importance given during the first 4 h, followed by observation. Important parameters observed include sedation, convulsions, hypothermia, grooming, hyperactivity and mortality.
3 Results and discussion
The fungus Penicillium europium producing yellowish pink pigment in Byrde medium was isolated in our laboratory. The TLC analysis of acetone extract of Penicillium europium cultural broth revealed the presence of two pigments. Further, the light pink pigment was separated into a pure form by subjecting acetone fraction to silica gel chromatography eluted with methanol. The purity was checked by TLC (Rf = 0.70) with a single spot.
The UV–visible spectrum shows absorption at 343 nm, indicating a (3-unsaturated ketone (Benzoquinone moiety) (Cooksey et al., 1998) (Fig. 1). The IR spectrum shows bands at 3406 cm−1 corresponding to the hydroxyl group (O–H), bands at 1641 cm−1 correspond to quinone structure (C = 0), bands at 1387 cm−1, 1337 cm−1 correspond to p-unsaturation and bands at 2935 cm−1 correspond to C–H stretching (Fig. 2).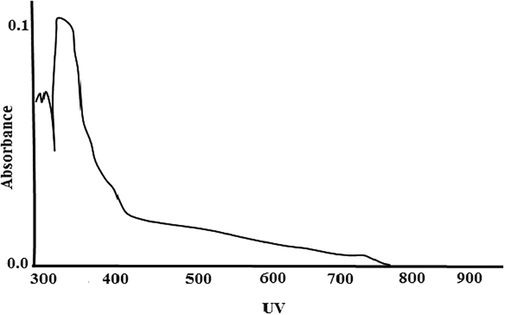
UV–Vis Spectra of pigment.
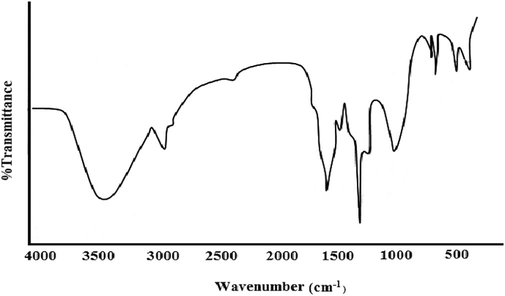
IR spectra of pigment.
Further 1H NMR (CDCl3) (Figure −3) shows peaks (9 ppm) at 0.89 (d, 6H, J = 8 Hz, 2CH3), 1.34 (d, 6H, J = 7 Hz, (CH2)3), 1.31 (d, 3H, J = 7 Hz, l'–CH3), 1.65 (s, 3H, 5-CH3), 1.75 (s, 3H, 3-CH3), 1.87 (s, 3H, –CH3), 2.10 (m, 1H, CH(CH3)2), 2.40 (m,1H, l'–CH), 3.5 (s, 1H, OH) exchangeable with D2O (Fig. 3).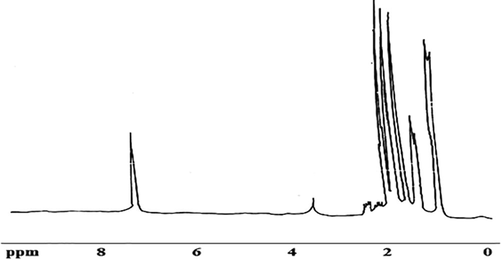
1H NMR spectra of pigment.
Table 1 shows the molecular ion peak at 264 M/Z (25%).
IR absorption bands cm−1
3406, 2935,1641,1387,1337,1109, 824
1H NMR δ ppm
3.5,2.40,2.10,1.87,1.75,1.65,1.31,0.89
Mass M/Z
264 (25%)
Molecular formula
C16H24O3
Molecular weight
264
Melting point
180–182 °C
UV–visible spectrum
343 nm
Thus based on UV, IR, PMR and mass spectra, the pigment was tentatively identified as 2- (1,5, dimethyl hexyl) −3, 5-dimethyl-6- hydro −1, 4 - benzoquinone having the molecular formula C16H24O3. (Fig. 4)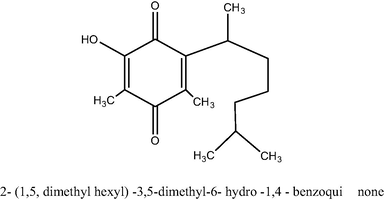
Structure of 2- (1,5, dimethyl hexyl) −3,5-dimethyl-6- hydro −1,4 – benzoquinone.
The proposed compound structure was further confirmed by comparison of its NMR spectral data with those published for synthetic O-methyl perezone (Perri et al., 1989; Escobedo-González et al., 2019) Such compound is believed to be isolated for the first time from the fungus.
Using the pigment (quinone), the toxicity was studied using LD50. The pigment was diluted in water and a single dose (300 mg/Kg) was administered orally to 3 Albino rats. Animals were observed individually after dosing at least once during the first 30 min, periodically during 24 h, with special attention given during the initial 4 h.
Even after 48 h of observation, there was no toxic effect of pigment on rats. Then three other rats were subjected for a limit test at one dose level of 5000 mg/Kg and kept under observation. After 48 h, there was no toxic effect of pigment on the rats.
Majority of the natural pigments obtained from fungi are reported to have a broad spectrum of pharmacological activities. These pigments render fungi diverse biological roles such as enzyme cofactors (flavins) (Rao et al., 2017); protection against the damaging effects of photo-oxidation (carotenoids) (Gmoser et al., 2017), and the protection against environmental stress (melanins) (Dufosse et al., 2014). Though these fungal pigments are associated with distinct biological activities, the factors regulating their production and their physiological role remain largely unstudied (Sen et al., 2019). Current advances in biotechnological and analytical tools employing computational and molecular techniques help decipher the components responsible for the pigment colour, de-novo pathways and genome accountable for its production.
To reap maximum benefit, alternative routes for these metabolites' mass production may be achieved using heterologous expression, manipulating culture conditions and co-culturing. Fungi proficient of producing pigments can be collected from various environmental ecosystems and habitats and explored as a source of commercial pigments.
4 Conclusions
Based on this study, we conclude that this pigment is non-toxic, and it can be used as food colourant and additive. The fungus Penicillium europium which transforms longifolene was also found to produce yellowish pink pigment in Byrde medium. This pigment was isolated and characterized tentatively as 2- (1,5, dimethyl hexyl) -3, 5-dimethyl 1-6-hydro-l, 4-benzoquinone having the molecular formula C16H24O3based on UV, IR, 1HNMR and Mass spectral studies. This compound was isolated for the first time from the fungus. The pigment is nontoxic, which was confirmed by the LD50 test. Thus our research has shown that this pigment can be used as food, feed, colourant, and as essential nutrient. Microbes producing pigments could contribute to biotechnology and add value to the food, feed, and pharmaceutical industries.
Acknowledgement
The Authors are thankful to Karnataka University, Dharwad, India and Ibn Sina National College, Jeddah, K.S.A. for constant support and encouragement.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J. Dairy Food Sci.. 2011;6(1):71-78.
- [Google Scholar]
- Anthraquinones and betaenone derivatives from the sponge-associated fungus microsphaeropsis species: novel inhibitors of protein kinases. Nat. Prod.. 2000;63(6):739-745.
- [Google Scholar]
- Tyrosinase kinetics: failure of the auto-activation mechanism of monohydric phenol oxidation by rapid formation of a quinomethaneintermediate. Biochem. J.. 1998;333(3):685-691.
- [CrossRef] [Google Scholar]
- Industrial microbiology. Science (New York, N.Y.). 1981;214(4524):987-995.
- [CrossRef] [Google Scholar]
- Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotech.. 2014;26:56-61.
- [CrossRef] [Google Scholar]
- Green approach extraction of perezone from the roots of acourtiaplatyphilla (A. Grey): a comparison of four activating modes and supercritical carbon dioxide. Molecules. 2019;24:3035.
- [CrossRef] [Google Scholar]
- New xanthorin glycosides from a Dermocybe species. J. Nat. Prod.. 1999;62:1298-1300.
- [Google Scholar]
- Gill M., Steglich W., 1987. In Progress in the Chemistry of Organic Natural Products, Springer, Vienna. 51, 1-317.
- Pigments of Fungi, Part 27. New Xanthorin derivatives from a fungus of the genus dermocybe. J. Nat. Prod.. 1992;55(4):517-520.
- [Google Scholar]
- Pigments of fungi. LXVI. The gentiobiosides of (R)- and (S)-Torosachrysone from Australian toadstools and methods for assaying the enantiomeric purity of natural torosachrysone. Aust. J. Chem.. 2000;53:213-220.
- [Google Scholar]
- Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol.. 2017;4:4.
- [CrossRef] [Google Scholar]
- Natural Food Colorants (2nd ed.). London: Blackie Academic; 1996.
- Fungi as a potential source of pigments: harnessing filamentous fungi. Front. Chem.. 2020;8:369.
- [CrossRef] [Google Scholar]
- Biotransformation of longifolene by Penicillium europium. Biocatal. Biotransform.. 2021;39(1):41-47.
- [Google Scholar]
- Colour Atlas and textbook diagnostic microbiology (5th ed.). Lippincott Company: Philadelphia; J.B; 1997.
- Kushwaha K, Saini A, Saraswat P, Agarwal MK, Saxena J., 2014. Colorful World of Microbes: Carotenoids and Their Applications“, Advances in Biology, Article ID 837891, 13 pages, 2014. https://doi.org/10.1155/2014/837891
- OECD., 2002. Test No. 423: Acute Oral toxicity - Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects, OECD Publishing. Available online at: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en
- The physics and chemistry of color: the fifteen causes of color, by Kurt Nassau, John Wiley and Sons, New York, 1983, p. 454. Color Res. Appl.. 1984;9 190 190
- [CrossRef] [Google Scholar]
- Perri S.T., DykeH.J., Moore H.W., 1989. Rearrangement of cyclobutenones to 2,5- and 2,6-dialkylated 1,4-benzoquinones. Synthesis of O-methylperezone and O-methylisoperezone. J. Org. Chem. 54, 9, 2032–2034
- Fungal and bacterial pigments: secondary metabolites with wide applications. Front. Microbiol.. 2017;8:1113.
- [CrossRef] [Google Scholar]
- Microbial pigments in the food industry—challenges and the way forward. Front. Nutr.. 2019;6:7.
- [CrossRef] [Google Scholar]
- Isolation and structural elucidation of a dihydroubiquinone-9 from the fungus Aureobasidiumpullulans. FEMS Microbiol. Lett.. 1992;92:120-132.
- [CrossRef] [Google Scholar]
- Occurrence, biochemistry and physiology of phenazine pigment production. Adv. Microbiol. Physiol.. 1986;27:211-275.
- [Google Scholar]
- Text book of practical organic chemistry (5th ed). Harlow: Pearson Education Limited; 1989.
- Anthraquinones and a 10-Hydroxyanthrone from Phialophoraalba. J. Nat. Prod.. 1994;57(2):317-319.
- [Google Scholar]







