Translate this page into:
Enzymatic activity and virulence of Cordyceps locustiphila (Hypocreales: Cordycipitaceae) on the South American locust Schistocerca cancellata (Orthoptera: Acrididae)
⁎Corresponding author. sebastianpelizza@conicet.gov.ar (Sebastian A. Pelizza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The first barrier, when penetrating an insect host, encountered by microorganisms like fungi is insects cuticle; fungi produce a wide variety of extracellular enzymes involved in the degradation of protein, chitin, and lipids. The main objectives were to assay the enzymatic activity of Cordyceps locustiphila anamorph, in solid and liquid medium at different temperatures and also to evaluate the pathogenicity against the South American locust, Schistocerca cancellata. Conidia of C. locustiphila anamorph were adjusted to 1 × 104, 1 × 106 and 1 × 108 conidia/ml and mortality was recorded for S. cancellata nymphs. The enzymatic activities were determined through a plate test and liquid medium. When assessing the pathogenicity, the fungus caused the highest mortality (84.5 ± 3.5%) at a dose of 1 × 108 conidia/ml. The proteolytic activity showed the highest values (1.56 ± 0.21) (U) at 26 °C. The highest lipolytic activity (1.13 ± 0.36) (U) was observed at 26 °C, while the highest chitinolytic activity was of 0.85 ± 0.1 (U) at 4 °C. Significant differences were observed for enzymatic production on liquid medium, the highest values were recorded for chitinolytic activity (1.63 ± 0.04) (U) and the lowest for caseinolytic activity (0.04 ± 0.001) (U). The results obtained on mortality as well as the quality and variety of enzymes produced by C. locustiphila suggest that this fungus has features that make it a good candidate to be used as a biological agent for the control of the South American locust.
Keywords
Biological control
Entomopathogenic fungi
Enzyme production
Mortality
Pest insect
1 Introduction
During the 18th century, fungal anamorphs were described ignoring their connection to teleomorphic stages. The awareness of the relationship between anamorphic fungi and their teleomorphs (sexual reproductive stage) came only in 1854 for Aspergillus glaucus (L.) (Kirschner, 2018). Most of the Cordyceps-like teleomorphs are included in the family Cordycipitaceae (Sung et al., 2007a). There are about 400 known Cordyceps species, although it is believed that this is not a real estimation of the diversity of the genus (Hawksworth and Rossman, 1997). Different names have been attributed to asexual stages in Cordycipitaceae, and different types of asexual reproductive morphologies have been related to Cordyceps species (Kepler et al., 2017), among which many have shown to be polyphyletic across Hypocreales (Luangsa-ard et al., 2004). Beauveria is the most renowned and well-studied asexually typified generic name in the Cordycipitaceae, mainly because of its insect- pathogenic nature and its value as a biological control tool for a wide diversity of insect pests (Kepler et al., 2017). Nevertheless, Cordyceps locustiphila, a neotropical species, does not have its asexual stage (anamorph) among Beauveria genus. Pelizza et al. (2018) recorded and described the first and southernmost C. locustiphila infecting the romaleid grasshopper Tropidacris collaris (Orthoptera: Acrididae: Romaleidae) in Argentina (Cigliano and Lange, 2019) and proposed that the anamorphic stage should not be classified as Beauveria. The entomopathogen, C. locustiphila has also been reported in several regions of the Amazon in Brazil, Colombia, Ecuador, and Peru infecting the grasshopper Colpolopha sp. (Sanjuan et al., 2014) and other species among the Romaleidae, the most diversified family of endemic neotropical grasshoppers (Cigliano et al., 2014).
Schistocerca cancellata (Serville) (Orthoptera: Acrididae) is a large-sized (♂ = 28–49 mm, ♀ = 39–66 mm) polyphagous pest that feeds on a variety of wild and cultivated plants (Song et al., 2019). It is widely distributed in the southernmost region of South America, including Uruguay and Paraguay, central and northern Argentina, southern Brazil, south-eastern Bolivia, and central and northern Chile (De Wysiecki and Lange, 2005). Since 1954, the plague had been in a state of recession, after efforts to diminish its populations using several tons of insecticides and thus reducing its infestation area that had come to invade down to Chubut province in Patagonia, Argentina (De Wysiecki and Lange, 2005). Despite this, from 2015 S. cancellata populations experienced historical proportions outbreaks, where large swarms of up to 25 km2 were frequently found, causing havoc in agricultural crops in northern and central Argentina and areas of Bolivia and Paraguay (Pocco et al., 2019). To mitigate the damage made by this insect, the only alternative employed up to this moment are chemical insecticides, regardless of their negative impact to the environment (Álvarez et al., 2013).
Most pathogens employed in biological control strategies enter the host indirectly by ingestion, instead entomopathogenic fungi are able to actively penetrate insect cuticle initiating host body invasion regardless of the insect feeding habits (Vega et al., 2012). To overcome this first barrier fungi produce different types of extracellular enzymes that can act degrading protein, chitin, and lipids (Pedrini et al., 2007; Muhammad et al., 2020a, 2020b).
As the enzymatic activity and virulence are two related features that characterize efficient biocontrol agents, the purpose of this study was to describe the production of main enzymes of C. locustiphila anamorph on different culture medium at different temperatures and to evaluate its biocidal capacity against S. cancellata.
2 Materials and methods
2.1 Fungal isolation
The entomopathogenic C. locustiphila was found infecting T. collaris, in an undisturbed area of the Yabotí biosphere reserve (26° 37′ S 53° 40′ W), a naturally protected region that includes part of the departments of Guaraní and San Pedro in Misiones province, Argentina. The grasshopper T. collaris colonized by C. locustiphila was collected in a plastic recipient with silica gel and posteriorly deposited at the Herbarium of the Spegazzini Institute of Botany, La Plata National University (access code LPS 49245). The entomopathogenic anamorph was also isolated from T. collaris and deposited at the Spegazzini-Institute–culture-collection (access number LPSc 1218) and PCR products obtained were sequenced and deposited at GenBank (accession numbers: MF185185; MF185186; MF185187; MF185188).
2.2 Pathogenicity assays
The C. locustiphila anamorph was cultured on potato-dextrose-agar medium for 10 days in the dark at 25 °C to promote conidia production. Posteriorly, conidia were collected (Fig. 1) with disposable cell scrapers (Fisherbrand™) and put into test tubes containing 0.01% (v/v) Tween 80™ (polyoxyethylene sorbitan monolaurate) (Merck). The resulting suspensions were vortexed for 2 min and subsequently filtered through four layers of sterile muslin. The concentration of solutions was adjusted to 1 × 104, 1 × 106 and 1 × 108 conidia/ml using a Neubauer haemocytometer (Marienfeld™; Germany) and conidial viability was established for each stock suspension according to Goettel and Inglis (1997). The average viability of the conidia was in all cases over 95%.
Electron microscopy photo of conidia the anamorphic form of Cordyceps locustiphila.
Third-instar S. cancellata nymphs were used for pathogenicity assay. Treatments consisted of different concentration of conidial suspensions (1 × 104, 1 × 106 and 1 × 108 conidia/ml). Three replicates of 10 individuals (on different dates) were sprayed with 1 ml of each treatment using a 35 ml glass atomizer. For each replicate and treatment, 10 control insects were atomized with 1 ml of 0.01% [v/v] Tween 80™ only. Treated and control locusts were placed in 50 × 9-cm acetate tubes with screened ends (Henry, 1985) and fed with lettuce, cabbage, and wheat bran. The acetate tubes were maintained in climatic chambers under controlled conditions (30 °C, 60% relative humidity and 14:10-h light:dark photoperiod). The cumulative mortality was recorded daily for 10 days. Mycosis was confirmed by examination of dead locusts under the microscope (ZEISS-Primus Star-Germany).
2.3 Production of enzymes at different temperatures on solid media
The lipolytic, proteolytic, amylolytic, cellulolytic, and chitinolytic activities were studied performing a plate test in agar media with specific substrates: Tween 20™, casein, starch, carboxymethylcellulose, and Chitin Azure, respectively (Hankin and Anagnostakis, 1975). The C. locustiphila strain was cultivated in Petri dishes containing malt extract agar at 26 °C, for seven days in the dark to obtain agar plugs (7 mm diameter) from the fungal active growth zone, which acted as inocula. Inocula was placed on the centre of 9 mm Petri dishes containing agar media and the different substrates to estimate lipolytic, proteolytic, amylolytic, cellulolytic, and chitinolytic of the funguns. Petri dishes were incubated in growing stove (Semedic I291PF) at 4 ± 1 °C, 15 ± 1 °C and 26 ± 1 °C for 15 days (Kathiresan and Manivannan, 2006; Saparrat et al., 2008; Sabu et al., 2012; Sudarkodi et al., 2015). The existence of a halo surrounding the fungal colony was recorded to estimate enzymatic activity. A total of three repetitions were performed for each treatment (enzymatic activity and temperature).
An opaque halo around the colony due to the precipitation of the calcium salt of the fatty acid was present when lipolytic activity was occurring, proteolytic activity was visualized as a transparent halo, a consequence of the degradation of casein (Koneman and Roberts, 1987), and the chitinolytic activity also was revealed when a transparent halo formed as Chitin Azure was depolymerized by the activity of the fungus (Howard et al., 2003). Amylolytic and cellulolytic activities were evident when a transparent enzyme degradation halo formed as well. To determine amylolytic activity, a 1% iodine solution in 0.2% KI solution (lugol) was used (Hankin and Anagnostakis, 1975). The cellulolytic activity was revealed with 0.2% Congo Red 5% acetic acid, and 1 M sodium chloride.
2.4 Production of enzymes in liquid medium
A Sabouraud dextrose liquid medium (150 ml) containing 0.5% (w/v) peptone meat, 0.5% (w/v) casein peptone, and 2% (w/v) glucose was prepared and four malt extract agar (MEA) plugs of C. locustiphila culture were incorporated. These were cultivated for eight days at 30 ± 1 °C, darkness and in orbital shaker (Labdiscount™) at 180 rpm. This constituted the crude extract for further determination of enzymatic activity (Mancillas-Paredes et al., 2019).
2.5 Enzyme extracts
The chitinolytic, keratinolytic, and caseinolytic activities were determined using the crude extract produced by C. locustiphila. The extract was filtered by vacuum pressure using OSMONICS borosilicate fiberglass discs (0.45 µm) and kept at 4 °C. To establish chitinolytic activity methods by Kuzu et al. (2012) with modifications were carried out. Briefly, 0.003 g of Chitin Azure was suspended in 0.6 ml of Tris-HCl buffer (0.1 M and pH 8) and then incorporated to 0.6 ml of the crude extract. This solution was incubated at 28 °C for 2 h and posteriorly the reaction was stopped in a cold bath and centrifuged (5000 g) for 15 min at 5 °C. The blanks were obtained by heat inactivation of the crude enzyme extract and the absorbance established at 595 nm. The absorbance variation per time unit was measured and one unit (U) of enzyme activity was defined as the amount of enzyme required to vary 0.01 absorbance in a minute, at 31 °C and pH 8. The proteolytic activity was determined using casein at 1% (w/v) in 0.1 M Tris-HCl buffer, pH 8.0 that was heated in a water bath for 20 min at 35 °C to accomplish its dissolution. The reaction mixture contained 50 µl of crude extract and 550 ml of casein. The reaction time was 30 min and stopped afterwards with 90 µl of TCA 5% (w/v). The blanks consisted of the same reaction mixture, but changing the order in which components were added: 1) crude extract, 2) TCA 5% and 3) casein. After stopping reactions, products were maintained for 15 min at 4 °C and posteriorly centrifuged at 4800g for 20 min at 25 °C (López et al., 2000). Absorbance was measured at 280 nm considering a caseinolytic unit (Ucas) as the amount of enzyme required to produce an increase of one absorbance unit at 280 nm per min. Finally, a mix containing 150 μl of filtered enzyme extract per 0.22 μm and 250 μl of a 1% w / v solution of azocasein (Sigma Chem. Co.) in 0.1 M Tris-HCl buffer, pH9 was prepared in order to establish the azocaseinolytic activity of the fungus. The solution was incubated for 30 min at 37 °C and the reaction stopped by adding 1 ml of 10% w/v trichloroacetic acid. The product was centrifuged at 3000g for 15 min and 0.9 ml of the supernatant mixed with 1 ml of 1 N NaOH. The spectrophotometric lectures were made at 440 nm. The crude extract was heated at 100 °C for 5 min to inactivate enzymatic activity and used as reaction blank. The amount of enzyme that caused an increase of 0.1 Abs per minute under the reaction conditions was considered the azocaseinolytic unit.
2.6 Statistical analysis
The Trimmed Spearman Karber analysis was performed to estimate LC50 of the entomopathogen with 95% confidence limits (CL). Data on the enzymatic activity of C. locustiphila at the different temperatures were analysed using a two-way ANOVA. Analyzes of variance were performed with the InfoStat 2007 software (InfoStat, 2001).
3 Results
The highest mortality observed was at a dose of 1 × 108 conidia/ml, causing the death of 84.5 ± 3.5% third-instar nymphs of S. cancellata. On the other hand, the lowest mortality was recorded at a dose of 1 × 104 conidia/ml with pathogenicity of 18.8 ± 2.2% (Fig. 2). The Trimmed Spearman Karber analysis allowed describing the relationship between the conidial concentrations of C. locustiphila strain and nymphal mortality. The LC50 values and confidence limits of each replicate of C. locustiphila strain against third-instar nymphs of S. cancellata are presented in Table 1.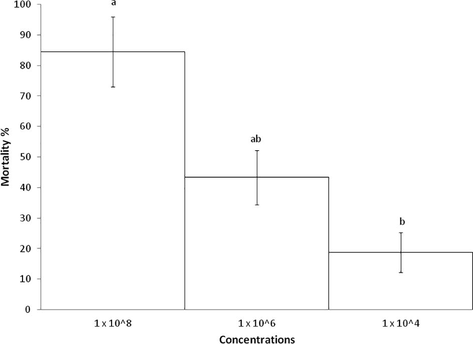
Cumulative mortality (%) ± SD of third-instar Schistocerca cancellata nymphs within 10 days after infection with 1x104; 1x106 and 1x108 conidia/ml of Cordyceps locustiphila (LPSc 1218). Different letters denote significant differences, according to the Tukey test (p < 0.05).
Bioassays
LC50
Confidence limits (95%)
Lower
Upper
1
7.195
1.565
3.096
2
2.156
65
8.576
3
9.545
1.515
5.95
Average
1.166
2.416
5.856
Significant differences were observed between enzymatic activity of the fungus at evaluated temperatures (F = 11.97; DF = 14; P < 0.0001) (Table 2). Moreover, the proteolytic activity showed the highest values: 1.56 ± 0.21 (U), 1.5 ± 0.32 (U), and 1.14 ± 0.12 (U) at 26 °C, 16 °C, and 4 °C, respectively (Table 3). The highest lipolytic activity was of 1.13 ± 0.36 (U) at 26 °C and the maximum registered chitinolytic activity (0.85 ± 0.1) (U) occurred at 4 °C (Table 3).
DF
F
P
Enzymes
7
18.86
<0.0001
Temperature
2
0.49
0,6154
Enzymes*Temperature
14
11.97
<0,0001
Enzymatic activity
Temperature
4 °C
16 °C
26 °C
Proteolytic
1.14 ± 0 c
1.5 ± 0.17 c
1.56 ± 0.25 c
Chitinolytic
0.85 ± 0.12 abc
0.21 ± 0.01 ab
0 a
Lipolytic
0.8 ± 0.7 abc
0 a
1.13 ± 0.98 c
Xynalase
0.66 ± 0.57 abc
1 ± 0 bc
0 a
Pectinase
0 a
1.16 ± 0.11 c
1.4 ± 0.1 c
Cellulase
1.1 ± 0 c
1.1 ± 0 c
0 a
Lacasa
0 a
0 a
0 a
Amylase
0 a
0 a
1.16 ± 0.05 c
As for the enzymatic activity in a liquid medium, significant differences were observed (F = 2046.83; DF = 3; P < 0.0001) with the highest values for chitinolytic activity (1.63 ± 0.04) (U) and the lowest values for caseinolytic activity 0.04 ± 0.001(U) (Fig. 3).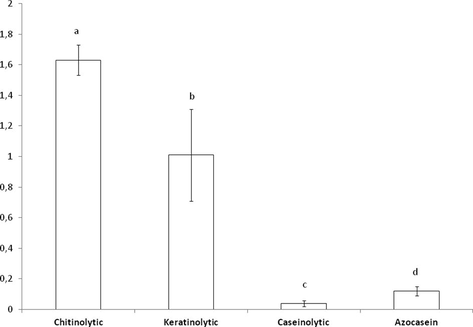
Results of the Chitinolytic, Keratinolytic, Caseinolytic and Azocasein activity of Cordyceps locustiphila (LPSc 1218) in liquid medium. Data are means of three replicates ± SD. Different letters denote significant differences, according to the Tukey test (p < 0.05).
4 Discussion
Insect pathogenic fungi possess certain features, such as high specificity, contact transmission, natural dispersion, safety for non-target organisms, and the ability to maintain control once established in the environment, that make them desirable candidates for microbial pest control programs (Barra-Bucarei et al., 2020). Moreover, entomopathogenic fungi produce multiple mycotoxins, which play an essential role in improving fungal pathogenesis and virulence (Muhammad et al., 2020a, 2020b) The pathogenic ability of these fungi is influenced by several factors including the nature of the host, the pathogen itself and environmental conditions (Muhammad et al., 2018). Fungal invasion of the insect body is promoted, among others mechanisms, by mechanical action exerted by hyphae and also by enzymatic activity (Mascarin and Jaronski, 2016). This study demonstrates for the first time the pathogenic efficacy of C. locustiphila anamorph to infect the South American locust S. cancellata. Furthermore, the enzymatic activity of this entomopathogen was described both qualitatively and quantitatively using solid and liquid substrates, respectively.
Entomopathogenic fungi can penetrate/go through the insect cuticle/exoskeleton not only using mechanical pressure but also employing several types of enzymes (Vega et al., 2012). These “extracellular cuticle-degrading” enzymes include proteases, chitinases, and lipases (Binod et al., 2007). Pedrini et al., 2007 proposed that since hydrolases degrade components containing C and N, which are the main sources for fungal growth, these enzymes could be considered as key factors in the process of fungal penetration. The highest mortality values caused by C. locustiphila reached 84.5 ± 3.5% at a dose of 1 × 108 conidia/ml. Moreover, high chitinolytic, proteolytic, and lipolytic activity values for the fungus were encountered when evaluated at different temperatures in both liquid and solid media. Chitinases are the main responsible for the degradation of the insect cuticle and therefore penetration into the host. Consequently, virulence attributed to fungal entomopathogens could be correlated with their capacity to produce chitin degrading enzymes (Dhawan and Joshi, 2017).
5 Conclusion
Despite the fact that, the present study was made exclusively under laboratory conditions and that, the pathogen-host system could work differently under field conditions, results obtained on pathogenicity as well as the quality and diversity of enzymes produced by the fungus, indicate that C. locustiphila shows some of the most desirable features to be found in a microbial biocontrol agent that could be useful as an alternative to chemicals for the control of the South American locust.
Acknowledgements
This study was partially supported by Consejo Nacional de Investigaciones Científicas y Tecnológicas (PIP 0018), Comisión de Investigaciones Científicas de la provincia de Buenos Aires (CICPBA), Universidad Nacional de La Plata (UNLP, 11/N 903), fPICT 2018-2100).
Disclosure statement
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Behavior of insecticide chlorpyrifos on soils and sediments with different organic matter content from Provincia de Buenos Aires, República Argentina. Water Air Soil Pollut. 2013;224:1453.
- [Google Scholar]
- Antifungal activity of Beauveria bassiana endophyte against Botrytis cinerea in two Solanaceae crops. Microorganisms. 2020;31(8):1-65.
- [Google Scholar]
- Evaluation of fungal culture filtrate containing chitinase as a biocontrol agent against Helicoverpa armigera. J Appl Microbiol.. 2007;103:1845-1852.
- [Google Scholar]
- Acridoideos (Orthoptera) de importancia agroeconómica en la República Argentina. In: Roig-Junent S.A., Claps L.C., Morrone J.J., eds. Biodiversidad de Artrópodos Argentinos, Universidad Nacional de Tucumán. Argentina: Editorial INSUE; 2014. p. :1-36.
- [Google Scholar]
- Quebrachera grasshopper, Tropidacris collaris (Stoll, 1813) (Orthoptera: Romaleidae) In: Lecoq M., Zhang L., eds. Encyclopedia of Pest Orthoptera of the World. Pekin, China: China Agricultural University Publisher; 2019. p. :230-234.
- [Google Scholar]
- De Wysiecki, M.L., Lange, C.E. 2005. The locust Schistocerca cancellata Serville (Orthoptera: Acrididae: Cyrtacanthacridinae) in Argentina: biology, ecology, history and control. In: Memories of the second international course: Integrated management of the Central American locust (Schistocerca piceifrons piceifrons, Walker) and acridoideos pest in Latin América. pp. 151-156.
- Enzymatic comparison and mortality of Beauveria bassiana against cabbage caterpillar Pieris brassicae. Vet Microbiol.. 2017;48:522-529.
- [Google Scholar]
- Fungi: Hyphomycetes. In: Lacey L.A., ed. Manual of Techniques in Insect Pathology. San Diego, CA: Academic Press; 1997. p. :213-248.
- [Google Scholar]
- The use of solid media for detection of enzyme production by fungi. Mycologia. 1975;67:597-607.
- [Google Scholar]
- Melanoplus spp. In: Singh P., Moore R.F., eds. Handbook of Insect Rearing. Amsterdam: Elsevier; 1985. p. :451-464.
- [Google Scholar]
- Detection and characterization of chitinases and other chitin-modifying enzymes. Appl. Microbiol. Biotechnol.. 2003;30:627-635.
- [Google Scholar]
- Manual del usuario, versión 1. Córdoba, Argentina: Universidad Nacional de Córdoba; 2001.
- Cellulase production by Penicillium fellutanum isolated from coastal mangrove rhizosphere soil. Res. J. Microbiol.. 2006;1(5):438-444.
- [Google Scholar]
- A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales) IMA Fungus. 2017;8(2):335-353.
- [Google Scholar]
- Practice Laboratory Mycology. Buenos Aires-Argentina: Editorial Médica Panamericana S.A.; 1987.
- Kuzu, S.B., Guvenmez, H.K., Denizci, A.A. 2012. Production of a thermostable and alkaline chitinase by Bacillus thuringiensis subsp. Kurstaki strain HBK-51. Biotechnol. Res. Int. 2012, 135498.doi: 10.1155/2012/135498.
- Purification and characterization of macrodontain I, a cysteine peptidase from unripe fruits of Pseudananas macrodontes (Morr.) Harms (Bromeliaceae) J. Food Biochem.. 2000;19:443-454.
- [Google Scholar]
- The order level polyphyletic nature of Paecilomyces sensu lato as revealed through 18S-generated rRNA phylogeny. Mycologia. 2004;96:773-780.
- [Google Scholar]
- Proteases and Chitinases Induced in Beauveria bassiana during Infection by Zabrotes subfasciatus. Southwest Entomol.. 2019;44:125-137.
- [Google Scholar]
- Temperature-dependent development of Asian citrus psyllid on various hosts, and mortality by two strains of Isaria. Microb. Pathog.. 2018;119:109-118.
- [Google Scholar]
- Comparative pathogenicity of four entomopathogenic fungal species against nymphs and adults of citrus red mite on the citrus plantation. Int. J. Trop. Insect Sci. 2020 https://link.springer.com/article/10.1007/s42690-020-00263-z
- [Google Scholar]
- Characterization of mycotoxins from entomopathogenic fungi (Cordyceps fumosorosea) and their toxic effects to the development of asian citrus psyllid reared on healthy and diseased citrus plants. Toxicon. 2020;188:39-47.
- [Google Scholar]
- Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Com. Biochem. Physiol.. 2007;146:124-137.
- [Google Scholar]
- Cordyceps locustiphila (Hypocreales: Cordycipitaceae) infecting the grasshopper pest Tropidacris collaris (Orthoptera: Acridoidea: Romaleidae) Nova Hedwigia. 2018;107(3–4):349-356.
- [Google Scholar]
- Density dependent phenotypic plasticity in the South American locust, Schistocerca cancellata (Orthoptera : Acrididae) Ann. Entomol. Soc. Am.. 2019;112(5):458-472.
- [Google Scholar]
- Solid-State Fermentation for Production of Phytase by Rhizopus oligosporus. Appl. Biochem. Biotechnol.. 2012;103:251-260.
- [Google Scholar]
- Entomopathogens of Amazonian stick insects and locust are members of the Beauveria species complex (Cordyceps sensu stricto) Mycologia. 2014;106:260-275.
- [Google Scholar]
- Celtis tala and Scutia buxifolia leaf litter decomposition by selected fungi in relation to their physical and chemical properties and the lignocellulolytic enzyme activity. Eur. J. Soil Biol.. 2008;44:400-407.
- [Google Scholar]
- South American locust, Schistocerca cancellata (Serville, 1838) (Orthoptera: Acrididae) In: Lecop M., Zhang L., eds. Encyclopedia of Pest Orthoptera of the World Pekin. China: China Agricultural University Publisher; 2019. p. :198-203.
- [Google Scholar]
- Production and optimization of protease by filamentous fungus isolated from paddy soil in Thiruvarur District Tamilnadu. J A B B. 2015;3(6):066-069.
- [Google Scholar]
- Fungal entomopathogenes. In: Vega F.E., Kaya H.K., eds. Insect Pathology. London: Elsevier; 2012. p. :171-220.
- [Google Scholar]







