Translate this page into:
Anticancer activity in HeLa and MCF-7 cells via apoptopic cell death by a sterol molecule Cholesta-4,6-dien-3-ol (EK-7), from the marine ascidian Eudistoma kaverium
⁎Corresponding author. jeshran@sathyabama.ac.in (Rajaian Pushpabai Rajesh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Eudistoma kaverium, an ascidian belonging to the family Polycitoridae is previously recorded from Indian waters. The work done on this species is limited only to taxonomy and ecology. As ascidians are potential source of anticancer compounds, we have purified fractions of E. kaverium using HPLC and evaluated its anticancer activity.
Method
Chromatography based fractionation of the crude extract was carried out and mass spectrometry was used to identify the active molecule. Cell viability was checked using MTT assay. Flow cytometry, ROS generation, Hoechst staining and DNA fragmentation was performed to confirm the anticancer activity of the compound.
Results
A column fraction EK-7, which was identified as a sterol Cholesta-4,6-dien-3-ol using GC–MS analysis, exhibited potent cytotoxicity with IC50 value of 6.5 μM in MCF-7 and 10.2 μM against HeLa cells. Moreover, EK-7 triggered the cell cycle arrest in the sub G0/G1 stage and induced apoptosis twenty-four hours post-treatment. Hoechst staining of EK-7-treated HeLa cells portrayed apoptotic events such as changes in cell morphology, chromatin condensation, membrane swelling, and development of apoptotic bodies. The changes in light scattering by the EK-7-treated HeLa cells indicate the general characteristics of cell death as a result of apoptosis. The EK-7-treated HeLa cells show cell cycle arrest in G0/G1, G2/M and S phases and the increased amount of sub G0/G1 population indicates the increase in the apoptotic induced cell population. The isolated compound Cholesta-4,6-dien-3-ol (EK-7), based on the GC–MS analysis and library search from E. kaverium displayed potent anticancer activity which is mediated through apoptosis targeting the G0/G1 phase.
Conclusion
Based on the experimental data, it shows that EK-7(Cholesta-4,6-dien-3-ol) a sterol derivative has potent anticancer activity mediated through apoptosis. If this research is continued further with preclinical and clinical trials, EK-7(Cholesta-4,6-dien-3-ol) could be possibly used as a chemotherapeutic agent in treating cancer.
Keywords
Eudistoma kaverium
Ascidian
HeLa
MTT
Flow cytometry
Apoptosis
1 Introduction
A good number of anti-cancer drugs currently used are of natural, especially from marine products or its derivatives (Simmons et al., 2005). According to the researchers, marine compounds have higher source and potential biological activity than terrestrial compounds (Gribble, 2015). Based on data from the National Cancer Institute, USA, more than 1% of marine-based compounds displayed antitumor potential, only 0.1% of terrestrial compounds are found to exhibit antitumor potential (Vijayakumar et al., 2020). Since marine organisms survive in different physical and biological conditions, they are equipped with greater chemical diversity (Frederick Grassle, 2013; Jensen et al., 2015), which justifies this 10-fold rise. Additionally, marine organisms keep competing for food and space, resulting in the production of secondary metabolites as a part of the defense and survival mechanism (Cragg and Newman, 2005). Several anticancer compounds have been tested with human clinical trials over the past decade that are sourced from the marine environment (Arif et al., 2004; da Rocha, 2001; Gordaliza, 2007; Molinski et al., 2009; Rawat et al., 2006). A total of 20,000 and more natural products have been separated from several marine organisms. Alkaloids, steroids, terpenoids, polypeptides, polysaccharides polyethers, and macrolides are molecules that have potential biomedical applications (Jiang et al., 2010; Menna, 2009). Numerous marine compounds have caught attention because of their activity against multiple types of tumours (Mayer and Gustafson, 2003; Newman and Cragg, 2004; Olano et al., 2009; Rocha et al., 2011). The anticancer drug Cytosar-U is isolated from Cryptotethya crypt, a marine sponge, which contains modified nucleosides, such as spongothymidine and spongouridine (Bergmann and Burke, 1955; Cragg and Newman, 2013; Wright, 2007). Marine organisms such as ascidians, mollusc and sponges have been a proven source of a copious number of anticancer, antibacterial and potent drug compounds (Datta et al., 2015; Gupta et al., 2013; Hughes and Fenical, 2010). Compounds such as didemnin B, ecteinascidin 743 and aplidine (Cragg and Newman, 2005; Lopes et al., 2003; Rinehart, 2000; Singh et al., 2013) are derived from ascidians, which have anticancer property and are into clinical trials.
In this study, the less explored ascidian Eudistoma kaverium is assessed for its anticancer activity in HeLa and MCF-7 cells, to discover a potential anticancer molecule.
2 Materials and methods
2.1 Extraction of ascidian from the marine environment 9.2770° N, 79.1252° E
The ascidian Eudistoma kaverium Meenakshi, 2002 was collected from the Mandapam coast (9.28230N, 79.20810E), from Southeast India's coast. The ascidian E. kaverium was identified using standard keys (Montaser and Luesch, 2011). Ascidians were extracted with methanol and dichloromethane. The crude extract is used for further analysis (Houssen and Jaspars, 2012).
2.2 Isolation of cytotoxic compound-chromatographic method
2.2.1 Column fractionation
The potent crude dichloromethane extract was fractionated as previously described by (Rajesh et al., 2010) with slight modification. The crude extract was fractionated, based on gradient-partitioning technique (Martins et al., 2014), with a step-gradient solvent system of increasing polarity from hexane (H) to ethyl acetate (EA) to methanol (M) (100% H- 100% EA- 100% M). A total of 34 fractions were collected. Among these fractions, 14 major fractions were analysed for cytotoxicity using MTT assay against HeLa cell line.
2.2.2 High-performance liquid chromatography (HPLC)
Column fraction EK-7 (75% H: 25% EA), which exhibited significant cytotoxicity activity against HeLa cell line, was further concentrated and analysed using analytical HPLC (Shimadzu SPD20A Prominence) with an analytical column Agilent ZORBAX 300SB-C18 (5 µM, 4.6́150 mm) USA. A step gradient binary solvent system from 95% water (0.1% trifluoroacetic acid) to 95% methanol (0.1% trifluoroacetic acid) was employed to analyse the column fraction EK-7 (Newman et al., 2003).
2.3 High-resolution-electrospray ionisation-mass spectrometry (HR-ESI-MS) and GC–MS analysis
HR-ESI MS analysis of column fraction EK-7 was performed in Bruker Daltonics Electrospray Ionisation Quadrupole Time of Flight (ESI Q TOF; Maxis Impact). The intact mass of the target molecule was obtained by directly infusing the sample into the mass spectrometer. GC–MS was performed in a gas chromatograph (Agilent 7890A) coupled with mass spectrometer (Agilent 5975C) (Agilent Technologies, USA). The mass spectra and GC–TIC profiles were obtained using the ChemStation data analysis software. In order to identify column fraction W-7, its mass spectra were matched with the corresponding entries in databases (NIST-08 MS library version 2.0f).
2.4 Chemicals and source of antibodies
Chemicals and reagents of cell-culture require molecular biology grade, as required, were used in this study. Most of the methods and molecular biology protocols adopted in this study are by the standard protocols detailed in Molecular Cloning (El Gamal, 2010). Most of the reagents needed for cell culture including media, antibiotics, Fetal Bovine Serum (FBS) and various other required supplements were from Invitrogen Life Sciences, USA or Sigma-Aldrich, USA. All tissue culture ware was procured from either Nunc or Corning. All experiments were performed in multiples for proper analysis of the results. Statistical significance for all experimental analyses was determined by t-test or one-way ANOVA using statics software Graph Pad Prism version 5.0. A p-value of ≤ 0.05 between the groups was considered significant.
2.5 Cell culture, maintenance of cell lines, growth medium and treatment conditions
MCF-7 (Breast adenocarcinoma), HeLa (cervical carcinoma) and HaCaT (Immortal keratinocytes) were from ATCC. Cell lines were maintained in Dulbecco's modified eagle medium (DMEM) with FBS(10%), penicillin (100 units/ml) and streptomycin (100 μg/ml) at 37 °C with 5% CO2. Cell culture was performed using standard procedures in a laminar airflow chamber (Bergmann and Feeney, 1951).
2.6 Cell viability determination by MTT assay
Cells of optimum density were inoculated into a 96-well plate and treated with varying concentrations of crude and purified fractions. The cells were incubated for 24 h, and then treated with 5 mg/ml MTT dye, followed by incubation for 3 to 4 h. The formazan crystals formed were measured spectrophotometrically in a plate reader at 570 nm (Houssen and Jaspars, 2012; Vera and Joullie, 2002).
2.7 Flow cytometric analysis of cells (apoptosis and necrosis)
Flow cytometric analysis was performed as described by (Shashi et al., 2006). Briefly HeLa and MCF-7 cells were treated with varying concentrations of EK-7. Following the instructions of the manufacturer, cells were washed followed by staining with annexin V -FITC antibody & PI. These cells were scanned for fluorescence intensity in FL-1 (FITC) and FL-2 (PI) channels. Quadrant statistics were used to analyse the cell population (apoptosis and necrosis) in which the lower right quadrant indicated apoptosis and the upper right quadrant indicates necrosis.
2.8 Determination of hypo-diploid sub G1DNA (G0) population- flow cytometric analysis
A total of 2x106/ml cells were seeded in a 6-well plate and treated with different concentrations (1, 5, 10, 15 μM) of EK-7 and incubated for 24 h. The cells were washed with 70% ice-cold alcohol in phosphate buffer saline (PBS) and digested with 400 μg/ml of DNase-free RNase at 37 °C. The cells were stained with PI (10 μg/ml) to determine DNA fluorescence and cell cycle phase distribution. The samples were then measured using (FACS)-Canto (BD Biosciences, USA) for hypo-diploid ≤ (sub-G1, ≤ 2n DNA) apoptotic cell population from FL2-A vs. cell counts (Houssen and Jaspars, 2012; Vermes et al., 1995).
2.9 Hoechst 33,258 staining of cells for nuclear morphology
Hoechst staining was performed as described in (Shashi et al., 2006) with slight modification. HeLa cells were grown in T-25 flasks, on reaching optimal confluence, the cells were treated with different concentrations of EK-7. Following which, the cells were isolated, washed and suspended in 100 µl PBS gently. They were further fixed in 400 µl cold acetic acid: methanol (1 + 3, v/v) and incubated at 4 °C overnight. Cells were again washed, spread on a clean slide and dried overnight at room temperature. Slides were stained using Hoechst 33,258 and incubated for 30 minand observed under microscope.
2.10 Measurement of intracellular peroxides- flow cytometric analysis
Intracellular peroxide and reactive oxygen species (ROS) levels were estimated using 2,7-dichlorofluorescein diacetate (DCFH-DA). The measurement of intracellular peroxidase is performed using flow cytometer following the procedure of Narayanaswamy et al. (2018). After various stages of treatments, cells were incubated with a medium containing DCFH-DA (5 µM; 10 mM stock in 100% ethanol) for 1 h. Cells were washed in PBS, centrifuged for 5 min at 100g and suspended in Dulbecco's PBS. PI (5 µg/ml; stock 1 mg/ml PBS) prior to analysis. The mean fluorescence peak (10,000 events) was analysed from the gated cell population. The green DCF-fluorescence was analysed in the FL-1 channel and PI fluorescence was analysed in the FL-2 channel (Rothe and Valet, 1990).
2.11 DNA fragmentation assay
Genomic DNA isolated from MCF-7 cells were subjected to DNA fragmentation assay. The cells (2x106/ml) are treated with EK-7 or without EK-7 at different concentrations (5, 10, 15, 20 μM) for 24 h and the treated DNA was electrophoresed on 1.8% agarose gel (Pittman et al., 1993).
3 Results
3.1 Collection, Identification, extraction and purification of ascidian secondary metabolites
The ascidian collected from Mandapam was identified as Eudistoma kaverium using standard keys (Meenakshi, 2002). The cold steeped organic solvent crude extracts using methanol and dichloromethane were used for preliminary screening against cancer cell lines for finding the cytotoxicity of the crude extract. The potent dichloromethane crude extract after column chromatography yielded several fractions (EK1- EK34). Each fraction was separately screened for the presence of cytotoxicity against cancer cell lines using the MTT assay (Fig. 1). Most of the fractions produced cytotoxicity, but among them, fraction EK-7, which produced significant activity was further analysed for specific anticancer assays.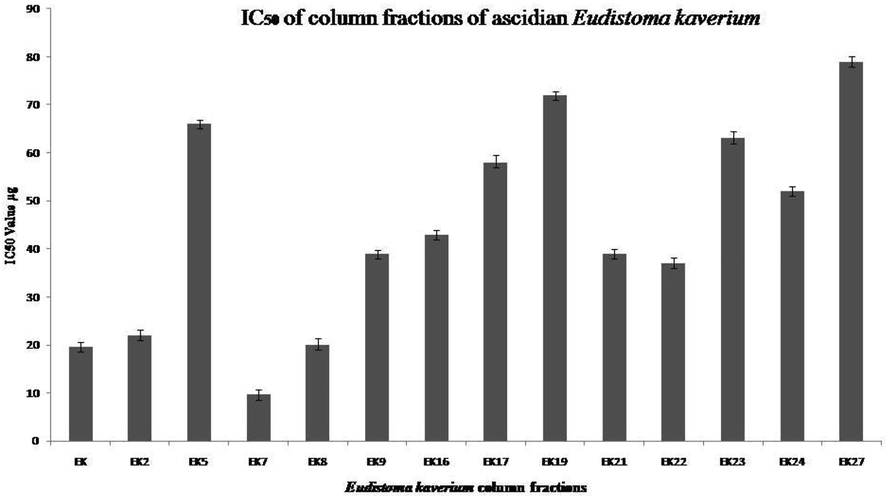
IC50 determination using MTT assay for the Column Fractions: IC50 value were calculated based on MTT assay, graph showing the IC50 values of different column fraction of E. kaverium extract. Data are means ± SD and representative of two similar experiments.
3.2 Hplcs of column fraction EK-7
HPLC analysis of EK-7 using an analytical C18 column showed a single peak at a retention time of 18.6 min (Fig. 2a) which indicates that the sample is pure. So this column fraction EK-7 was used for further studies.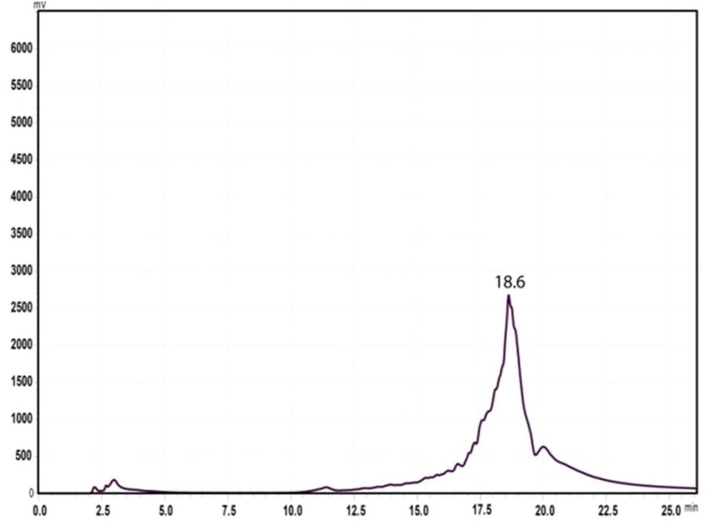
HPLC spectrum of column fraction EK-7 indicates single peak at the retention time of 18.6 min.
3.3 Mass spectrometry of EK-7
A high-resolution mass spectrum analysis of EK-7 indicates that the molecule has a molecular mass of 385.291(M + H)+ (Fig. 2b). GC–MS analysis spectrum presented in Fig. 2c identified the fraction EK-7 as Cholesta-4,6-dien-3-ol.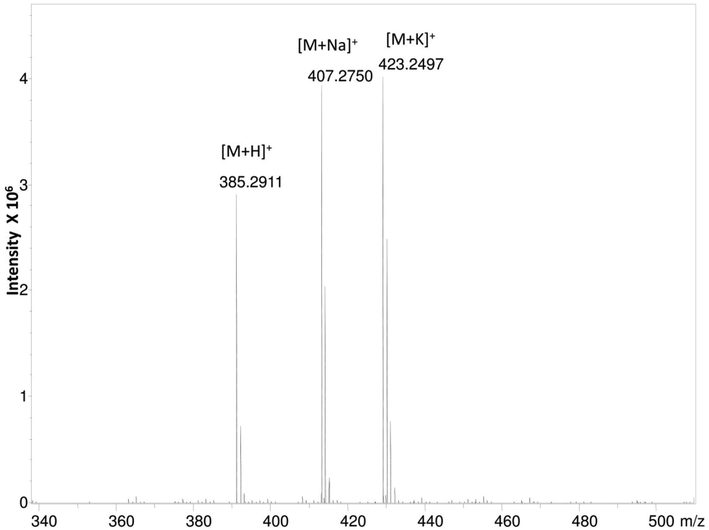
HR ESI-MS spectrum of column fraction EK-7 showing the molecular weight of 385.291(M + H) +.
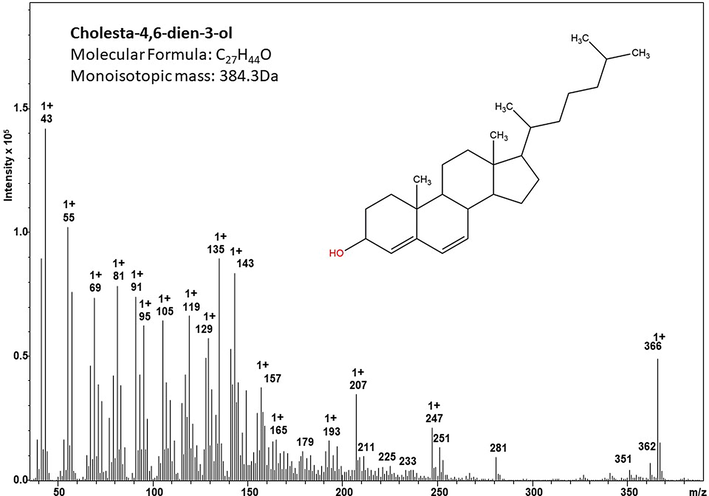
GC–MS spectrum of the column fraction EK-7, NIST-08 MS library version 2.0f based profiling and identification of the compound cholesta-4,6-dien-3-ol.
3.4 Determination of IC50 values and cell viability
The inhibitory concentration (IC50) was 19.5 μg for the crude extract of E. kaverium. Cell growth inhibition induced by purified fraction EK-7 displayed varied activity with different cell lines. IC50 value against MCF-7 was 6.5 μM and 10.2 μM against cervical cancer cell lines. EK-7 treated HeLa and MCF-7 cells showed a dose-dependent inhibition in cell proliferation at low concentration (Fig. 3), comparatively not inhibiting the growth of the control cell line (HaCaT).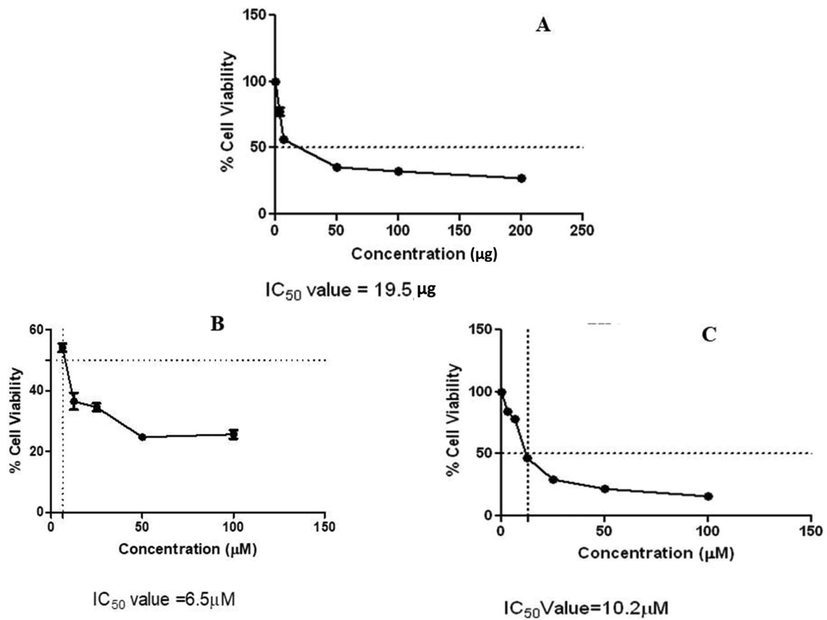
Cytotoxicity of EK-7 on HeLa and MCF-7 cell proliferation: (a) Crude extract of E. kaverium showing a IC50 value of 19.5 µg (b) MCF-7 treated with EK-7 showing a IC50 value of 6.5 µM (c) HeLa treated with EK- 7 showing a IC50 value of 10.2 µM.
3.5 Effect of EK-7 flow cytometry analysis using Annexin-V binding
Cell death induced by the test compound EK-7 is evaluated using Annexin-V binding experiments, which were carried out in both MCF-7 and HeLa cell lines. Both cell lines were separately treated with different concentrations of EK-7 and incubated for 24 h, cell death by apoptosis was determined by flow cytometry, based on binding of Annexin-V to the phosphatidylserine of the exposed cells (Fig. 4). We observed an increase in apoptotic and post-apoptotic cells, with increasing incubation period. In MCF-7 cells, 76% of apoptotic cells and 10.31% post-apoptotic cells were observed, while in HeLa cells 76% of cells were alive, 10.05% were apoptotic cell and 4.58% were necrotic cells. We concluded that MCF-7 cells appeared to be more sensitive to EK-7 than the HeLa cells, and also indicate that EK-7 induced apoptotic pathway for cell death.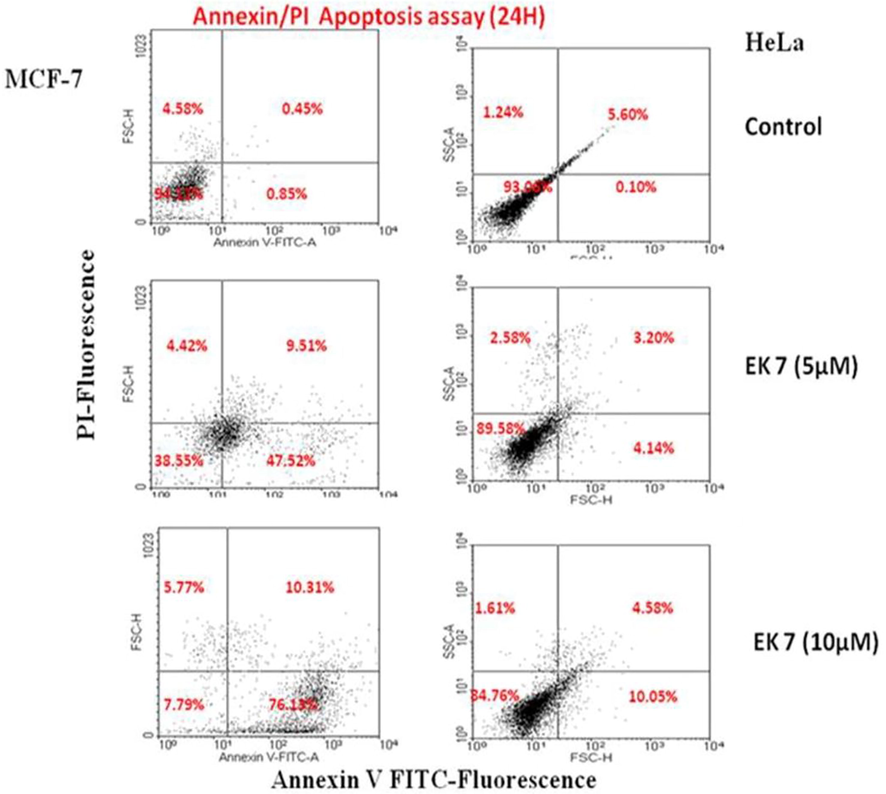
Flow cytometric analysis of EK 7 induced apoptosis in HeLa and MCF-7 cells using annexin V-FITC and PI double staining: The cancer cell lines were treated with 5 and 10 µM of EK-7 and after 24 h cells were stained and flow cytometric analysis was performed. Cells in the lower right quadrant represented apoptosis and in the upper right quadrant indicated necrosis (represented by percentage). Apoptosis is high in MCF-7 cells (76.13%) in response to EK-7 than the HeLa cells (10.05%).
3.6 EK-7 increased G0 fraction cell cycle
EK-7 treated (5, 10 and 15 µM) HeLa and MCF-7 cells exhibited high G0 fraction after 24 h, which includes both debris and apoptotic fractions (Fig. 5). Higher EK-7 concentration demonstrated cell death or increase in G0 fraction. HeLa cells treated with 15 µM EK-7 showed an increase of 40% G0 fraction when compared to control, there was a 86% increase in G0 fraction for MCF-7 cells after 24 h of treatment. There is no significant effect observed on the G2/M fraction after 24 h, from which we conclude that EK-7 does not produce any delay in the cell cycle or block in the mitotic pathway.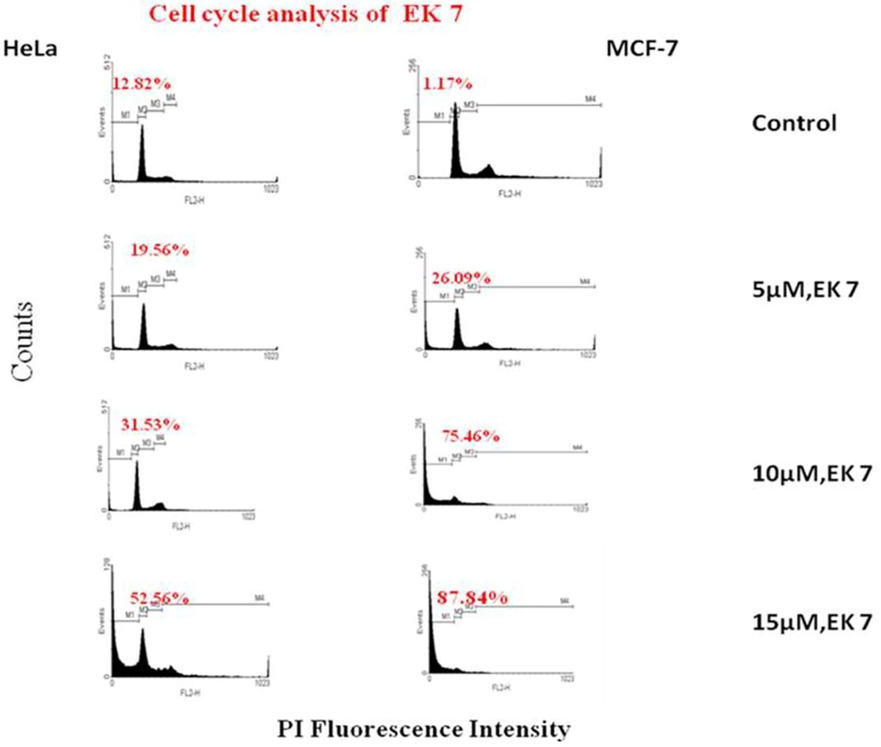
DNA cell cycle analyses in HeLa and MCF-7 cells exposed to EK 7. Cells (1 × 106/ml) in culture were treated with different concentrations of EK 7 for 24 h. Cells were stained with PI to determine DNA fluorescence and cell cycle phase distribution. The cells appear more as dead and as debris in the sample leading to the distribution in the G0 phase, which is evident for cells treated with 15 µM of EK-7. In concentration of 10 µM the cell were in G0 phase and there was no change observed in the G2/M phase, implying that EK-7 does not act by hindering the mitotic pathway.
3.7 Hoechst staining of EK-7 induced HeLa cells
Untreated cell nuclei were round and normal. EK-7-treated cells displayed early condensation of nuclei and concurrently morphological changes were observed in some cells. Upon extended exposure of 10 µM EK-7 in the HeLa cells, morphological changes and an increased number of apoptotic bodies were observed (Fig. 6).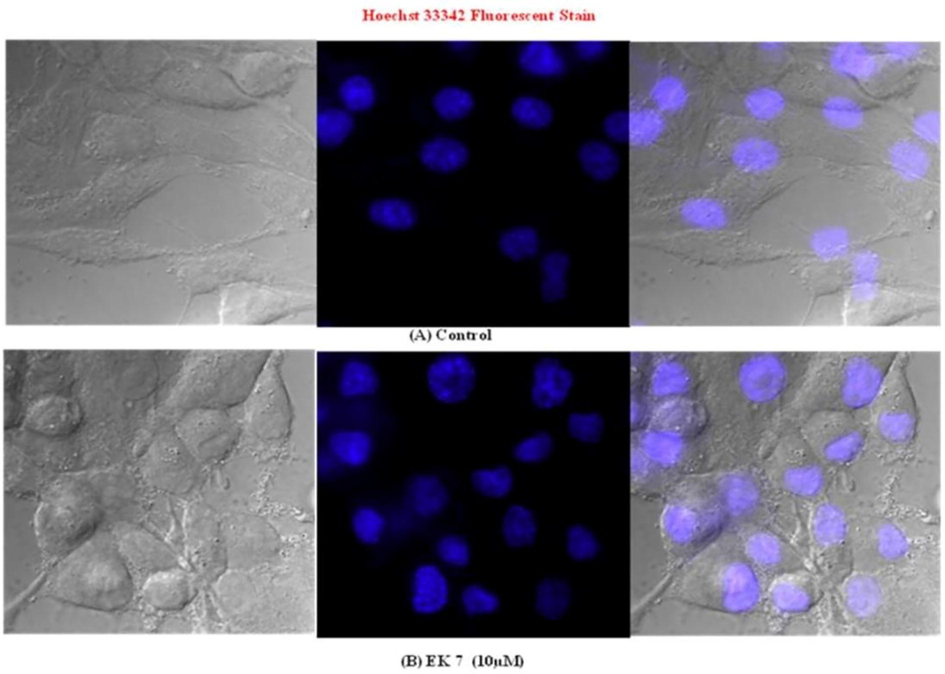
Hoechst 33,258 fluorescence staining of HeLa cells for nuclear morphology and apoptotic body formation. Cells were incubated with EK 7 for indicated time periods. Condensed nuclei and the apoptotic bodies are indicated by arrows. (A) Untreated control -HeLa; (B) HeLa cells treated with 10 µM EK 7 for 24 h which indicate condensed nuclei.
3.8 EK-7 treated in HeLa and MCF-7 cells mediates the generation of intracellular peroxides
Cancer cell lines were incubated with different concentrations of EK-7 and stained using DCFH-DA followed by analysis in flow cytometry (Fig. 7). Forward and side scattered gated cells were analysed in FL1 vs. FL2, and FL1 vs. cell count channels for fluorescence intensity. There is no significant difference in DCF fluorescence on comparing untreated and lower concentrations (1 and 5 µM) of EK-7-treated cells. There is a moderate increase in the DCF-positive cell population, which is derived based on the doubling of log fluorescence intensity over control at high concentration of EK-7.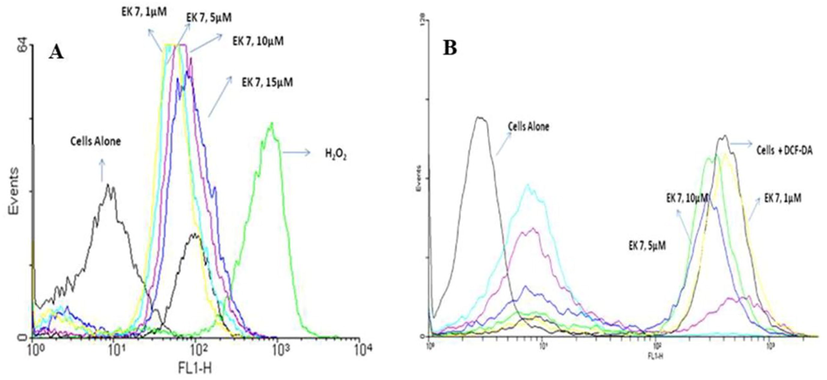
EK 7 mediated generation of peroxides in (a) HeLa and (b) MCF-7 cells. Cells (1 × 106/ml/well) after various treatments with EK 7 in a 12-well culture plate for indicated time periods were incubated with DCFH-DA (5 µM) for 1 h. Cells were washed in PBS, centrifuged at 100g for 5 min and suspended in DPBS and 10,000 events were analyzed for DCF-fluorescence on flow cytometer in the FL-1 vs. FL-2 channels. Shift in fluorescence peek when treated with increasing concentration of EK-7(1, 5, 10, 15 µM) againt Hela and MCF-7 cell lines.
3.9 EK-7-mediated DNA fragmentation in MCF-7 cells
DNA fragmentation analysis was performed for both EK-7 treated and untreated cancer cell lines. The agarose gel (Fig. 8) of the treated and untreated cells illustrated that EK-7 treated cells induced DNA fragmentation. Apoptotic cells are characterized by the presence of fragmented DNA, which is observed as a DNA apoptotic ladder in a gel. Observed results clarify that DNA from EK-7-treated cells exhibited DNA fragments which reflect those from apoptosis. A fainted apoptotic DNA ladder was observed after 24 h of treatment when treated with low concentration of EK-7. In prolonged exposure or higher concentration of EK-7, the apoptotic DNA ladder was prominent, indicating a standard apoptosis pattern linked with minimal post-apoptotic necrosis. DNA isolated from untreated control did not show apoptotic DNA ladder (Fig. 8).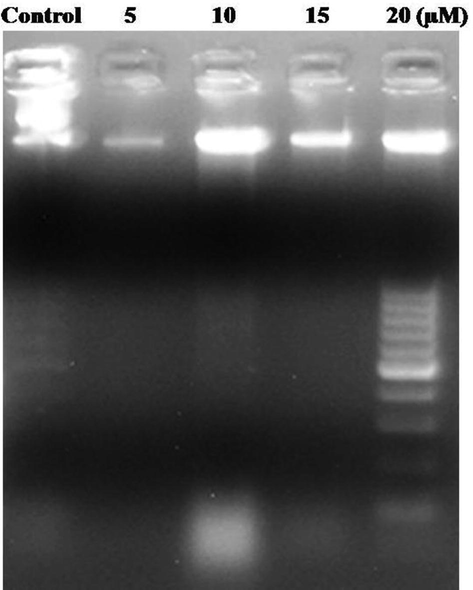
EK-7 mediated DNA Fragmentation in MCF-7 Cells. Cells (1 × 106/ml/well) after various treatments with EK-7 in 12-well culture were collected and the DNA was run in 1% agarose gel to identify the DNA fragmentation event. Lane 1 control, Lane 2–5 MCF-7 Cells treated with 5, 10, 15 and 20 µM of EK-7.
4 Discussion
Most of the medicinal drugs isolated from natural products are of terrestrial origin; however, marine organisms are exceptional because of the possibility to produce novel chemical and biological products. As a result, recently, several marine-based medicinal products have been successfully introduced into the market and many others in clinical trials. The mechanisms by which marine products induce apoptosis have become the subject of great attention in the process of developing anti-cancer agents. In this study, we report for the first time that a sterol derivative Cholesta-4,6-dien-3-ol, an ascidian fraction (EK-7), from E. kaverium, induces apoptosis in HeLa and MCF-7 cells. This is also evident from the fact that EK-7 induced the formation of apoptotic bodies in HeLa and MCF-7 cells, which was confirmed by flow cytometry. All these experimental end-points indicated that the process of apoptosis is activated by EK-7.
The isolated novel ascidian-derived cytotoxic compound is identified from the 14 column fractions, which were screened in HeLa cell line. EK-7 is the most potent fraction, which arrested the growth of HeLa cells, with a IC50 value of 10.2 µM (Fig. 3). We found that EK-7 showed a concentration-dependent growth inhibition on human cancer cell lines. The differential cytotoxicity of EK-7 on human cancer cell lines HeLa and MCF-7(Singh et al., 2021) can help in understanding its cytotoxic effect on different types of progressive cancers. In the case of the breast cancer cell line (MCF-7), the calculated IC50 value was of 6.5 μM, thereby revealing the high efficiency of EK-7. In this regard, the effect of EK-7 was studied for its apoptotic property to understanding the mechanism leading to the activation of apoptosis and/or necrosis in HeLa and MCF-7 cells. It was observed that EK-7 did not cause apoptosis even at the highest concentration in normal human cells lines (HaCaT), which is as desirable as an ideal chemo-preventive agent that should not exert toxicity to normal cells while eliminating the cancer cells. Experiments were performed to find the way how EK-7 induced death in HeLa and MCF-7 cell lines. The cell death was activated via apoptosis, which was determined based on the Annexin-V staining, the exposed cells that are detected using flow cytometry (Fig. 4). Comparing the basal apoptotic population in the untreated cultures, EK-7 produced a concentration-dependent increase in the apoptotic cells and it is noteworthy that MCF-7 cells showed a significant higher effect towards EK-7 treatment than HeLa cell lines.
As a process of determining the anti-proliferative mechanism and understanding the regulatory pathway of EK-7 on cancer cell lines,cell cycle analysis was carried out. It was identified that EK-7 induced G1 arrest and apoptosis in MCF-7 cells, EK-7 treatment showed a rise in sub-G0 DNA population (<2n DNA) when compared to control.G1 regulation is associated with many factors that play an important role in proliferation, differentiation, oncogenic transformation and programmed cell death (Owa et al., 2012; Robbs et al., 2013). Nuclear endonuclease(s) activation is the first step in signal transduction and plays an important role in preventing cancer (Pittman et al., 1993), teratogenesis (Guru et al., 2021; Hays, 1981; Owa et al., 2012) and cell differentiation (Bursch et al., 1990; Cinciripini et al., 1997). Activation the endonuclease pathway will start a series of apoptotic event leading to the death of cancer cell. This apoptotic DNA fragment observed in the MCF 7 when treated with EK-7 is a first step of triggering the cell death. Thus, this sterol derivative Cholesta-4,6-dien-3-ol,extracted from ascidian provides a novel strategy which can be therapeutically effective against malignancy by selectively sensitizing the cancer cells.
5 Conclusion
In this study, we report for the first time, that a purified fraction EK7, possibly a sterol derivative Cholesta-4,6-dien-3-ol, from the marine ascidian E. kaverium, possesses cytotoxic effect in HeLa and MCF-7 cell line through apoptosis induction. Several experimental studies like microscopic observation, flow cytometry-based cell cycle analysis, Annexin-V-FITC assay, ROS generation and DNA fragmentation inferred with the proof that the cell death of the cancer cells were induced via proper mechanism. The evidence hints towards the use of EK-7 fraction of E. kaverium as a lead molecule in the discovery of potent anticancer drug in near future.
Ethical approval
This article does not contain any studies with animal and human participants performed by any of the authors.
Acknowledgements
The first and corresponding author thanks DST SERB- Young Scientist program with grant support No. SB/FT/LS-268/2012. The authors thank the Deanship of Scientific Research, Prince Sattam bin Abdul Aziz University, Al Kharj, Kingdom of Saudi Arabia. We thank the Mass Spectrometric facility at MBU, IISc for providing support to acquire mass spectrometric data. RPR thank Prof. Siddhartha. P. Sarma, MBU, IISc and Siva Krishna.N IISc, for their kind help and laboratory support throughout this study. All authors thank Mr. Carlton Ranjith for preparing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel Marine Compounds: Anticancer or Genotoxic? J Biomed Biotechnol.. 2004;2004(2):93-98. PMID: 15240919; PMCID: PMC548801.
- [CrossRef] [Google Scholar]
- Contributions to the study of marine products. XXXIX. The nucleosides of sponges. III. Spongothymidine and spongouridine. J. Org. Chem.. 1955;11:1501-1507.
- [Google Scholar]
- Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis (London). 1990;11:847-853.
- [Google Scholar]
- Plants as a source of anti-cancer agents. J Ethnopharmacol.. 2005 Aug 22;100(1–2):72-79. PMID: 16009521
- [CrossRef] [Google Scholar]
- Natural products: a continuing source of novel drug leads. Biochim Biophys Acta.. 2013 Jun;1830(6):3670-3695. Epub 2013 Feb 18. PMID: 23428572; PMCID: PMC3672862.
- [CrossRef] [Google Scholar]
- Scheduled reduced smoking: effects on smoking abstinence and potential mechanisms of action. Addict Behav.. 1997 Nov-Dec;22(6):759-767. PMID: 9426793.
- [CrossRef] [Google Scholar]
- Natural products in anticancer therapy. Curr. Opin. Pharmacol.. 2001;1:364-369.
- [CrossRef] [Google Scholar]
- Bioactive Compounds from Marine Invertebrates for Potential Medicines - An Overview. Int. Lett. Nat. Sci.. 2015;34:42-61.
- [Google Scholar]
- Frederick Grassle, J., 2013. Marine Ecosystems, in: Encyclopedia of Biodiversity. Elsevier, pp. 45–55. https://doi.org/10.1016/B978-0-12-384719-5.00290-2
- Natural products as leads to anticancer drugs. Clin. Transl. Oncol.. 2007;9:767-776.
- [CrossRef] [Google Scholar]
- A recent survey of naturally occurring organohalogen compounds. Environ. Chem.. 2015;12:396.
- [CrossRef] [Google Scholar]
- Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev. Comp. Immunol.. 2021;114:103863
- [CrossRef] [Google Scholar]
- Teratogenesis: a review of the basic principles with a discussion of selected agents: Part III. Clin. Pharm. 1981
- [CrossRef] [Google Scholar]
- The marine actinomycete genus Salinispora: a model organism for secondary metabolite discovery. Nat. Prod. Rep.. 2015;32:738-751.
- [CrossRef] [Google Scholar]
- Marine Natural Products and their Synthetic Derivatives for Cancer Therapy, in: Alternative and Complementary Therapies for Cancer. 2010. p. :613-643.
- [CrossRef]
- Marine-derived anticancer agents in clinical trials. Expert Opin. Investig. Drugs. 2003;12:1367-1383.
- [CrossRef] [Google Scholar]
- Marketed marine natural products in the pharmaceutical and cosmeceutical industries: tips for success. Mar. Drugs. 2014
- [CrossRef] [Google Scholar]
- Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int. J. Cancer. 2003;105:291-299.
- [CrossRef] [Google Scholar]
- Occurrence of a new species of colonial ascidian - Eudistoma kaverium sp. nov. and four new records of Eudistoma to Indian coastal waters. Indian J. Mar. Sci. 2002
- [Google Scholar]
- Antitumor potential of natural products from Mediterranean ascidians. Phytochem. Rev.. 2009;8:461-472.
- [CrossRef] [Google Scholar]
- Drug development from marine natural products. Nat. Rev. Drug Discov.. 2009;8:69-85.
- [CrossRef] [Google Scholar]
- Elucidation of Mechanisms of Anticancer Plant Compounds Against the Tumor Cells. In: Anticancer Plants: Mechanisms and Molecular Interactions. 2018. ISBN : 978-981-10-8416-4
- [Google Scholar]
- Marine natural products and related compounds in clinical and advanced preclinical trials †. J. Nat. Prod.. 2004;67:1216-1238.
- [CrossRef] [Google Scholar]
- Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003
- [CrossRef] [Google Scholar]
- Antitumor compounds from marine actinomycetes. Mar. Drugs. 2009;7:210-248.
- [CrossRef] [Google Scholar]
- Cell Cycle Regulation in the G1 Phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr. Med. Chem. 2012
- [CrossRef] [Google Scholar]
- A system for characterizing cellular and molecular events in programmed neuronal cell death. J. Neurosci.. 1993;13:3669-3680.
- [CrossRef] [Google Scholar]
- Anticancer activity of the ascidian polyclinum indicum against cervical cancer cells (HeLa) Mediated through Apoptosis Induction. Med. Chem. (Los. Angeles). 2010 Nov;6(6):396-405. PMID: 21054277.
- [CrossRef] [Google Scholar]
- Marine Peptides and related compounds in clinical trial+. Anticancer Agents Med. Chem.. 2006;6:33-40.
- [CrossRef] [Google Scholar]
- The transcription factor NFAT1 induces apoptosis through cooperation with Ras/Raf/MEK/ERK pathway and upregulation of TNF-α expression. Biochim. Biophys. Acta - Mol. Cell Res. 2013
- [CrossRef] [Google Scholar]
- Cnidarians as a source of new marine bioactive compounds - an overview of the last decade and future steps for bioprospecting. Mar. Drugs. 2011
- [CrossRef] [Google Scholar]
- Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2’,7’-dichlorofluorescin. J. Leukoc. Biol. 1990
- [CrossRef] [Google Scholar]
- A novel lignan composition from Cedrus deodara induces apoptosis and early nitric oxide generation in human leukemia Molt-4 and HL-60 cells. Nitric Oxide - Biol. Chem. 2006
- [CrossRef] [Google Scholar]
- Pharmacologic downregulation of protein arginine methyltransferase1 expression by adenosine dialdehyde increases cell senescence in breast cancer. Eur. J. Pharmacol.. 2021;891:173697
- [CrossRef] [Google Scholar]
- Clinical status of anti-cancer agents derived from marine sources. Anticancer Agents Med. Chem. 2013
- [CrossRef] [Google Scholar]
- Natural products as probes of cell biology: 20 years of didemnin research. Med. Res. Rev. 2002
- [CrossRef] [Google Scholar]
- A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995
- [CrossRef] [Google Scholar]
- Shrimp shells extracted chitin in silver nanoparticle synthesis: expanding its prophecy towards anticancer activity in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol.. 2020;165:1402-1409.
- [CrossRef] [Google Scholar]
- Wright, A.E., 2007. Marine organisms as a source of novel lead structures for drug development. pp. 113–125. https://doi.org/10.1039/9781847550231-00113







