Translate this page into:
Microbial degradation of plastics: Sustainable approach to tackling environmental threats facing big cities of the future
⁎Corresponding author. mushahid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Most microorganisms are used as a foundation of bioplastic production and also used for the decomposition of plastics. Although bioplastics production is considered expensive than artificial plastic, it has many advantages over them. Some bio-polymers have also gained public acceptance and are now being produced. The useful breakdown of plastic bags takes more than a thousand years. For the decomposition of plastics, microorganisms should be calculated extensively so that solid wastes can be decomposed. Thus, microbes have been played an important role in decompose as well as the production of plastics.
Keywords
Microorganisms
Decomposition
Bioplastic
Solid wastes
1 Introduction
Plastic usage has transformed our life in various ways. The production and utilization of plastics are always increasing due to the rising demand. They are inexpensive, strong, lightweight, corrosion-resistant, duration and electrical insulation properties and have high thermal (Aruna and Shanthi, 2015). Five hundred billion to one trillion/annum PE (polythene) covers have been under regular use worldwide. The useful breakdown of plastic bags takes more than a thousand years. Plastic causes global warming and pollution not only as a major issue of waste disposal but then also releases dioxides and CO2 while burning (Swapnil et al., 2015).
Microplastic is being formed due to the photodegradation of large plastic by the sunlight, converting it to be even toxic, thereby contaminating the sand and water. It can be accidentally be consumed (both micro/macro forms) by the terrestrial and aquatic animals and enter the food chain/web. Especially microplastic has become a mandatory product in the food chain of marine biota nowadays (Denuncio et al., 2011). In the aquatic ecosystem, PE waste is the principal risk factor for marine animals. It causes abdominal obstruction in the fishes, birds and other aquatic and terrestrial animals (Spear et al., 1995; Secchi and Zarzur, l999). As per the report of Coe and Rogers (1997), plastic contaminants dumped in the aquatic environment affect at least 276 species, including all animals, especially 44% of seabirds and 86% of sea turtles. The postpartum of the dead terrestrial animals, have been reported to have PE carry bags in the intestine (Ghosh and Singh, 2005), which is considered to be a major root cause for its death. This PE has blocked the entire alimentary canal and its secondary toxin and intermediate digestive products of the PE. The undigested PE has been the major cause of problems in various animals (Ghosh and Singh, 2005). It has been reported that the PE obstructed digestive function, such as fermentation via blocking the other components from blending with digestive enzymes and juices, resulting in indigestion (Hartman, 1975). The blockage of the opening between the reticulum and omasum due to the obstruction of PE becomes highly fatal for the animal, if the PE is not being removed (Smith, 1964).
The digestive salts react with the PE and form complexes that obstruct the digestive tract's food passage, leading to irritation in the rumen and pain due to immunosuppression (Derraik, 2002). The plastic accumulated in the alimentary canal reduced the immunity and led to secondary infection such as hemorrhagic septicemia in the dead cow. At a global scale, nearly 10% of the Municipal Waste contains used plastic covers and materials (Barnes et al., 2009). Based on a survey, every year, 100,000 tons of plastic products have been dumped into the oceans, sea and aquatic environment, resulting in various animals death (Rutkowska et al., 2002). During the biodegradation of plastic, the plastic it enters into the food chain and produces secondary toxic material and toxins, which is fatal for aquatic animals.
Natural decomposition is a common phenomenon in which complex organic matter is transformed into a simple organic substance by living organisms, especially microbes. The microorganism obtains nutrients from the plastic via the enzymatic degradation process. Plastic waste serves as the source of energy and carbon required for their growth and development. It is a key part of recycling materials by the natural ecosystem (Joel, 1995). The plastic materials can be degraded either under anaerobic or aerobic conditions. Aerobic bacteria utilize O2 as an electron acceptor and break down the complex organic chemicals into simpler forms, often producing CO2 and water as the end product (Seymour, 1989). Aerobic and anaerobic decomposition act as a major environmental component for the natural remediation of contaminants at many harmful waste sites. In anaerobic decomposition, the microbial mechanism degrades the organic pollutants without the involvement of O2. Certain anaerobic bacteria involve carbon dioxide, manganese, sulphate, iron, nitrate, organic chemicals during the degradation process as electron acceptors to form simple products (Datta et al., 1998).
2 Available methods for study of plastic biodegradation
Polymer degradation is defined as any alteration in its physical or chemical properties due to environmental factors, including light, heat and moisture, or biological activity (Pospisil and Nespurek, 1997). There are three types of polymer degradation methods, photodegradation, thermo-oxidative degradation and biodegradation based upon the factors involved (Shah et al., 2008).
Rutkowska et al. (2002) reported that microorganisms such as bacteria, fungi, and algae could degrade polymer materials through their metabolic activity, the so-called “biodegradation,” without the involvement of heat energy under aerobic or anaerobic conditions. In the aerobic biodegradation, the end products produced were CO2 and H2O in the soil composite method. The anaerobic biodegradation of landfills and sediments includes methane, H2O and CO2 as the end products. Usually, it is a complex process to produce water and CO2 from the long-chain polymer, which needs various steps and different microbial activity. In each step, a particular microbial community will break the polymer into granules and the others will utilize the monomers and excrete them. The microbial community of the detritus food chain utilizes the excreted waste. It is an eco-friendly, cost-effective, globally accepted method, however, the efficiency is moderate. (Shah et al., 2008). A study in the biodegradation of three types of PE carry bags have been experimentally tested in aquatic ecosystem. Ordinary polythene, along with two other types of plastic carry bags, were subjected for degradation and found that 2% of degradation at the surface of the biodegradable polythene carry bags have been reported after forty weeks under experimental conditions. The degradation of standard polythene was reported to be negligible (O’Brine and Thompson, 2010).
3 Plastic degradation by bacteria and fungi
There were various reports available on polytene degradation by microbe (Table. 1). Aswale and Ade (2008) reported the biodegradation of carry bags. Bacterial isolates from the dumping areas was used and tested for the characterization of tensile strength, surface corrosion, percentage of weight are the parameters analyzed. The sample exposed under the experimental condition for three months with regular shivering of the polythene discs showed surface corrosion, reduction in tensile strength, and a maximum rate of 12.5% of weight loss by Pseudomonas sp. and Bacillus cereus were the two strains identified using biochemical tests and morphological keys, actively involved in this degradation experiment. Biodegradation of degradable plastic polyethylene by fungi Phanerochaete and bacteria Streptomyces species have been reported by Lee et al., 1991. The methods used for testing plastic degradation are molecular weight distribution, weight loss, change in tensile strength and changes in percentage of elongation. The type of microorganism used was lignocellulose degrading fungal and bacterial strains. The fungi Phanerochaete chrysosporium and bacterial strains including Streptomyces, S. setonii 75Vi2 and Viridosporus T7A, S. badius 252 actively degraded the test sample with 50% reduction in tensile strength. The initial test sample contained 6% starch and pro-oxidant, which facilitated the degradation process.
Sl. No.
Polythene Tested
Parameters checked
Microbes/ enzymes used for degradation
References
1.
Carry bags
Tensile strength, surface corrosion, weight percentage
Pseudomonas sp; Bacillus cereus
Aswale and Ade, 2008
2.
Biodegradable plastic with starch and pro-oxidant
Molecular weight distribution, weight loss, tensile strength, elongation percentage
Streptomyces;Phanerochaete chrysosporium; S. setonii 75Vi2; Viridosporus T7A; S. badius 252,
Lee et al., 1991
3.
Plastic cups and polythene bags
Loss of weight
Pseudomonas; A.nidulance; B.subtilis; P.vulgaris; S.aureus; A.niger; S.lactis; A.glaucus; A.flavus; Penicillium; M. luteus
Priyanka and Archana, 2011
4.
Branched low density polyethylene
Gravimetric and molecular weight loss
B.borstelensis strain 707
Hadad et al., 2005
6
Powdered form of low density polyethylene
Sturm test and SEM analysis.
Aspergillus sp; Aspergillus versicolor
Pramila and Vijaya Ramesh, 2011
7
Low density polythene films
Weight measurements, Tensile strength, SEM, FTIR, GC-MS
P.aeruginosa; P.putida; P.syringae
Kyaw et al., 2012
8
Linear low density polyethylene torque blended using starch
SEM, DSC, TGA, FTIR spectroscopy, loss in weight.
P.funiculosum; G.virens; P.pullulans; A.niger; C.globosum
Gilan et al., 2004
9
Low Density Polythene and LinearLow Density Polythene
GC-MS and FTIR
B. megaterium; Brevibacillus; B.cereus; B. subtilis
Abrusci et al., 2011
11
Branched Low Density polyethylene
FTIR, SEM, Average Weight loss.
Rhodococcus ruber C208
Chandra and Rustgi, 1997
12
LDPE, HDPE and LLDPE with a balanced contented of antioxidants and pro oxidants
FTIR, H NMR, SEM
Rhodococcus rhodochrous ATCC 29,672
Fontanella et al., 2009
14
HDPE and LDPE
Average heaviness
Listeria; Bacillus; Micrococcus; Vibrio
Kumar et al., 2007
16
PE carry bags
Loss of weight
Serretia marscence
Aswale and Ade, 2009
17
Low density polythene
Percentage elongation, FTIR, Tensile strength, SEM, Weight loss and spectroscopy,
Aspergillus oryzae
Konduri et al., 2011
18
Commercial environmentaly degradable polythene
Epifluorescence microscopy, SEM and FTIR
Cladosporium cladosporides ATCC 20251; Nocardia steroids GK 911; Rhodococus rhodocorous ATCC 29,672
Bonhomme et al., 2003
19
Extruded low density polyethylene (LDPE)
FTIR and SEM
Staphylococcus epidermis
Chatterjee et al., 2010
20
High density polyethylene (HDPE)
Loss of weight, Crystallinity percentage and FTIR
Arthrobacter; Pseudomonas sp
Balasubramanian et al., 2010
21
PE carry bags and cups
Tensile strength and Weight loss
Bacillus; Staphylococcus; Streptococuus;, Diplococcus; Micrococcus; Pseudomonas; Moraxella; A. ornatus; A. nidulans; A. flavus, A. candidus, A. cremeus, fungi (Aspergillus niger & A. glaucus
Reddy, 2008
22
BPE 10 & Low density polythene and
Elongation prcentage, Tensile strength, FTIR, SEM, Surface energy, and Contact angle.
Bacillus cereus (C1)
Suresh et al., 2011
23
Polyethylene bag waste and water sachets
Weight loss percentage
Aspergillus niger; Pseudomonas putida; Bacillus subtilis; Pseudomonas aeruginosa
Nwachukwu et al., 2010
24
Disposable plastic films
Tensile strength, Percentage of elongation and Average weight loss
M. rouxii NRRL 1835; Streptomyces strains; Aspergillus flavus
El-Shafei et al., 1998
25
Plastic cups and bags.
Weight loss
Streptococcus; Staphylococcus; Moraxella; Micrococcus; Pseudomonas; A. glaucus; A. niger
Kathiresan, 2003
26
High molecular weight polyethylene
Tensile strength, Relative elongation
Trametes versicolor IFO 7043 and IZU-15413; Phanerochaete chrysosporium ME-446
Iiyoshi et al., 1998
27
Degradable polyethylene
Strum test and Percentage of weight loss.
Bacillus mycoides; Penicillium frequentans
Seneviratne et al., 2006
28
Plastic carry bags
Loss of weight
Aspergillus niger
Aswale and Ade, 2011
29
Starch polyethyleneprooxidant degradable plastics
Mechanical properties, molecular weight distribution and FTIR
Streptomyces setonii 75Vi2; Streptomyces badius 252; Streptomyces viridosporus T7A
Pometto et al., 1992
30
LDPE powder
Loss of weight
Streptomyces KU1; Streptomyces KU8; Streptomyces KU6; Streptomyces KU5; Pseudomonas sp; Bacillus sp; Staphylococcus sp; A. flavus; Aspergillus nidulans
Usha et al., 2011
31
PE carry bags
FTIR, GC-MS, TLC and Weight loss.
B.cereus; S.marcescens, P.aeruginosa; S.aureus B-324; A.glaucus; M.lylae B-429; A.niger; P.chrysosporium; P.ostretus
Aswale, 2010
32
Ordinary polyethylene (vegetable starch 6%) and artificial polyethylene
Weight loss
Pseudomonas spp.
Nanda et al., 2010
33
HDPE flims
Elongation, FTIR, Tensile strength, Elongation break.
A.oryzae; A. niger; A.flavus
Konduri et al., 2010
34
Powdered Low Density Polythene
XRD, DSC, SEM and FTIR
Penicillium pinophilum; A.niger
Volke-Sepulveda et al., 2002
Priyanka and Archana (2011) evaluating the biodegradation potential of microbial isolates obtained from various soil sources, such as agricultural, sludge zone, energy park, sewage water, medicinal garden etc. The experimental sample used for analysis was plastic cups and polythene bags, which was incubated with fungal and bacterial strains for 31 days. The maximum biodegradation rate shown was 12.25% and 12.5% by the fungal strain Aspergillus niger and Streptococcus lactis bacteria respectively. Other microbes such as Pseudomonas, Aspergillus nidulance, Bacillus subtilis, Proteus vulgaris, Staphylococcus aureus, Aspergillus niger, Streptococcus lactis, Aspergillus glaucus, Aspergillus flavus, Penicillium, Micrococcus luteus also showed the potency for degrading the plastic cups and polythene bag. Biochemical and morphological tests were used for the identification of microbial strains. The rate of biodegradation has been evaluated using the loss of the weight method. The bacterial strain Brevibaccillus borstelensis 707 was isolated from bare soil, had been experimentally reported for its active degradation of branched low-density polyethylene 0.92 gcm−3. It has been reported that the rate of biodegradation was found to be 11% in gravimetric and 30% in molecular weight loss methods. The rate of biodegradation was calculated using the gravimetric, molecular weight-loss method and FTIR analysis (Hadad et al., 2005).
Rutkowska et al, 2002 investigated the biodegradation potency of microbial isolates from the Baltic sea and tested with various polymers. Samples include clear polyethylene with 5% of starch, modified polyethylene films containing 8% and polyethylene with 20% pro-degradant additives. Tensile strength, change in weight and morphology of polymer were the methods used for evaluating the rate of biodegradation. The samples were incubated in the Baltic Sea water for 20 months. The polyethylene blends showed only minimal degradation during the summer and winter. Around 26% of biodegradation has been reported for polyethylene with the additive during summer which was calculated by weight loss method. Pramila and Ramesh (2011) reported the biodegradation of powdered form of the low-density polyethylene (LPDE) using Aspergillus sp. and Aspergillus versicolor, which was analysed in SEM and sturm test to calculate the rate of biodegradation by the volume of CO2 release and found to be maximum of 4.1594 g CO2/l/week. Low-density polythene (LDPE) films biodegradation have been reported by Kyaw et al., 2012 and tested for weight measurements, testing of tensile strength, scanning based on electron microscope analysis, FTIR-ATR spectrophotometer, and gc-ms for calculating biodegradation rate. Four bacterial strains including Pseudomonas aeruginosa, PAO1 (ATCC 15729) Pseudomonas aeruginosa, (KT2440 ATCC 47054) Pseudomonas putida and (DC3000 ATCC 10862) Pseudomonas syringae showed 20% biodegradation rate for the LDPE sample.
Linear low density polyethylene torque blended using starch was inoculated with various microbial strains such as Penicilliurn funiculosum, Gliocladiurn virens and Pullularia pullulans, Aspergillus niger, Chaetomium globosum SEM, DSC, TGA, FTIR spectroscopy, loss in weight are the techniques for calculation of the biodegradation rate. It has been identified that the presence of starch content in the sample polymer is highly responsible for its decomposition by microbial consortia. The rate of degradation is directly proportional to the starch content in the mixture. Therefore, the higher the starch content, the greater will be the degree of degradation (Gilan et al., 2004). The biodegradation potency of the following strains such as Bacillus cereus, B. subtilis, B. megaterium, borstelensis, and Brevibacillus have been evaluated by Abrusci et al., 2011. The sample includes Low-Density Polythene and Linear Low-Density Polythene. The polythene films were sprinkled in agricultural fields for the segregation of microbes after 30 days. Polythene films with Fe stearate (75–85%) and Ca stearate (31–67%) at 45 °C indicated a reduction in carbonyl groups. The Rhodococcus ruber (C208) showed 7.5% of biodegradation in the branched low density polyethylene (0.92 gcm−3) after incubating for about 8 weeks. The films were analysed by using SEM, loss of weight, extracellular protein formation and polysaccharide in biofilm. Another study with Rhodococcus ruber C208 showed active biodegradation of branched low density polyethylene at a rate of 8% after incubating for 28 days and analysed in FTIR, scanning electron microscopy and loss of weight for calculating the biodegradation rate (Chandra and Rustgi, 1997).
Fontanella et al. (2009), conducted an experiment to evaluate the biodegradation rate of the different polymer samples such as LDPE, HDPE and LLDPE with a balanced content of antioxidants and pro-oxidants. Polymer samples were incubated with Rhodococcus rhodochrous ATCC 29,672 and Pseudomonas stutzeri for 45 days and analysed in FTIR, SEC measurements, HNMR spectroscopy, SEM, elongation, extension percentage after incubation to estimate the rate of biodegradation. The rate of biodegradation is highly depend on pro-oxidant additive and ecology conditions. The polymers such as LDPE (Low-density polythene) and PE (polythene) were degraded by Pseudomonas stutzeri. The PE elongation change had been recorded at regular intervals of 15, 30 and 45 days with an elongation rates of 1.8 cm in 30 days and a maximum rate of 73.38% in 45 days. The modification in the tensile strength was recorded to be 0.01 N/cm2. An experiment conducted by Kumar et al., 2007, involved in degrading the polymers HDPE and LDPE. Soil samples from Suva, Fiji Islands were used for the isolation of microbial consortium used in polymer degradation such as Listeria, Bacillus, Micrococcus, and Vibrio showed 5% biodegradation rate. Average heaviness analysis were used for the calculation of biodegradation rate.
A study to bioremediate the plastic waste in municipal solid waste were done by isolating the microbe from the soil samples collected fro municipal solid waste compost. From which, 250 strains were isolated and screened for plastic degradation and found that the fungi Trichoderma viride showed active bioremediation potential. The fungal strain was incubated for a period of 60 days and analysed for average weight loss and the strain was identified by using biochemical and morphological tests. Production of cellulose enzyme and loss of weight were the parameters used for calculating the bioremediation rate and found to have weight loss of 20.10% and 33.35% with plastic plates and 33.35% with compost pile.
An experiment with PE carry bags have been conducted by Aswale and Ade (2009). PE carry bags have been collected from dumping sites and incubated with Serretia marscence and found to have 22.22% degradation potential. The carry bags with S.marscence was incubated at 24 ˚C with pH 4.0. and incubated in shaking condition. Weight loss was analysed to determine the biodegradation efficiency. Another experiment with LDPE was conducted by Konduri et al, in the year 2011 and found that Aspergillus oryzae has the capability to degrade LDPE with a molecular weight of 1,80,000 Da. The parameters analysed after degradation experiments were elongation percentage, FTIR, tensile strength, SEM and weight loss. The tensile strength, weight loss and percentage elongation was found to be 51%, 47.2% and 62% respectiviely after 90 days of incubation. Sample were exposed to UV radiation and manganese stirrer treatments before inoculation.
Microbe such as Cladosporium cladosporides ATCC 20,251 and Nocardia steroids GK 911 and Rhodococus rhodocorous ATCC 29,672 showed biodegradation of commercially produced biodegradable polythene after incubating for a period of 243 days (Bonhomme et al., 2003). Staphylococcus epidermis enzymatically degraded the low-density polyethylene (LDPE) of 20 µm thickness. Extracellular enzymes secreted by the bacteria were responsible for the biodegradation process and causes enzymatic breakdown results in the creation of holes in the shredded polythene samples which was identified in SEM and FTIR analysis (Chatterjee et al., 2010). Commercially available High-density polyethylene (HDPE) was actively degraded by Arthrobacter sp. and Pseudomonas species. Samples were collected from polythene dumping sites and incubated with the selected microbe for about 30 days and characterized for weight loss, crystallinity percentage and in FTIR. The Biodegradation capability of Arthrobacter sp. and Pseudomonas sp were found to be 12% and 15% respectively (Balasubramanian et al., 2010).
A study to degrade the PE carry bags and cups were conducted in the year 2008. Two different samples were used in this experiment. 1. The sample polythene strips were buried in the soil of the MSW pile. 2. Naturally dumped polythene cups and carry bags were collected and buried at the onsite municipal composite. A microbial consortia includes the bacterial strains such as Bacillus sp., Staphylococcus sp., Streptococcus sp., Diplococcus sp., Micrococcus sp., Pseudomonas sp. and Moraxella sp., and fungal strains the Aspergillus nidulans, A. flavus, A. candidus, A. cremeus, A. niger and A. glaucus were isolated from the soil. In the presence of mixed microbial consortia, a maximum biodegradation rate of 11.54% weight loss was recorded in LDPE. The High-density polythene showed maximum reduction in tensile with a incubation period of 12 months (Reddy, 2008).
The BPE 10 and Low-density polythene (with 10% oxo-biodegrading additive) materials were degraded by using the bacterial strain Bacillus cereus (C1). Microbial isolates from the municipal composting yard were inoculated with the sample and subjected to 90 days of incubation. BPE10 was pre-treated before the incubation. The techniques for testing the biodegradation rate were percentage elongation, change in tensile strength, FT-IR spectroscopy, SEM analyses, surface energy and contact angle. Around 17.036% change in tensile strength along with 17.40 decrease in contact angle were recorded (Suresh et al., 2011). The bacteria consortial strains Pseudomonas putida, Bacillus subtilis and Pseudomonas aeruginosa and fungi Aspergillus niger showed 1.19% biodegradation of the polythene bags. About 1.19% of weight loss was recorded when treated with 0.5 M HNO3 which was monitored by change in colour (Nwachukwu et al., 2010). Another study conducted an experiment with disposable plastic films with microbial strains such as streptomyces, Aspergillus flavus, Mucor rouxii NRRL 1835. An extracellular enzyme production from the microbe was found to be responsible for the biodegradation of samples. After incubation, the change in tensile strength, percentage of elongation and Average weight loss were analysed to calculate the rate of biodegradation. The elongation percentage showed a reduction of 28.5% and 46.5% for fungal and bacterial strains respectively. All the samples were heated for about 10 days before incubating with microbial inoculants (El-Shafei et al., 1998).
Pseudomonas sp and Aspergillus glaucus species showed biodegradation of plastic cups and bags at a rate of 20.54 ± 0.13 and 28.80 ± 2.40, respectively. The percentage of weight loss/month is the method used for measuring the rate of biodegradation. The samples plastic cups and bags were incubated with the microbial strains isolated from mangroves rhizosphere soil and incubated in shaking condition. Eventhough other strains such as Streptococcus, Staphylococcus, Moraxella, Micrococcus, Aspergillus. niger showed biodegradation potential, the maximum rate of biodegradation has been recorded for Psedumonas sp. and Aspergillus glaucus (Kathiresan, 2003). According to the report by Iiyoshi et al., 1998, microbial strains Trametes versicolor IFO 7043 and IZU-15413 and Phanerochaete chrysosporium ME-446 will actively degrade the High-molecular-weight polyethylene. Tensile strength changes, relative elongation (Strograph-R3) and polyethylene molecular weight distribution (Waters model 150 -C) were used to measure the biodegradation rate. About 100.0 ± 1.3% change in relative tensile strength, 91.2 ± 9% relative elongation was recorded. Another research with various types of polythene waste materials were conducted by burying the soil containing indigenous microorganisms for 2–4 years. Among the isolated microbial strains, Bacillus mycoides and Penicillium frequentans found to possess degradation capability of biodegradable polyethylene samples. The combination of B. mycoides and P. frequentans showed a biodegradation rate of 7.15% of weight loss for sample preheated at 70 °C and 6.65% for the unheated sample after the incubation period of 60 days. Among the indigenous microbial consortia Bacillus mycoides and Penicillium frequentans were found to be the most effective strains (Seneviratne et al., 2006).
The Aspergillus niger showed active biodegradation of plastic carry bags. The loss of weight is the method used for calculating the biodegradation rate. Microbial isolates from polythene dumping sites were collected and inoculated with polythene carry bags. About 25% of the weight was observed after an incubation period of 32 weeks in shaking condition. The morphological identification revealed that Aspergillus niger was responsible for the biodegradation of PE carry bags (Aswale and Ade, 2011). Starch polyethylene pro-oxidant degradable plastics were used for evaluating the biodegradation potency of Streptomyces setonii 75Vi2, Streptomyces badius 252 and Streptomyces viridosporus T7A. Lignocellulose degrading microorganism was incubated with the sample polymers. The extracellular enzyme degradation has been reported for the Streptomyces setonii 75Vi2, Streptomyces badius 252, and Streptomyces viridosporus T7A. The biodegradation rate was tested using the following methods, mechanical properties, polyethylene molecular weight distribution, FTIR, percentage elongation, strain energy (Kg mm) and tensile strength (kg/mm2) etc. FTIR analysis revealed the presence of enzyme concentrates on the degraded areas (Pometto et al., 1992). LDPE (Low-density polyethylene) powder was subjected to the biodegradation assay by using the microbial isolates obtained from plastic waste dumping sites. The loss of weight method was used to calculate the biodegradation rate. The rate of biodegradation in weight loss was found to be 46.16% by Actinomycetes (Streptomyces KU8), Aspergillus flavus 37.09%, and bacteria Pseudomonas sp, 20.63% after an incubation period of 6 months (Usha et al., 2011).
The Phanerochaete chrysosporium and Pseudomonas aeruginosa showed extreme biodegradation potency for the PE carry bag samples. The rate of biodegradation reported was 50% by Phanerochaete chrysosporium and 35% for Pseudomonas aeruginosa under the experimental conditions of 24 °C with 4 pH. After incubation, samples were analysed in FTIR, GC-MS, TLC, and weight loss percentage to determine the biodegradation rate. The following microbe have also showed positive results, including Bacillus cereus, Serratia marcescens 724, Pseudomonas aeruginosa, Streptococcus aureus B-324, Aspergillus glaucus, Micrococcus lylae B-429, Aspergillus niger, Phanerochaete chrysosporiu, Pleurotus ostretus (Aswale 2010). Pseudomonas sp. (P1, P2, and P3), showed active degradation of two plastic samples, the natural polythene with 6% starch and artificial polyethylene. About 46.25% of weight loss for natural polythene and 29.1% weight loss for artificial polythene has been recorded using Pseudomonas sp (Nanda et al., 2010). Aspergillus oryzae degraded the HDPE films (0.1 μm width) at a rate of 72% after 90 days of incubation. High-Density Polythene film subjected to biodegradation showed carbonyl peak at 1718.32 cm was analysed in FTIR, tensile strength and percentage elongation (Konduri et al., 2010). Powdered Low-Density Polythene was subjected to biodegradation test using the microbial strains Penicillium pinophilum and Aspergillus niger. Analytical characterization was done by using X-ray diffraction, DSC, SEM, and FTIR. After 31 months of incubation, a maximum rate of 5% reduction in crystallinity was seen by A.niger and 11.07% of the change in glassy with Pencillium pinophilum has been recorded. The samples were incubated under 2 different conditions, such as the presence and absence of ethanol. Mineralization was found to be high for Pencillium pinophilum when incubated with ethanol (Volke-Sepulveda et al., 2002).
4 Microbial mechanism of PE degradation
Decomposition of PE has been initiated with its attachment to the microbial cell surface (Fig. 1). Various bacteria, including Streptomyces setonii 75Vi2 and Streptomyces viridosporus T7A and Streptomyces badius 252 and fungi, secrete extracellular enzymes that facilitate the decomposition of PE (Iiyoshi et al., 1998; Pometto et al., 1992; Kim et al., 2005). In fungi, the ligninolytic system's extracellular enzymes contain laccases, oxidases enzymes and catalases that produce the extracellular hydrogen peroxide (Ruiz-Dueñas and Martínez, 2009). Based on the microbe, culture conditions, strains, and enzymes involved the degradation of plastic may vary (Seneviratne et al., 2006). The decomposition of lignin involves three enzymes, the MnO2 (Manganese peroxidase) and lignin peroxidase (LiP) and phenoloxidase, with copper, called laccase. (Iiyoshi et al., 1998; Maciel et al., 2010). Based on this ligninolytic enzyme's potency, they have been used widely in different industries such as chemical, textile, fuel, agricultural, food, cosmetic, paper, and used to remediate xenobiotic compounds dyes (Maciel et al., 2010). During the degradation of phenolic compounds, the lignin materials are degraded via oxidation under MnO2 (MnP) and H2O2. MnO2 oxidizes monomeric phenols and Mn-II to Mn-III (Gilan et al., 2004), and Mn-III oxidizes synthetic lignin (Wariishi et al., 1991) and phenolic lignin dimmers (Wariishi et al., 1989) via the formation of phenoxy radicals (Kim et al., 2005). The production of various secondary products from PE degradation is based on biodegradation conditions. Under aerobic conditions, CO2, H2O is the end product produced and anaerobic degradation leads to methane (under methanogens), water and carbon dioxide as final products. H2S is produced under the existence of sulfate-reducing bacteria (Arutchelvi et al., 2008).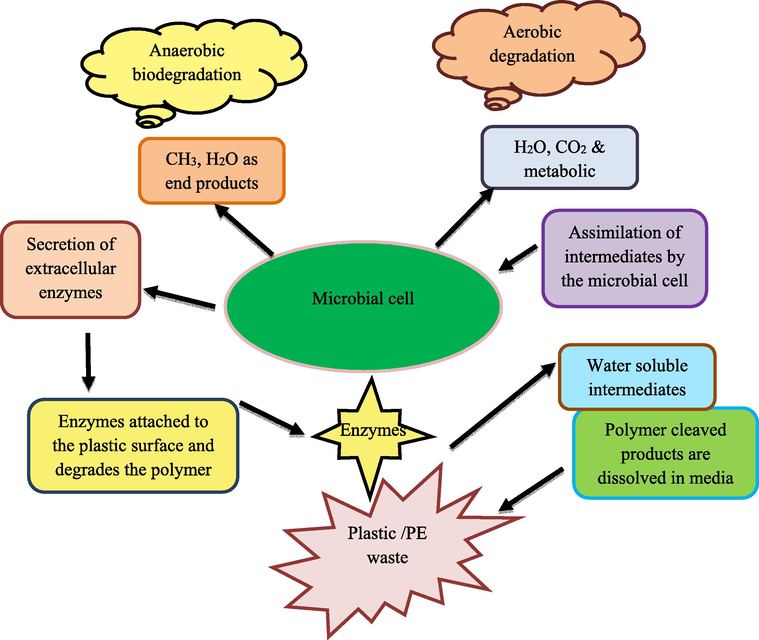
Microbial degradation of plastic polymers.
5 Investigating the techniques involved in the bioremediation of plastic.
Natural decomposition of the polymer can be characterized by uptake of O2, the rate of CO2 released alterations in the polymer's physical and chemical properties, and microbial growth rate (Mohan and Srivastava, 2010) used different assessments method for evaluating the polymer degradation based on the following reasons (Table 2).
-
CO2 production may result in the decomposition of the polythene's low molecular weight fraction, with no degradation in the elongated chain.
-
Loss of additives or minor changes in chemical makeup may affect the plastic strength.
-
Loss of weight may be due to the percolating of additives with plasticizers.
| S. No. | Variations in characteristics of polymer | Various Types of technique | References |
|---|---|---|---|
| 1 | Mechanical: Modulus of the polymer & Tensile strength -Elongation at fail | Dynamic Mechanical Analysis (DMR) | Huang et al., 2005; |
| 2 | Physical: Morphology- Microcracks | Scanning Electron Microscope (SEM) | Kathiresan, 2003 |
| Density, Molecular Weight Distribution, Contact angle, Viscosity | HT-GPC (High-Temperature Gel Permeation Chromatography) | Kathiresan. 2003 | |
| Glass Transition temperature and Melting | DSC, Thermogravimetric analysis. | Zuchoswka et al., 1999 | |
| Amorphous region and Crystalline | X-diffraction, Small and Wide-angle X ray Scattering. | Albertson et al., 1995 | |
| 3 | Chemical properties | Fourier Transformed Infrared Spectroscopy (FTIR) | Doble et al., 2008. |
| 4 | Molecular Weight | Gas Chromatography-Mass Spectrometry (GC-MS), Gas Chromatography (GC), Thin-layer Chromatography (TLC), Nuclear Magnetic Resonance (NMR), Matrix-Assisted Laser Desorption Ionization-Time Of Flight, Chemiluminescence. | Albertson et al., 1995; Deguchi et al., 1997. |
| 5 | Evolution test of CO2 | Gas Chromatography (GC) | Albertson et al., 1995; Seneviratne et al., 2006 |
| 6 | Metabolic force of the cell | protein analysis, Fluorescein Diacetate (FDA), Adenosine triphosphate (ATP) | Gilan et al., 2004; Kounty et al., 2006 |
6 Toxicity of biodegradable plastic
Pure plastics are less toxic due to their relative chemical inertness and insolubility in water. Adipates and phthalates are plasticizers added as an additive in several plastic products such as polyvinyl chloride (PVC) to change its tensile strength and percolate these compounds out of the product in trace amounts. Polystyrene in the food containers has been predicted to be leached and entered into the human body resulting in hormonal imbalance, highly carcinogenic, and adverse effects in the living organism. The entirely produced parent polymers are free from toxicity, but the monomers used during the production process are highly toxic. In 2010, Aswale monitored the effect of biodegraded polythene on seed germination in plants including soya bean, safflower, groundnut, sesame and sunflower. During the observation, the pre-treated seeds show a decreased percentage in seed germination. The degradation of polythene bags, cups were studied using P. aeruginosa, Streptomyces sp, A. niger, S. aureus, Rhizopus sp. and biodegraded polythene toxicity level (Pramila and Ramesh, 2011; Seneviratne et al., 2006) Carbon dioxide gas is the main product produced during the degradation of PE (polythene). The granules from the bio-treated polythene showed adverse effects on the roots, which leads to abnormalities in the production of polysaccharides, proteins & also nutrient uptake (Abrusci et al., 2011). Protein was the only product produced in Nocardia asteroids GK911(Bacterium) reported by Bonhomme in the year 2003. Kannahi and Sudha (2013) observed the production of carboxylic acids, ketones, and aldehyde in the smoke of film extrusion of low-density polythene (LDPE).
7 Conclusion
Awareness must be created about plastic pollution and its adverse effects on living organisms. Mass level through monthly campaigns and eminence of PE (polythene) pollution should be updated area wise to create awareness among the public. People should be encouraged to use eco-friendly products. Methods for Proper disposal of plastics must disseminate among people using all available media platforms. Selection of appropriate microbial strains, adapting suitable in-situ and ex-situ remediation techniques, continuous monitoring of remediation site, and proper maintenance such as providing proper aeration, nutrients necessary for microbial growth and physicochemical conditions are highly required. Genetic, molecular analysis for identifying genes responsible for producing plastic degrading enzymes and recombinant DNA technology can improve and accelerate remediation of plastic waste and its disposal. The awareness should be highly created at the school level by guiding the students to properly separate the biodegradable and non-biodegradable plastic waste before its disposal.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the Project no. (IFKSURP-1435-012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int. Biodeterior. Biodegrad.. 2011;65:451-459.
- [Google Scholar]
- Degradation product pattern and morphology changes as means to differentiate abiotically and biotically aged degradable polythene. Polymer. 1995;36:3075-3083.
- [Google Scholar]
- Biodegradation of Plastics – A Brief Review. Int. J. Pharm. Sci. Rev. Res.. 2015;31(2):204-209.
- [Google Scholar]
- Biodegradation of polyethylene and polypropylene. Ind. J. Biotechnol.. 2008;7:9-22.
- [Google Scholar]
- Aswale, P,N,, Ade, A.B., 2009. Effect of pH on biodegradation of polythene by Serretia marscence, The. Ecotech. 1, 152-153
- Studies on bio-degradation of polythene. Aurangabad, India: Dr Babasaheb Ambedkar Marathwada University; 2010. PhD thesis
- Aswale, P., Ade, A., 2011. Polythene degradation potential of Aspergillus niger. In:
- High-density polyethylene (HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar. India. Lett. Appl. Microbiol.. 2010;51:205-211.
- [Google Scholar]
- Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. Royal. Soc. B. Biol. Sci.. 2009;364:1985-1998.
- [Google Scholar]
- Biodegradation of maleated linear low-density polyethylene and starch blends. Polym. Degrad. Stab.. 1997;56:185-202.
- [Google Scholar]
- Enzyme-mediated biodegradation of heat treated commercial polyethylene by Staphylococcal species. Polym. Degrad. Stab.. 2010;95:195-200.
- [Google Scholar]
- Marine Debris: sources, impacts, and solutions. Springer, New York: Sci; 1997.
- Popular Plastics And Packaging. New Delhi, India: Mahindra Publishers; 1998. p. :73.
- Nylon Biodegradation by Lignin-Degrading Fungi. Appl. Environ. Microb.. 1997;63(1):329-331.
- [Google Scholar]
- Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar Pollut Bull 2011
- [Google Scholar]
- The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull.. 2002;44:842-852.
- [Google Scholar]
- Marine microbe mediated biodegradation of low and high density polyethylenes. Int. Biodeter. Biodeg.. 2008;61(3):203-213.
- [Google Scholar]
- Biodegradation of disposable polyethylene by fungi and Streptomyces species. Polym. Degrad. Stab.. 1998;62:361-365.
- [Google Scholar]
- Comparison of the biodegradability of various polyethylene films containing pro-oxidant Additives. Polym. Degrad. Stab.. 2009;95:1011-1021.
- [Google Scholar]
- A review on phytoremediation of heavy metals and utilization of its byproducts. Appl. Ecol. Environ. Res.. 2005;3:1-18.
- [Google Scholar]
- Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol.. 2004;65:97-104.
- [Google Scholar]
- Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J. Appl. Microbiol.. 2005;98:1093-1100.
- [Google Scholar]
- An evaluation of land treatment of municipal wastewater and physical siting of facility installations. Army, Washington DC: Office of the Chief of Engineers; 1975.
- Effect of compatibilizer on the biodegradation and mechanical properties on high content starch /low density polyethylene. Polym. Degrad. Stab.. 2005;90:95-105.
- [Google Scholar]
- Polyethylene degradation by lignindegrading fungi and manganese peroxidase. J. Wood. Sci.. 1998;44:222-229.
- [Google Scholar]
- Joel, F.R., 1995. Polymer Science & Technology: Introduction To Polymer Science, Eds. 3, Pub: Prentice Hall PTR Inc, Upper Saddle River, New Jersey 07458, 4-9.
- Screening of polythene and plastic degrading microbes from Muthupet mangrove soil. J. Chem. Pharm. Res.. 2013;5(8):122-127.
- [Google Scholar]
- Polythene and plastic degrading microbes from mangrove soil. Rev. Biol. Trop.. 2003;51:629-633.
- [Google Scholar]
- Cloning of a manganese peroxidase cDNA gene repressed by manganese in Trametes versicolor. J. Microbiol.. 2005;43:569-571.
- [Google Scholar]
- Synergistic Effect of Chemical and Photo Treatment on the Rate of Biodegradation of High Density Polyethylene by Indigenous Fungal Isolates. Int. J. Biotechnol. Biochem.. 2010;6:157-174.
- [Google Scholar]
- Effect of Pro-Oxidants on Biodegradation of Polyethylene (LDPE) by Indigenous Fungal Isolate, Aspergillus oryzae. J. Appl. Polym. Sci.. 2011;120:3536-3545.
- [Google Scholar]
- Kounty, M., Lemaire., Delort, A.M., 2006. Biodegradation of polyethylene films with pro-oxidant additives, Chemo. 64, 1243-1252.
- Diversity and effectiveness of tropical mangrove soil microflora on the degradation of polythene carry bags. Rev. Biol. Trop.. 2007;55:777-786.
- [Google Scholar]
- Biodegradation of Low Density Polythene (LDPE) by Pseudomonas species. Ind. J. Microbiol.. 2012;52(3):411-419.
- [Google Scholar]
- Biodegradation of degradable plastic polyethylene by phanerochaete and streptomyces species. Appl. Environ. Microbiol.. 1991;57:678-685.
- [Google Scholar]
- Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: A review. Electron. J. Biotechnol.. 2010;13
- [Google Scholar]
- Microbial deterioration and degradation of polymeric materials. J. Biochem. Tech.. 2010;2(4):210-215.
- [Google Scholar]
- Studies on the biodegradation of natural and synthetic polyethylene by Pseudomonas spp. J. Appl. Sci. Environ. Manage.. 2010;14:57-60.
- [Google Scholar]
- Occurrence and recalcitrance of polyethylene bag waste in Nigerian soils. Afr. J. Biotechnol.. 2010;9:6096-6104.
- [Google Scholar]
- Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull.. 2010;60:2279-2283.
- [Google Scholar]
- Production of an Extracellular Polyethylene-Degrading Enzyme(s) by Streptomyces Species. Appl. Environ. Microbiol.. 1992;58:731-733.
- [Google Scholar]
- Highlights in chemistry and physics of polymer stabilization. Macromol. Symp.. 1997;115:143-163.
- [Google Scholar]
- Biodegradation of low density polyethylene (LDPE) by fungi isolated from marine water- a SEM analysis. Afr. J. Microbiol. Res.. 2011;5:5013-5018.
- [Google Scholar]
- Biodegradation of low density polyethylene (LDPE) by fungi isolated from municipal landfill area. J. Microbiol. Biotech. Res.. 2011;1(4):131-136.
- [Google Scholar]
- Biodegradability of Polythene and Plastic by the Help of Microorganism: A Way for Brighter Future. J. Environ. Analytic. Toxicol.. 2011;1:111.
- [Google Scholar]
- Impact of soil composting using municipal solid waste on biodegradation of plastics. Ind. J. Biotechnol.. 2008;7:235-239.
- [Google Scholar]
- Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol.. 2009;2:164-177.
- [Google Scholar]
- Biodegradability of Polyethylene Starch Blends in Sea Water. Pol. J. Environ. Stud.. 2002;11:267-274.
- [Google Scholar]
- Polyethylene biodegradation by a developed Penicillium-Bacillus biofilm. Curr. Sci.. 2006;90:20-22.
- [Google Scholar]
- Polymer Science Before and After 1899: Notable Developments during the Lifetime of Maurtis Dekkar. J. Macromol. Sci. Chem.. 1989;26:1023-1032.
- [Google Scholar]
- Biological degradation of plastics: A comprehensive review. Biotechnol. Adv.. 2008;26:246-265.
- [Google Scholar]
- Smith, W.M., 1964. Manufacture of plastic, Volume 1. Technology and Engineering, Reinhold Pub. Corp, USA.
- Spear, L,B,, Ainley, D.G., Ribic, C.A., l995. Incidence of plastic in seabirds from the tropical pacific l984-1991: Relation with distribution of species, sex, age, season, year and body weight, Mar. Environ. Res. 40, 123-146.
- Investigation on biodegradability of polyethylene by Bacillus cereus strain Ma-Su isolated from compost soil. Int. Res. J. Microbiol.. 2011;2:292-302.
- [Google Scholar]
- Swapnil, K.K., Amit, G., Deshmukh., Mahendra, S.D, Vikram, B.P., 2015. Microbial degradation of plastic: a review, J. Biochem. Tech. 6(2), 952-961.
- Screening of polyethylene degrading microorganisms from garbage soil. Libyan. Agric. Res. Cen. J. Intl.. 2011;2:200-204.
- [Google Scholar]
- Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J. Appl. Polym. Sci.. 2002;83:305-314.
- [Google Scholar]
- Oxidative cleavage of a phenolic diarylpropane lignin model dimer by manganese peroxidase from Phanerochaete chrysosporium. Biochem.. 1989;28:6017-6023.
- [Google Scholar]
- In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem. Biophys. Res. Commun.. 1991;176:269-276.
- [Google Scholar]
- Physical Structure of polyolefin starch blends after ageing. Polym. Degrade. Stab.. 1999;64(3):39-347.
- [Google Scholar]







