Translate this page into:
Potential therapeutic effect of synthesized AgNP using curcumin extract on CCl4-induced nephrotoxicity in male mice
⁎Corresponding author. habdrabou@ksu.edu.sa (Hossam Ebaid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nephrotoxicity is a particularly important challenge in a clinical context. Oxidative stress, plays an important role in the pathogenesis of kidney. Curcumin has a potential antioxidant impacts, despite of its low water solubility and bioavailability which restricts its uptake. Here, we elevated curcumin solubilization and bioactivity by including in a modified silver nanoparticles. This study is aimed at assessing the protective impacts of silver nanoparticles with curcumin (AgNP-curcumin) against CCl4-induced injury in mice kidney. C57BL/6 mice were categorized into three groups. Group 1 mice received vehicle only. Group 2 mice received a single dose of 1 mL/kg CCl4 in paraffin. Group 3 mice were treated with 2.5 mg/kg AgNP-curcumin twice a week for 3 weeks after receiving CCl4 challenge. Results showed that CCl4 disrupted oxidative markers. Reduced glutathione was significantly decreased and the lipid peroxidation indicator MDA was significantly elevated in the CCl4 group. Urea, creatinine and gamma glutamyl transferase (GGT) were significantly elevated in the CCl4 group. However, lactate dehydrogenase (LDH) was significantly decreased. Severe histopathological changes were observed in the renal tissues in the CCl4 group compared with the findings in the control group. AgNP-curcumin was found to significantly restore the oxidative balance and kidney structure and function. The comet assay showed that CCl4 increases nuclear DNA tail length (DNA damage) by 76.36% compared with the control, while AgNP-curcumin demonstrated a decrease in tail length by 30.53% relative to that in the CCl4 group. Through its antioxidant activity, AgNP-curcumin showed a potential role in preventing the deleterious effects of CCl4 on DNA in renal tissues.
Keywords
Carbon tetrachloride
Nephrotoxicity
Oxidative stress
Toxicity markers
Silver nanoparticles-curcumin
1 Introduction
Drug-induced nephrotoxicity is a common complication of several medications and diagnostic materials (Nolin and Himmelfarb, 2010). Inflammation, oxidative stress, and apoptosis play important roles in the pathogenesis of CCl4-induced kidney damage (Safhi, 2018; Hassan et al., 2020). Scientific interest is increased in herbal medicine (Badr et al., 2011; Ebaid et al., 2011; Alhazza et al., 2019) and natural products (Ebaid, 2014a, 2014b; Ahmed et al., 2015; Al-Tamimi et al., 2018) and their beneficial pharmacological effects. Curcumin (Curcuma longa) has been used for centuries as a herbal medicine to treat diabetic ulcers, rheumatism, anorexia, sinusitis, and cough (Mahmoud et al., 2014; Marslin et al., 2018). It has beneficial effects on the renal, hepatic, and cardiac systems, exerts hypoglycemic, neuroprotective, and anti-rheumatic effects, and also protects against myocardial infarction and inhibits blood clotting (Naksuriya et al., 2014). In addition, curcumin can play an important role in protecting against and treating kidney disease. A study showed that curcumin with vitamin E exerted protective effects against CCl4-induced nephrotoxicity in mice (Venkatanarayana et al., 2012). The role of curcumin has been reported to be associated with its anti-apoptotic profile (Farkhondeh et al., 2019), prevention of respiratory complex I activity (Tapia et al., 2014) and antioxidant and anti-inflammatory effects (Farkhondeh et al., 2019). Moreover, the nephroprotective effect of curcumin also was reported to be associated with autophagy and decreased mitochondrial fission (Molina-Jijón et al., 2016).
Curcumin has low plasma levels because its poor absorption, rapid metabolism, and rapid systemic elimination. To improve the curcumin bioavailability, numerous approaches have been undertaken (Anand et al., 2007). Nanotechnology is a growing field that can potentially provide a range of new applications for drugs, food preservation, medicine and diagnostic technologies (Mortezaee et al., 2019). Recently, nanotechnology has been used to improve the stability and bioavailability of curcumin, and it has been synthesized in the form of micro-formulations to treat a variety of diseases (Sivasami and Hemalatha, 2018). Nanomaterials possess unique physico-chemical properties, making them ideal for a range of new nanotechnology applications (Zhao et al., 2018). Nanoparticles can show novel properties depending on their size, morphology, and shape, which enable them to interact with microbes, plants, and animals (Siddiqui et al., 2018; Hassan et al., 2021). In addition, nanoparticles can play a role in treating many recalcitrant diseases, including infection with human immunodeficiency virus (HIV) and cancer, as well as having the ability to deliver drugs to target sites (De la Harpe et al., 2019).
Silver nanoparticles (AgNPs) were recently synthesized (Siddiqui et al., 2018). They possess a broad spectrum of antifungal, antibacterial, and antiviral properties (Yin et al., 2020) and exhibit many other bioactivities. Therefore, AgNPs occupy an important position among the nanoparticles developed to date (Ahamed et al., 2010). Silver compounds in its nano size have received more research interest due to their effect as antibacterial and/or antifungal. In addition, silver nanoparticles has broader medical impacts as antioxidant (Prakash et al., 2013; Jyoti et al., 2016). In this context, the combination of silver nanoparticles with curcumin leads to an improvement in the curcumin and silver bioavailability and stability for better medication performance (Chen and Schluesener, 2008; Ravindra et al., 2012; Moghadamtousi et al., 2014; Yang et al., 2016; Gao et al., 2020). The present study investigated the therapeutic impacts of the AgNPs-curcumin against CCl4-nephrotoxicity mouse model.
2 Materials and methods
2.1 Fabrication of silver nanoparticles stabilized with curcumin matrix
The silver nanoparticles stabilized with curcumin were prepared according to Jyoti et al. (2016) with some modifications. Silver nitrate was added to polyvinylpyrrolidone and well mixed in an ethylene glycol solution. Then, 100 mL curcumin dissolved in ethylene glycol was added to the reaction solution under magnetic stirring for 60 min. The reaction heat was raised slowly to reach 100 °C and then kept for 180 min. The formed silver nanoparticles stabilized with curcumin matrix were collected in acetone and washed with ethyl alcohol and water.
2.2 Animals
Twenty-four healthy male C57BL/6 mice (30 g, 12–14 weeks old) were purchased from King Faisal Hospital, Riyadh, Saudi Arabia. They were placed in plastic cages under controlled conditions (22 ± 3 °C; 72%–77% relative humidity; 12 h day/night cycle) in the Animal House (Department of Zoology, KSU) on a pellet diet with fresh tap water ad libitum. The animals were randomly divided into three groups (n = 8): Group I (control): mice were intraperitonealy injected with 1 mL/kg saline. Group 2 mice received a single intraperitoneal injection of 1 mL/kg CCl4 in liquid paraffin (1:1 v/v) to achieve an ultimate dose of 0.1 mL/animal according to Makni et al. (2011). Groups 3 was treated with 2.5 mg/kg AgNPs-curcumin, twice a week for 3 weeks (Hassan et al., 2019) after receiving CCl4 challenge. After completion of the treatments, animals were sacrificed for collecting their blood and kidneys for further use. The study was approved by the Ethical Committee in the King Saud University (KSU-SE-20-38).
2.3 Preparation of samples
The animals were sacrificed and the kidneys were collected and washed with 0.1 mol/L phosphate-buffered saline (pH 7.4). Then, the samples were homogenized (Ika-Werke, Germany) in 25 mmol/L Tris-KCl buffer (pH 7.36). The supernatants were separated and kept at − 80 °C until use. The blood samples were collected in vacuum polystyrene tubes (USA), centrifuged at 1000×g and serum was stored at −20 °C. Moreover, one kidney from each animal was used for the comet assay and histopathological study.
2.4 Assessment of kidney functions
Urea and creatinine assays were carried out using Quimic Clininica Aplicada diagnostic kits (S.A. Spain) according to the manufacturer instructions.
2.5 Measurement of lactate dehydrogenase (LDH)
The level of lactate dehydrogenase was used as an indicator for assessing the mode of cell death. LDH was estimated by the commercial kits in the tissue samples, in accordance with the manufacturer’s manual using Quimica Clinica Aplicada diagnostic kits (S.A., Spain).
2.6 Assessment of toxic insult on the renal tissues
Gamma-glutamyl transferase (GGT) and albumin were used as an indicator for assessing the toxic burden on the target organ (Ahmad et al., 2015) using commercial kits (Quimica Clinica Aplicada diagnostic kits, S.A., Spain) in the tissue samples, in accordance with the manufacturer’s manual.
2.7 Measurement of reduced glutathione (GSH) and oxidation of lipids
The GSH level was evaluated by as previously described method (Jollow et al., 1974). In addition, the lipid oxidation was determined by measuring the level of MDA as previously described in the method of Buege and Aust (1978).
2.8 Comet assay of kidney samples
In this study, the comet assay of kidney samples was performed using a slightly modified version of the protocol of Singh et al. (1988) under alkaline conditions (Hassan et al., 2019, 2020).
2.9 Histopathological assessment of kidney
Sections from renal tissues were prepared and stained with hematoxylin and eosin as previously described (Ebaid et al., 2012). Renal tissue was examined using a Leica DMRB/E light microscope (Heerbrugg, Switzerland) with a camera (MC 170 HD; Leica, Singapore).
2.10 Statistical analysis
All of the data are expressed as the mean ± SD and were analyzed by GraphPad Prism 5 software. One-way ANOVA with Tukey’s post hoc multiple comparison test was applied. In the present study, a p value < 0.05 was considered to indicate statistical significance. Superscript letters *, **, and *** are used to indicate significant differences from the negative control (CN−, Group I) at p values of < 0.05, <0.005, and < 0.001, while #, ##, and ### are used to indicate significant differences from the CCL4 group at the same p value thresholds.
3 Results
3.1 Silver nanoparticles stabilized with curcumin matrix
The prepared silver nanoparticles stabilized with curcumin matrix are examined by SEM which indicates the spherical and agglomerated structure (Fig. 1). In addition, the entire structure of the silver nanoparticles stabilized with curcumin matrix was characterized by the XRD (Fig. 2). Four clear peaks have been detected at 20° of 38°, 45°, 65° and 78° which are characteristic for the formation of crystalline silver nanoparticles (Dipankar and Murugan, 2012; Ahmed et al., 2016; Jyoti et al., 2016; Verma and Mehata, 2016).
The Scanning electron microscopy (SEM) of the silver nanoparticles stabilized with curcumin matrix (AgNP-curcumin), showing the morphology of the surface at 20000.
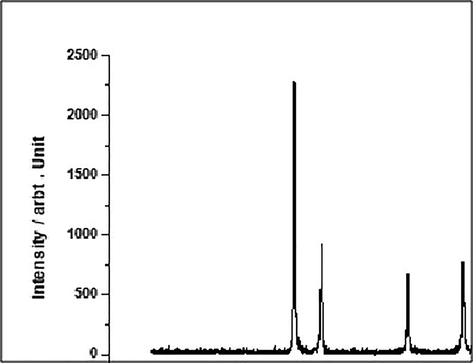
The XRD patterns off the silver nanoparticles stabilized with curcumin matrix.
3.2 Effect of AgNP-curcumin on oxidative stability
3.2.1 a. Effect on MDA levels
The CCl4 group showed a significant increase in the MDA level by 32% (control value vs treated value; P ≤ 0.05), while the CCl4 + AgNP-curcumin group showed a decline in its level by 9.23% compared with that in the CCl4 group (Fig. 3).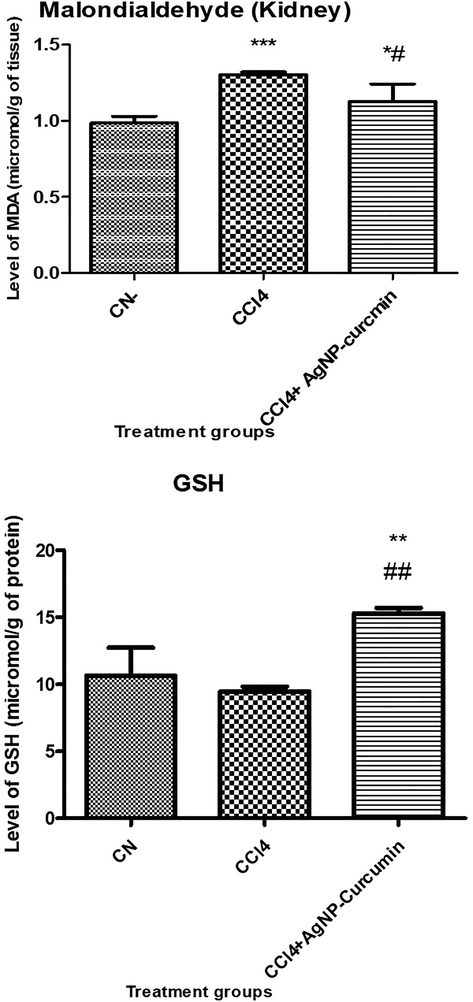
Bar graph showing the levels of oxidative stress, reduced glutathione, and lipid peroxidation. All data are expressed as mean ± SD. An asterisk shows a significant difference from the negative control (CN) at p < 0.05, while a hash symbol shows a significant difference from the positive control (CCl4) at p < 0.05.
3.2.2 Effect on GSH levels
The CCl4 group showed a decline in its GSH level by 22.57% (P ≤ 0.05), while the CCl4 + AgNP-curcumin group showed elevation in its level by 72.88% (P ≤ 0.05) compared with that in the CCl4 group (Fig. 3).
3.3 Effect of AgNP-curcumin on toxicity markers
To assess the toxic burden in vivo, the following markers were estimated to assess the toxic load after exposure to a toxic substance.
3.3.1 Albumin
No significant changes in the albumin concentration were identified among the different mouse groups. The CCl4 group showed no significant elevation in its albumin level in relative to the control group, while the CCl4 + AgNP-curcumin group showed no significant decrease in its level in comparison to the CCl4 group (Fig. 4).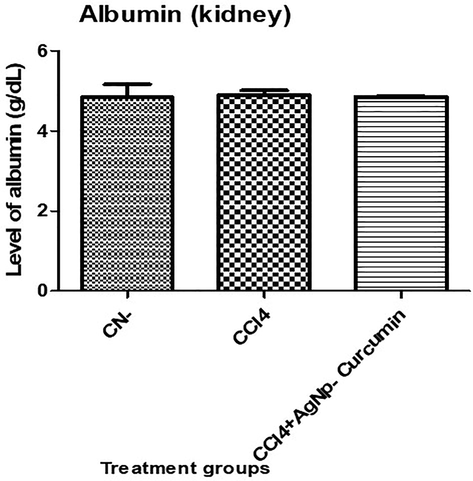
Bar graph showing the levels of albumin in the serum samples. An asterisk shows a significant difference from the negative control (CN) at p < 0.05, while a hash symbol shows a significant difference from positive control (CCl4) at p < 0.05.
3.3.2 Gamma glutamyl transferase
The CCl4 group showed a significant (P value < 0.05) increase in its GGT activity by 13.48% concerning to the control mice, while the CCl4 + AgNP-curcumin group showed a significant (P value < 0.05) decrease in its activity by 37.95% (P ≤ 0.05) compared with that in the CCl4 group (Fig. 5).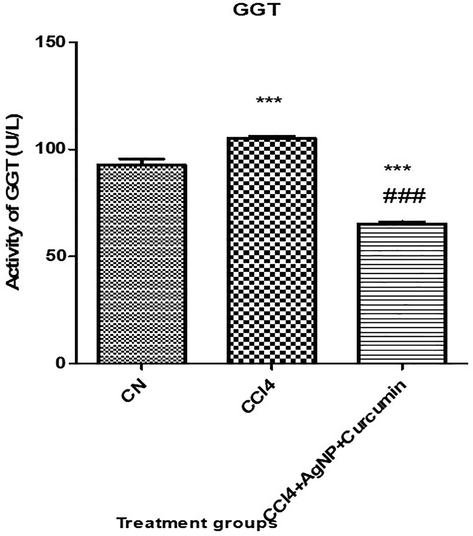
Bar graph showing the levels of gamma-glutamyl transferase (GGT) in the serum samples. An asterisk shows a significant difference from the negative control (CN) at p < 0.05, while a hash symbol shows a significant difference from positive control (CCl4) at p < 0.05.
3.4 Assessment of necrosis by lactate dehydrogenase
Lactate dehydrogenase (LDH) is an indicator of necrosis in living systems (Chan et al., 2013). In the present study, the CCl4 group showed a significant (P value < 0.05) decline in its LDH activity by 10.99%, while the CCl4 + AgNP-curcumin group showed a significant (P value < 0.05) decline in its level by 36.85% compared with that in the CCl4 group (Fig. 6).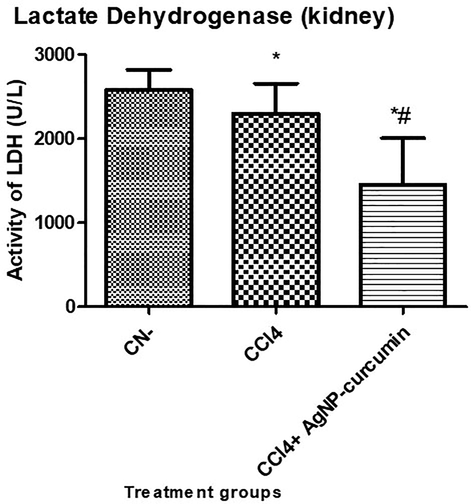
Bar graph showing the level of lactate dehydrogenase (LDH) in the serum samples. An asterisk shows a significant difference from the negative control (CN) at p < 0.05, while a hash symbol shows a significant difference from the positive control (CCl4) at p < 0.05.
3.5 Effect of nanoparticles on renal function markers
In the present study, urea and creatinine were chosen to assess the efficacy of NPs in the amelioration of CCl4-induced renal toxicity in vivo.
3.5.1 Urea
Group II showed a significant (P value < 0.05) elevation in its urea level by 59% compared with that in the control Group I. However, in the animals treated with NPs, namely, Group III, after CCl4 treatment there was a significant (P value < 0.05) decrease in urea level by 30.55% compared with that in Group II (Fig. 7).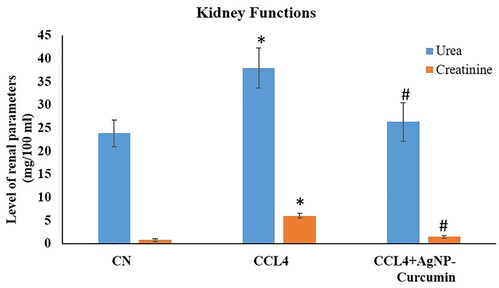
Bar graph showing the levels of urea and creatinine in the serum samples in kidney function tests. An asterisk shows a significant difference from the negative control (CN) at p < 0.05, while a hash symbol shows a significant difference from the positive control (CCl4) at p < 0.05.
3.5.2 Creatinine
Group II demonstrated a significant (P value < 0.05) increase in its creatinine level by 685.33% in comparison with that in the control group, while Group III displayed a significant (P value < 0.05) decrease in the creatinine level by 75.38% compared with that in Group II (Fig. 7).
3.6 Assessment of damage to nuclear DNA by comet assay
The CCl4 group exhibited an extension in tail length by 76.36% in comparison to CN. However, the CCl4 + AgNP-curcumin group displayed a shortening of the tail length by 30.53% relative to the CCl4 group (Fig. 8).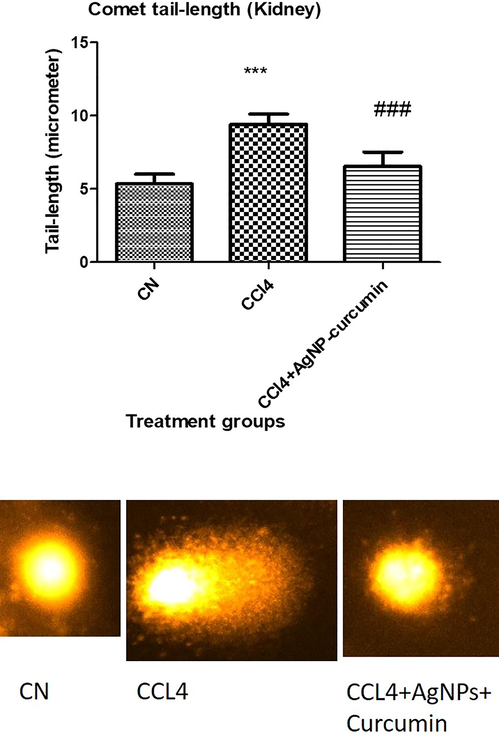
A representative image of the comet of kidney cells of the indicated animal groups. All data of the comet tail length are expressed as mean ± SD. Three asterisks indicate a significant difference from the negative control (CN) at p < 0.001, while three hash symbols show a significant difference from the positive control (CCl4) at p < 0.001.
3.7 Effect of nanoparticles on kidney histopathology
Fig. 8A, B shows the normal structural integrity of the kidney. However, nephrotoxicity induced by CCl4 was histologically clear in terms of severe alterations were observed in these mice (Fig. 8C, D). Examinations revealed dilated blood vessels associated with hemorrhage in the CCl4 mice. In the renal tissue of these mice, the glomeruli were found to be edematous with very narrow urinary spaces. Cells of many proximal convoluted tubules were eosinophilic with a narrow lumen (Fig. 8D). AgNP-curcumin treatment was found to markedly restore the deteriorated histological architecture of the kidney (Fig. 8E, F).
4 Discussion
It has been proved that curcumin possesses significant antioxidant, anti-inflammatory, and antitumor activities (Mahmoud et al., 2014). It was suggested that curcumin derivatives caused cell death by apoptosis and autophagy in tumors (Tseng et al., 2019). Recently, silver nanoparticles have been investigated for potential bioactivity. For example, AgNPs (green-synthesized) in combination with a curcumin derivative exhibited antitumor efficacy without acute toxicity (Murugesan et al., 2019). Numerous reports have proved that curcumin has excellent antioxidant properties that can be utilized to nullify the toxicity of many xenobiotics and counteract many diseases (Loo et al., 2016; Alves et al., 2018; Jaiswal and Mishra, 2018). Here, we investigated the effects of AgNPs in association with curcumin on CCl4-induced renal toxicity (See Fig. 9).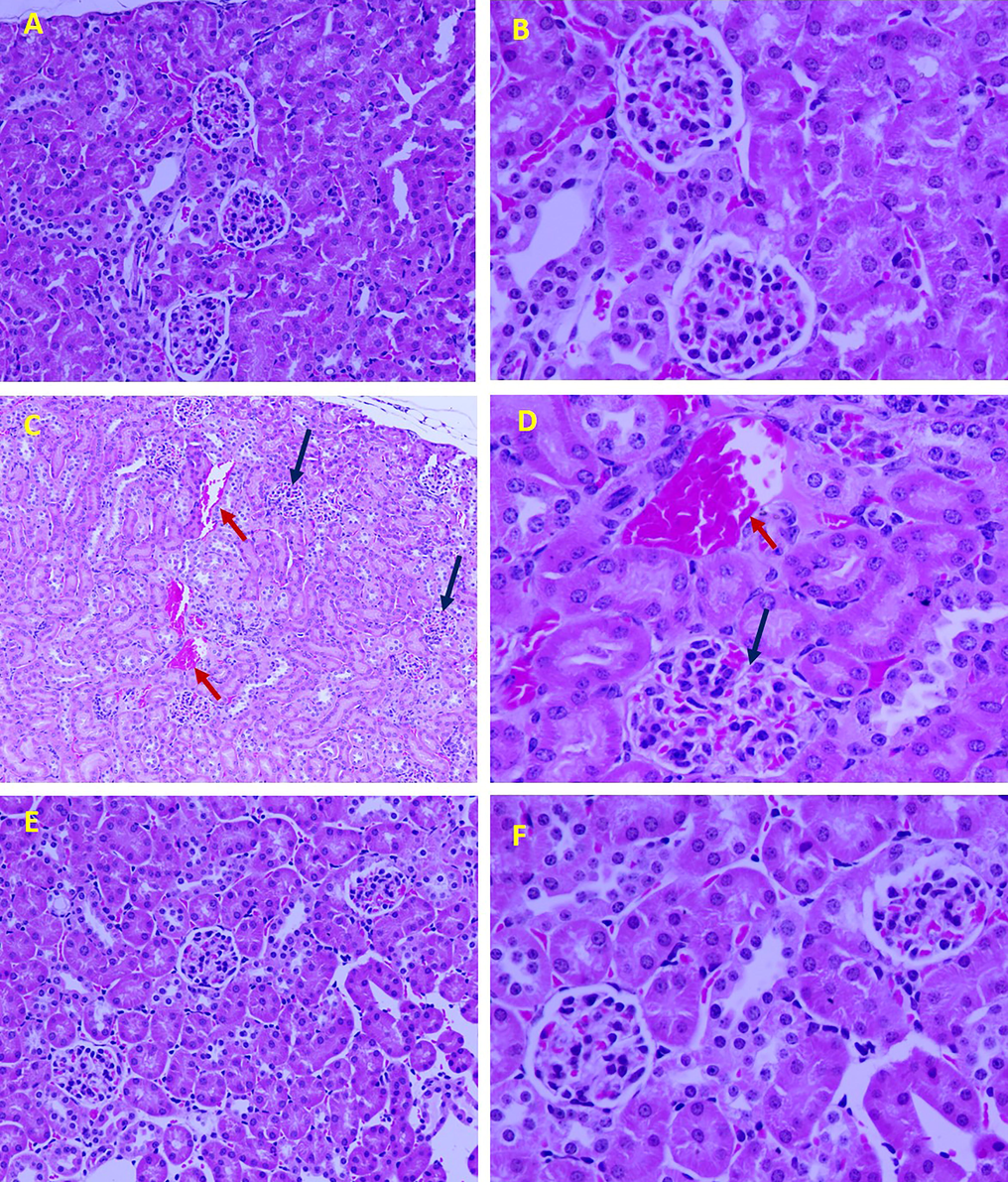
Representative histomicrographs of kidney tissue of the three groups, control (A: 200X, B: 400X), CCl4 (dilated blood vessels: red arrows; edematous glomeruli: blue arrows; C: 100X; D: 400X), and CCl4 treatment with AgNP-curcumin (E: 200X; F: 400X). Sections were stained with hematoxylin and eosin.
The therapeutic effect of AgNP-curcumin against CCl4-induced nephrotoxicity was investigated in terms of the significant increase in the activity of glutathione, as well as a decline in major markers of toxicity and kidney functions. LDH, an indicator of cell necrosis, was found to be declined in the CCl4 treated mice. Thus, AgNP-curcumin may induce programmed cell death upon CCl4-induced damage in kidney cells. Curcumin possesses significant antitumor and protective effects on nephrotoxicity (Mahmoud et al., 2014). It was found that curcumin induces cell death mainly through the apoptotic pathway (Tseng et al., 2019). Similarly, here, the cell death may be one of the mechanisms by which AgNP-curcumin could restore the damaged renal tissue. Furthermore, the comet assay and histological examinations confirmed our findings of biochemical indicators.
The toxicant CCl4 elicits severe toxic insults during its ROS-mediated biotransformation in vivo. These species disturb the redox balance and structural integrity in the target tissues (Shanmugavel et al., 2020). This was confirmed with the compromised antioxidant glutathione in the CCl4-treated rats here. Oxidative stress elevated MDA and compromised the level of GSH (Ebaid et al., 2020a, 2020b). in the target tissues. MDA, a major aldehyde resulting from lipid peroxidation, has been used as an indicator of tissue damage (Maheshwari et al., 2011). Moreover, free radicals invade the cell membrane and the intracellular organelles, including mitochondria, lysosome, Golgi apparatus, and endoplasmic reticulum, of target tissues. An increase in the MDA level in the tissue suggested the enhancement of the degree of peroxidation, leading to damage in the tissue and the failure of the antioxidant defense system (Kang 2015). Therefore, the DNA damage in the CCl4 treated mice group as confirmed by comet assay was due to the effect of oxidative stress radicals. Histological analysis of the kidney sections provided clear evidence in support of this CCl4-induced damage. On the other hand, because curcumin possesses potential antioxidant effects (Shaheen et al., 2014), our results demonstrate the restorative effect of AgNP-curcumin on the levels of MDA and reduced glutathione in the homogenates of renal tissues. Antioxidant enzymes play an important role in preventing tissue damage induced by CCl4. Here, the oxidative stability induced by AgNP-curcumin restored the intracellular organelle structure, especially the nucleus and DNA. This is proven by the results of the comet assay, which showed restoration of the renal tissue in mice treated with AgNP-curcumin.
CCl4 reduces the biological efficacy of many biomolecules, distorting numerous cellular functions and structures (Ebaid et al., 2012). GGT has been used as an important indicator of cardiovascular diseases in obese children (Bobrus-Chociej et al., 2018) and in nephrotoxicity (Kocaoğlu et al., 1997). In mice with CCl4-induced nephrotoxicity, GGT was significantly elevated relative to that in the control mice. Here, AgNP-curcumin restored the GGT level to normal in CCl4 mice. Similarly, the administration of curcumin and curcumin analog abrogated the increase in GGT level (Rukkumani et al., 2004).
The kidney additionally displays dysfunction related to toxicity. As metabolic products, urea and creatinine are removed from circulation by the kidney. Increases in the serum levels of these substances are regarded as an indication of renal dysfunction (George et al., 2014). Significant increases in serum creatinine and urea were seen in CCl4-treated mice in this study. Creatinine and urea are important serum markers of renal dysfunction (Nabi et al., 2018). Kidney dysfunction is reflected in the histology of early nephropathy, including diffuse glomerular basement membrane thickening, increased mesangial cellularity, and mesangial expansion (Ebaid et al., 2007; Huang et al., 2014). In present work, kidney dysfunction in CCl4-treated mice was restored, as evidenced by decreases in the levels of urea and creatinine by treatment with AgNP-curcumin. Supplementation of the diet with AgNP-curcumin in diabetic rodents was also reported to delay diabetic nephropathy by stimulating selenoproteins and modifying endogenous antioxidants (Nabavi et al., 2015).
In conclusion, our results provide evidence for the potential effect of AgNp-curcumin in the treatment of nephrotoxicity, suggesting that it reverses oxidative stress by suppressing lipid peroxidation and restoring glutathione, and down regulating the CCl4 toxicity markers, resulting in improved kidney structure and function.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1436-004).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Silver nanoparticle applications and human health. Clin. Chim. Acta. 2010;411(23–24):1841-1848.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci.. 2016;9:1-7.
- [Google Scholar]
- Chemoprotective effect of taurine on potassium bromate-induced DNA damage, DNA-protein cross-linking and oxidative stress in rat intestine. PloS ONE. 2015;10(3):e0119137
- [Google Scholar]
- Up-regulation of Hsp72 and keratin16 mediates wound healing in streptozotocin diabetic rats. Biol. Res.. 2015;48(1):54.
- [Google Scholar]
- Thymoquinone ameliorates Pachycondyla sennaarensis venom-induced acute toxic shock in male rats. BMC Pharmacol. Toxicol.. 2019;20(1):1-9.
- [Google Scholar]
- Samsum Ant Venom Exerts Anticancer Activity Through Immunomodulation In Vitro and In Vivo. Cancer Biother. Radiopharmaceut.. 2018;33(2):65-73.
- [Google Scholar]
- Association of silver nanoparticles and curcumin solid dispersion: antimicrobial and antioxidant properties. AAPS Pharm SciTech.. 2018;19(1):225-231.
- [Google Scholar]
- Bioavailability of curcumin: problems and promises. Mol. Pharm.. 2007;4(6):807-818.
- [Google Scholar]
- Perinatal supplementation with thymoquinone improves diabetic complications and T cell immune responses in rat offspring. Cell. Immunol.. 2011;267(2):133-140.
- [Google Scholar]
- Estimation of gamma-glutamyl transferase as a suitable simple biomarker of the cardiovascular risk in children with non-alcoholic fatty liver disease. Acta Biochim. Pol.. 2018;65(4):539-544.
- [Google Scholar]
- Detection of necrosis by release of lactate dehydrogenase activity. In: Immune Homeostasis. Totowa: Humana Press; 2013. p. :65-70.
- [Google Scholar]
- The hemocompatibility of nanoparticles: a review of cell–nanoparticle interactions and hemostasis. Cells. 2019;8(10):1209.
- [Google Scholar]
- The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces. 2012;98:112-119.
- [Google Scholar]
- Neutrophil depletion in the early inflammatory phase delayed cutaneous wound healing in older rats: improvements due to the use of un-denatured camel whey protein. Diagnost. Pathol.. 2014;9(1):46.
- [Google Scholar]
- Promotion of immune and glycaemic functions in streptozotocin-induced diabetic rats treated with un-denatured camel milk whey proteins. Nutr. Metabol.. 2014;11(1):31.
- [Google Scholar]
- Acute endotoxemia during gestation induces organ dysfunction and tissue damage in mouse offspring. Pakistan J. Zool.. 2012;44(3):765-776.
- [Google Scholar]
- Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metabol.. 2020;17(1):6.
- [Google Scholar]
- Piroxicam-induced hepatic and renal histopathological changes in mice. Libyan J. Med.. 2007;2(2):82-89.
- [Google Scholar]
- Role of Nigella sativa in ameliorating chloramphenicol induced tissue damage in rats. J. Med. Plants Res.. 2011;5(2):208-288.
- [Google Scholar]
- Curcumin-containing Silver Nanoparticles prevent carbon tetrachloride induced hepatotoxicity in mice. Comb. Chem. High Throughput Screen.. 2020;23:1-11.
- [Google Scholar]
- Protective effects of curcumin against nephrotoxic agents. Cardiovasc. Haematol. Disord. Drug Targets. 2019;19(3):176-182.
- [Google Scholar]
- Gao, M. Long, X. Du, J. Teng, M. Zhang, W. Wang, Y. Wang, X. Wang, Z. Zhang, P. Li, J. 2020. Enhanced curcumin solubility and antibacterial activity by encapsulation in PLGA oily core nanocapsules. In Proceedings of the Food and Function; Royal Society of Chemistry, 11, 448–455.
- George, G.S. Wakasi M.E., Egoro E. 2014. Creatinine and urea levels as critical markers in end-stage renal failure. J. Med. Health Scien., 3, 41–44.
- Ameliorative effect of zinc oxide nanoparticles against potassium bromate-mediated toxicity in Swiss albino rats. Environ. Sci. Pollut. Res.. 2019;26(10):9966-9980.
- [Google Scholar]
- Hassan, I., Ebaid, H., Alhazza, I.M., Al-Tamimi, J., 2020. The Alleviative Effect of Vitamin B2 on Potassium Bromate-Induced Hepatotoxicity in Male Rats. BioMed Research International 2020.
- Selenium nanoparticles mitigate diabetic nephropathy and pancreatopathy in rat offspring via inhibition of oxidative stress. J. King Saud Univers. Sci.. 2021;33(1):1-7.
- [Google Scholar]
- Huang L., Belousova T., Pan J.S., Du J., Ju H., Lu L., Zhang P., Truong L.D., Nuotio-Antar A., Sheikh-Hamad D., 2014. AKI after conditional and kidney-specific knockdown of stanniocalcin-1. J. Am. Soc. Nephrol. 25(10), 2303–2315. doi: 10.1681/ASN.2013070690. Epub 2014 Apr 3.PMID: 24700878
- Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol.. 2018;207(1):39-53.
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci.. 2016;9:217-227.
- [Google Scholar]
- Antioxidant activity of ethanol and water extracts from lentil (Lens culinaris) J. Food Nut. Res.. 2015;3(10):667-669.
- [Google Scholar]
- Acute acetaminophen nephrotoxicity and urinary gamma-glutamyl transferase activity in rats. Drug Metabol Drug Interact.. 1997;14(1):47-54.
- [Google Scholar]
- C.Y. Loo R. Rohanizadeh P.M. Young D. Traini R. Cavaliere C.B. Whitchurch W.H. Lee Combination of Silver Nanoparticles and Curcumin Nanoparticles for Enhanced Anti-biofilm Activities J Agric Food Chem. 30 2016 64 (12):2513–22
- Maheshwari D.T., M.S.Y. Kumar, S.K. Verma, V.K. Singh, S.N. 2011. SinghAntioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L. leaves. Food Chem. Toxicol., 49, 2422-2428.
- Thymoquinone and curcumin attenuate gentamicin-induced renal oxidative stress, inflammation and apoptosis in rats. Excli J.. 2014;13(13):98-110.
- [Google Scholar]
- Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol.. 2011;668:133-139.
- [Google Scholar]
- Oral delivery of curcumin polymeric nanoparticles ameliorates CCl4-induced subacute hepatotoxicity in wistar rats. Polymers. 2018;10(5):541.
- [Google Scholar]
- Review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed. Res. Int.. 2014;2014:12.
- [Google Scholar]
- The nephroprotection exerted by curcumin in maleate-induced renal damage is associated with decreased mitochondrial fission and autophagy. Biofactors.. 2016;42(6):686-702.
- [Google Scholar]
- Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chemico-biological interactions. 2019;312:108814
- [Google Scholar]
- Effects of green synthesised silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models. Int. J. Nanomed.. 2019;16(14):5257-5270.
- [Google Scholar]
- Curcumin: a natural product for diabetes and its complications. Curr. Top. Med. Chem.. 2015;15(23):2445-2455.
- [Google Scholar]
- Renal cell carcinoma: a review of biology and pathophysiology. F1000Res.. 2018;12(7):307.
- [Google Scholar]
- Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365-3383.
- [Google Scholar]
- Mechanisms of drug-induced nephrotoxicity. Handb. Exp. Pharmacol.. 2010;196:111-130.
- [Google Scholar]
- Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces. 2013;108:255-259.
- [Google Scholar]
- Development and characterization of curcumin loaded silver nanoparticle hydrogels for antibacterial and drug delivery applications. J. Inorg. Organomet. Polym. Mater.. 2012;22:1254-1262.
- [Google Scholar]
- Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress. J Pharm Pharm Sci.. 2004;7(2):274-283.
- [Google Scholar]
- Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci.. 2016;9:109-115.
- [Google Scholar]
- Safhi, M.M., 2018. Nephroprotective effect of Zingerone against CCl4-induced renal toxicity in Swiss albino mice: molecular mechanism. Oxidative medicine and cellular longevity, 2018.
- Shanmugavel, V., Komala, Santhi, K., Kurup, A.H., Kalakandan, S., Anandharaj, A., Rawson, A. 2020. Potassium bromate: effects on bread components, health, environment and method of analysis: a review. Food Chem., 311:125964.
- Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep.. 2018;2018(8):8323.
- [Google Scholar]
- A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res.. 1988;175(1):184-191.
- [Google Scholar]
- Augmentation of therapeutic potential of curcumin using nanotechnology: Current perspectives Artificial cells. Nanomed. Biotechnol.. 2018;46(sup1):1004-1015.
- [Google Scholar]
- Curcumin prevents maleate-induced nephrotoxicity: relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free Rad. Res.. 2014;48(11):1342-1354.
- [Google Scholar]
- Curcumin and tetrahydrocurcumin induce cell death in Ara-C-resistant acute myeloid leukemia. Phytother. Res.. 2019;33(4):1199-1207.
- [Google Scholar]
- Protective effects of curcumin and vitamin E on carbon tetrachloride-induced nephrotoxicity in rats. EXCLI J.. 2012;11:641.
- [Google Scholar]
- Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8:3040-3048.
- [Google Scholar]
- The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed.. 2020;15:2555.
- [Google Scholar]
- Fungal silver nanoparticles: synthesis, application and challenges. Crit. Rev. Biotechnol.. 2018;38(6):817-835.
- [Google Scholar]







