Translate this page into:
Comparative evaluation of a live E. coli vaccine and cefotaxime treatment against three E. coli serotypes in broilers
⁎Corresponding author at: Department of Botany and Microbiology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. imoussa1@ksu.edu.sa (Ihab M. Moussa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Avian Pathogenic Escherichia coli (APEC) infections concern economically the poultry industry inducing different disease syndromes leading to high mortality and condemnations. There is tremendous diversity in O serotypes within even a limited geographic region. It has been reported previously on a live E. coli vaccine’s ability to protect against different O78 APEC in commercial broilers. Due to the diversity of APEC serotypes in the field, this study was conducted to measure that live attenuated E. coli (Poulvac® E. coli) vaccine’s ability to cross-protect against three of the heterologous (non-O78) APEC isolates; O27, O8, and O115 compared to cefotaxime treatment through experimental infections in commercial broiler chickens. While the vaccination gave significant protection against the three serotypes tested there were at least subtle differences between them based on clinical signs, post-mortem lesions and mortality rate for 14 days post-challenge (dpc), E. coli re-isolation and histopathological examination at 4, 10 and 14 dpc., final body weight and feed conversion ratio at 35 days of age.
Keywords
E. coli = Escherichia coli
Antibiotic
Poulvac® E. coli vaccine
Protection
1 Introduction
Escherichia coli, a commensal inhabitant of the gastrointestinal tract of mammals and birds, is the causative agent of several diseases in animals and human worldwide. Pathogenic E. coli strains have been divided into intestinal pathogenic E. coli and extra intestinal pathogenic E. coli (ExPEC) depending on the location of the infection they are causing. These ExPEC strains can spread into various internal organs leading to variety of systemic or localized syndromes as, acute colisepticemia, airsacculitis or chronic respiratory disease (CRD), perihepatitis, pericarditis, swollen-head syndrome, fibrino-purulent polyserositis (peritonitis), salpingitis, omphalitis, synovitis, cellulitis, osteomyelitis, enteritis and coligranuloma (Nolan et al., 2013; Rahimi and Haghighi, 2012; Trampel et al., 2007; Harry and Hemsley, 1965; Cloud et al., 1985).
Controlling the predisposing factors is one of the strategies of treatment in addition to the early use of antibiotics. Unfortunately, the well-developed plasmid, transposons, and class 1 integrons (Singh et al., 2005) in E. coli lead to high frequency of resistance to some antibiotics specially macrolide and tetracycline groups. Furthermore, the reduced usage of antibiotics in poultry industry after banning may give the chance for E. coli vaccines to be an alternative way to reduce E. coli infection losses (Chansiripornchai et al., 1995).
The tremendous diversity in O serotypes of APEC within even a limited geographic region considered a high challenge that limits the widespread use of the vaccines against APEC. several vaccines based on killed and live attenuated strains have been tested experimentally. In general, they gave enough protection against infection with homologous strains while different levels of protection against heterologous strains have been reported as well (Dho-Moulin and Fairbrother, 1999; Heller et al., 1990; Melamid et al., 1991).
On the other hand the ability of live E. coli vaccine (O78: K80 aroA gene mutant) to protect commercial broilers and turkeys against different APEC O78 strains have been proved in different studies (Cookson and Davis, 2007; Cookson et al., 2008; La Ragione et al., 2013). In addition, cross protection of the Poulvac® E. coli against the more common heterologous APEC serotypes; O1, O2 and O18 has been reported by Cookson et al. (2009), while the 3 vaccination strategies (at either day 1, day 18 or days 1& 18) gave significant protection against the three serotypes tested, there were at least subtle differences between them.
The stability and safety of live AroA mutant E. coli vaccine strain (Poulvac® E. coli) was confirmed through European Medicines Agency (EMEA, 2013) with no risk to the environment or humans.
Because of the diversity of APEC serotypes in the field, this study was conducted to measure the efficacy of live attenuated commercial E. coli vaccine (Poulvac® E. coli) against experimental infection with 3 selected ExPEC serotypes (O27, O8, and O115) in commercial broiler chickens versus cefotaxime treatment.
2 Material and methods
Ethical statement
All experiments and methods were performed in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Committee on the Ethics of Animal Experiments of Damanhour University (DMU) including chicken vaccination and histopathological examination (and relevant protocols). Ethical Approval code of Damanhour University was (DMU/VetMed-2015/0067).
2.2 Experimental design
2.2.1 Birds
A total of 220 one-day-old commercial broiler chicks (Ross 308) of both sexes obtained from a commercial hatchery were used. chicks were randomised and evenly assigned to different treatment groups, resulting in 11 groups of twenty, each of which consisted of two replicates. All birds were floored reared in separate clean pens, fed and watered ad-libitum during the whole experiment. The birds were tested on the first and 20th days of age for the presence of APEC and revealed negative results.
Chicken groups number (no.) G1, G4 and G7 were non-vaccinated for E. coli, challenged with E. coli serotypes O27, O8, and O115, respectively and non-treated. Groups no. G2, G5 and G8 were vaccinated-challenged with the 3 serotypes, respectively and non-treated. While groups no. G3, G6 and G9 were non-vaccinated, challenged with E. coli serotypes O27, O8, and O115, respectively and treated with cefotaxime. Chickens in G10 were vaccinated for E. coli, non-challenged and non-treated, while in G11 were non- E. coli vaccinated, non-infected and non-treated.
2.2.2 Vaccines
A commercially available live attenuated aroA gene deleted vaccine (Poulvac® E. coli, Zoetis), containing E. coli serotype O78 with each one dose of reconstituted vaccine contains 5.2 × 106 to 9.1 × 108 CFU, was used at 1 day old via coarse spray.
Also, the protective vaccination schedule for viral diseases in all bird groups was, Newcastle disease + infectious bronchitis vaccine (Nobilis® Clone 30 + Ma5, MSD) applied by eye drop and Newcastle disease + avian influenza H5N1 vaccine (MeFluvac ND®, MEVAC) by S/C injection on the 7th day of age. Gumboro intermediate plus (Bursine Plus® vaccine, Zoetis) at 14th day and LaSota (Nobilis® ND LaSota, MSD) on the 18th day of age, both vaccines were applied via eye drops.
2.2.3 E. coli serotypes used for experimental infection:
Three different pathogenic E. coli serotypes O27 (strain No: L1-13), O8 (strain No: B4-13) and O115 (strain No: B8-14) previously identified at department of poultry and fish diseases, faculty of veterinary medicine, Damanhour university, Egypt. Pathogenicity and antibiotic sensitivity profile of these 3 APEC serotypes were previously tested and reported by Ellakany et al. (2019). The three serotypes were highly pathogenic and sensitive to cefotaxime. Challenge was applied at 21 days old with a dose of 0.5 ml of bacterial suspension containing 1.2 × 109 CFU/ml through intratracheal route (Rawiwet and Chansiripornchai 2009).
2.2.4 Treatment
Cefotaxime (Cefotax® -Egyptian international pharmaceutical industries) treatment was applied through intramuscular injection a dose of 20 mg/kg body weight (Bwt) daily for 2 successive days post challenge (dpc).
2.2.5 Evaluation parameters
-
Body weight (Bwt), Feed Intake (FI), Feed Conversion Ratio (FCR) and mortality in each group have been evaluated at 35 days old.
-
Clinical signs and post-mortem (PM) lesions scoring for 2 weeks post challenge.
-
Re-isolation of E. coli: 10 tracheal and 10 cloacal swabs were collected from each chicken group at 4, 10 and 14 dpc then streaked onto MacConkey’s agar. Colonies were further identified biochemically according to Konemann et al. (1997) and Quinn et al. (2002) and serologically for serotyping using slide agglutination test and specific polyvalent and monovalent sera (Edwards and Ewing, 1986).
-
Histopathological examination: Trachea, lung, liver and intestinal samples from 4 sacrified birds per each group, after euthanasia with intravenous injection of sodium pentobarbital (50 mg/kg), were collected at 4th dpc and sections were fixed in 10% neutral–buffered formalin (Bancroft and Layton, 2013).
3 Results
3.1 Results of experimental infection with APEC serotype O27 in G1, G2 and G3
3.1.1 Final Bwt, FI, FCR and mortality rate (Table 1)
Regarding Bwt, FI and FCR at 35 days old, there was significant improvement in Bwt and FCR (p ≤ 0.05) in favor of chickens in G2; vaccinated, challenged and non-treated compared to other chicken groups (G1& G3) with a body weight of 1874 g and a FCR of 1.5, where no significant difference (p ≥ 0.05) has been observed between this group (G2) and the vaccinated, non-challenged and non-treated G10. Mortality was 10% in chickens in G1 versus 0% in G2. Note: * Means within the same column carry different superscripts are significantly differed at level p ≤ 0.05. Note: **: Percentage of Bwt loss or gain compared to control negative chickens of G11.
Treatment
N
Bwt. (g)
FCR
FI
Mortality %
Bwt%**
G1
15
1297.00 ± 127.43c
1.96 ± 0.28a
2250.00
2/20 (10%)
79.67%
G2
15
1874.50 ± 57.47a
1.50 ± 0.04b
2792.50
0/20 (0%)
115.14%
G3
15
1647.00 ± 45.06b
1.61 ± 0.04ab
2638.00
0/20 (0%)
101.17%
G10
15
1841.50 ± 49.72a
1.50 ± 0.04b
2743.00
0/20 (0%)
113.11%
G11
15
1628.00 ± 44.37b
1.84 ± 0.05ab
2977.50
0/20 (0%)
100%
Clinical signs of diseased birds included decreased feed intake, whitish-brownish diarrhea, coughing and sneezing. These signs were most prominent in chicken G1. Post-mortem lesions included fibrinous pericarditis, perihepatitis and airsacculitis with increased severity in chicken G1.
3.1.2 Re-isolation of APEC serotype O27 after experimental infection
At 14 dpc, the high percentage of re-isolation of the APEC O27 from collected tracheal and cloacal swabs was observed in chicken G1 (60%), followed by G3 (20%) where G2 showed 0%. All tracheal and cloacal swabs samples from chicken G10 & G11 were negative for re-isolation of APEC (Table 2).
% of cloacal re-isolation
% of tracheal re-isolation
Chicken groups
14 dpc
10 dpc
4 dpc
14 dpc
10 dpc
4 dpc
60
80
80
60
80
80
G1
0
20
40
0
20
40
G2
20
40
60
20
20
40
G3
60
80
80
60
80
80
G4
20
40
60
20
40
60
G5
20
40
60
20
20
40
G6
60
80
80
60
80
80
G7
20
40
60
20
40
60
G8
20
40
60
20
20
40
G9
0
0
0
0
0
0
G10
0
0
0
0
0
0
G11
3.1.3 Histopathology
The histopathological findings of chicken groups at 4 dpc were illustrated in Table 3. Trachea: −, Normal tracheal mucosa and submucosa; +, Mild epithelial hyperplasia and congestion of the mucosal blood vessels; ++, Moderate epithelial degeneration, blood vessels, higher no. of mucosal cyst and submucosal inflammatory cell infiltration. Lung: -, Normal bronchi and respiratory portions; +, Congestion, edema of the interstitial blood vessels; ++, Marked congestion and hemorrhages, interstitial edema and exudates; +++, Marked increase of the interstitial fibrin exudate (lobular); ++++, Diffuse (Lobar) pulmonary pneumonia, fibrin exudation, interstitial edema and leukocytic infiltration. Liver: -, Normal hepatocytes and normal hepatic sinusoids and blood vessels; +, Mild degree of hepatocytes degeneration, hepatic blood sinusoid congestion, and leukocytic infiltration; ++, Moderate degree of hepatocytes degeneration, hepatic blood sinusoid congestion and leukocytic infiltration especially heterophils; +++: Coligranuloma in addition to severe hepatocyte degeneration and necrosis. Intestine: -, Normal villi and mucosal lining; +, Mild degree of enteritis (mild mucosal hyperplasia, epithelial degeneration, and leukocytic infiltration); ++, Moderate degree of enteritis; +++, Severe degree of enteritis.
Intestine
Liver
Lung
Trachea
Organs Chicken groups
+++
+++
+++
++
G1
+
+
+
+
G2
+
++
++
++
G3
++
+++
++++
+
G4
+
++
+
+
G5
+
++
++
+
G6
+
++
++
++
G7
+
++
+
+
G8
+
+
+
+
G9
–
–
–
–
G10
–
–
–
–
G11
Birds challenged with serotype O27 in G1 showed the most severe lesions where the trachea showed epithelial hyperplasia associated with a mild degree of submucosal leukocytic infiltration in addition to the presence of multiple mucous-filled cysts. Also, the examined lung samples of chickens in the same group revealed marked epithelial hyperplasia of secondary bronchi with collapsed peribronchial air capillaries and inflammatory cell infiltration within the parabronchial wall. Liver showed multifocal areas of haemorrhages and focal liquifactive hepatic necrosis. Intestinal examination revealed marked intestinal lining epithelial and glandular hyperplasia, degeneration of intestinal lining epithelium of the villi (Fig. 1.a) as well as mononuclear and heterophilic inflammatory cell infiltration in the serosal layer of the intestine.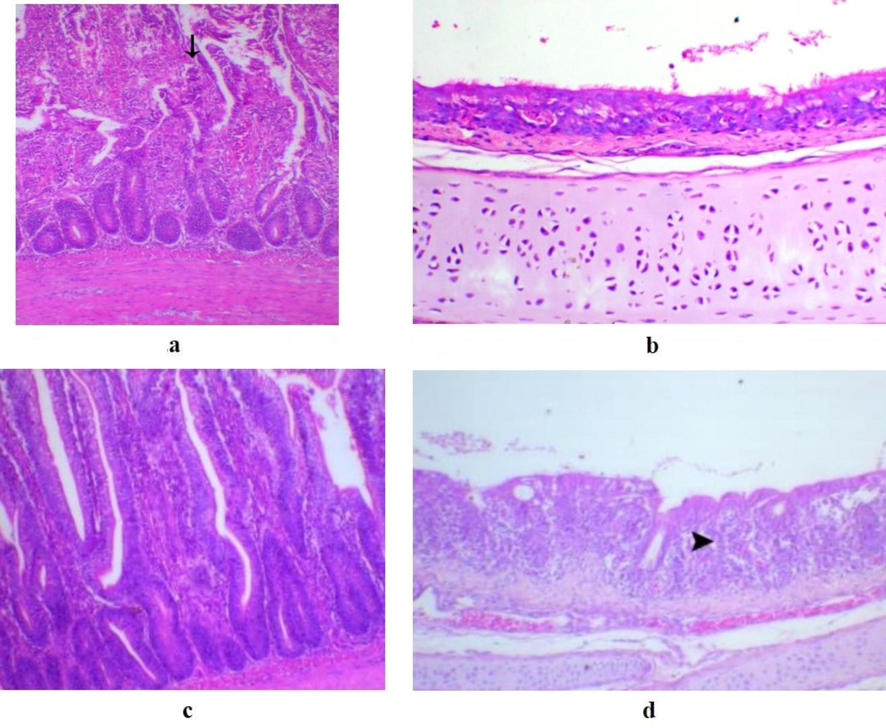
a) Intestine of a bird in G1 showing marked degeneration of intestinal villi structures (arrow), H&E, X200. b) Trachea of a bird in G2 vaccinated challenged with E. coli serotype O27 showing normal lining mucosa, H&E, X200. c) Normal intestinal tissue of a bird in G2, H&E, X200. d) Trachea of a bird in G3 non-vaccinated infected with E. coli serotype O27 and treated with cefotaxime showing tracheitis (arrowhead indicates marked leukocytic infiltration), H&E, X200.
On the other hands, the histopathological findings for birds in G2 showing the trachea with normal ciliated epithelial lining (Fig. 1.b). The lungs showed patent wide funnel-shaped atria which branched out to normal air capillaries with markedly decreased interstitial inflammatory reaction. The liver and intestine (Fig. 1.c) showed very mild lesions and mostly were within the normal limits.
Chicken G3 had markedly noticeable lesions with a moderate degree of tracheitis with leukocytic infiltration (Fig. 1.d). The pulmonary lesions showed severe interstitial exudation and heterophillic infiltration with noticeable inflammation within the parabronchial walls, focal hepatitis (focal hepatocytes degeneration associated with infiltration of heterophils and lymphocytes) and normal intestinal mucosa.
3.2 Results of experimental infection with APEC serotype O8 in G4, G5 and G6
3.2.1 Final Bwt, FI, FCR and mortality rate (Table 4)
Chicken G4 had the highest mortality rate of 3/20 (15%) compared to only 10% in G5. At 35 days old, chickens of G5 showed significant higher Bwt and better FCR (p ≤ 0.05) than the other 2 groups (G4& G6) with a body weight of 1692 g and a FCR of 1.53 respectively, while there was no significant difference observed between this group G5 and the vaccinated, non-challenged and non-treated G10. Note: * Means within the same column carry different superscripts are significantly differed at level p ≤ 0.05. Note: **: Percentage of Bwt loss or gain compared to control negative chickens of G11.
Treatment
N
Bwt. (g)
FCR
FI
Mortality %
Bwt%**
G4
15
1418.50 ± 68.16b
1.90 ± 0.10a
2652.00
3/20 (15%)
87.13%
G5
15
1692.00 ± 53.37a
1.53 ± 0.05b
2568.00
2/20 (10%)
103.13%
G6
15
1322.00 ± 116.67b
1.82 ± 0.26ab
2163.70
0/20 (0%)
81.20%
G10
15
1841.50 ± 49.72a
1.50 ± 0.04b
2743.00
0/20 (0%)
113.11%
G11
15
1628.00 ± 44.37a
1.84 ± 0.05ab
2977.50
0/20 (0%)
100%
A decreased feed intake, signs of diarrhea, coughing and sneezing, as well as fibrinous pericarditis, perihepatitis and airsacculitis were prominantely reported in the non-vaccinated, infected, and non-treated G4.
3.2.2 Re-isolation of APEC serotype O8 after experimental infection
Re-isolation from tracheal and cloacal swabs was present up to 14 dpc in all groups G4, G5 and G6 with a higher percentage (60%) in G4 (previously described in Table 2).
3.2.3 Histopathology
The trachea of birds challenged with APEC serotype O8 (G4) showed epithelial hyperplasia associated with multiple mucous-filled cysts while the lungs revealed marked exudation within the interstitial tissue, associated with leukocytic infiltration within the parabronchus wall (Fig. 2.a). The liver of chickens within this group showed severe coagulative necrosis, but intestinal examination revealed a mild degree of enteritis.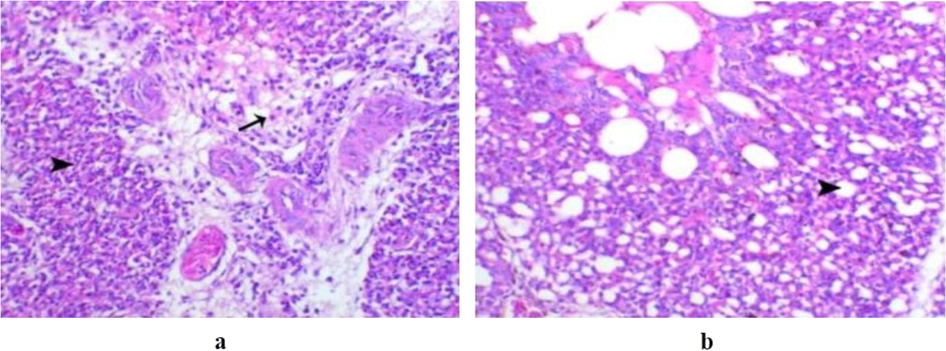
(a) Lung of a bird in G4 non vaccinated infected with E. coli serotype O8 and non-treated showing marked exudation within the interstitial tissue associated with leukocytic infiltration (arrow) within the parabronchus wall (arrowhead), H&E, X200. (b) Lung of a bird in G5 vaccinated challenged with E. coli serotype O8 and non-treated showing normal patent air capillaries (arrowhead), H&E, X200.
Birds vaccinated and challenged with E. coli serotype O8 (G5) showed normal ciliated epithelial lining of the trachea. The lung showed hyperplasia of the para bronchi lining epithelium with normal air and blood capillaries within the respiratory parabronchial walls and markedly limited interstitial reaction as edema and heterophils (Fig. 2.b). The liver revealed moderate congestion of blood sinusoids with focal hepatocytes degeneration. Intestinal lesions of this group appeared as very mild degeneration of the intestinal glands and mucosal lining.
Chicken G6 (Challenged and treated) had a moderate degree of tracheitis with leukocytic infiltration and the pulmonary lesions showed severe interstitial exudation and heterophilic infiltration with noticeable inflammation within the parabronchial walls. Focal hepatitis (focal hepatocyte degeneration associated with infiltration of heterophils and lymphocytes) and a mild degree of enteritis.
3.3 Results of experimental infection with APEC serotype O115 in G7, G8 and G9
3.3.1 Final Bwt, FI, FCR and mortality rate (Table 5)
Chicken G7 had significantly lower Bwt (p ≤ 0.05) compared to the other groups (G8 and G9) while there was no significant difference in FCR between all the groups challenged with E coli serotype O115 at 35 days old. Ten percent mortality was reported in chicken G8 (vaccinated, challenged and non-treated) while 5% mortality was observed in the challenged, non-vaccinated and non-treated G7. Also, there were very mild clinical signs and PM lesions in all groups challenged by serotype O115. Note: * Means within the same column carry different superscripts are significantly differed at level p ≤ 0.05. Note: **: Percentage of Bwt loss or gain compared to control negative chickens of G11.
Treatment
N
Bwt. (g)
FCR
FI
Mortality %
Bwt%**
G7
15
1405.00 ± 87.19b
1.65 ± 0.15a
2213.00
1/20 (5%)
86.3%
G8
15
1645.00 ± 106.95a
1.67 ± 0.19a
2568.00
2/20 (10%)
101%
G9
15
1631.00 ± 64.06a
1.62 ± 0.06a
2611.00
0/20 (0%)
100.18%
G10
15
1841.50 ± 49.72a
1.50 ± 0.04a
2743.00
0/20 (0%)
113.11%
G11
15
1628.00 ± 44.37a
1.84 ± 0.05a
2977.50
0/20 (0%)
100%
3.3.2 Re-isolation of APEC serotype O115 after experimental infection
Tracheal and cloacal swabs yielded positive results for re-isolation up to 14 dpc in chicken G7, G8 and G9 with a higher percentage (60%) in G7 (previously described in Table 2).
3.3.3 Histopathology
The histopathological lesions of birds in G7, G8 and G9 were less severe than birds infected with serotype O27 and O8. The tracheas of birds challenged with O115 (G7) showed deciliated epithelial lining with marked leukocytic infiltration within the lamina propria, while, the lungs had exudation and leukocytic infiltration within the interstitial tissue. Livers from chicken G7 showed moderate hepatic congestion, heterophilic infiltration and on examination of the intestine, revealed a mild degree of enteritis.
Regarding G8 and G9, the tracheas showed normal ciliated epithelial lining. Lung and intestinal tissues were normal, while liver samples showed mild focal leukocytic infiltration and moderate degree of diffuse periportal heterophilic infiltration in birds of G8 (Fig. 3.a) and G9 (Fig. 3.b), respectively.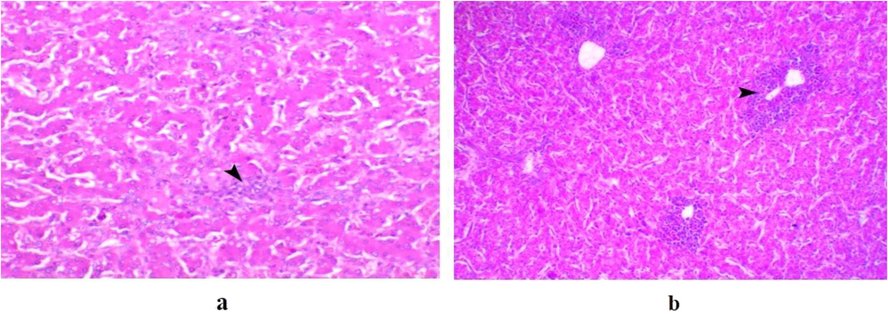
a) Liver of a bird in G8 showing mild focal leukocytic infiltration (arrowhead), H&E, X 200. b) Liver of a bird in G9 infected bird with E. coli serotype O115 and treated with cefotaxime showing periportal heterophils infiltration, H&E, X200.
4 Discussion
The massive distribution of E. coli in different poultry flocks is always related to many factors such as poor biosecurity levels, bad management (inadequate ventilation, high stocking density, poor litter conditions, poor hygiene, high ammonia levels), higher incidence of respiratory viral diseases and immunosuppressed birds. Also, the continuous evolution of antimicrobial resistant E. coli strains due to massive use of antibiotics without routine sensitivity testing and, the weak biosecurity programs, especially in broiler farms (Blanco et al., 1998; Ibrahim et al., 2019).
Antimicrobial resistant ExPEC strains resemble a serious problem for both poultry and public health, since these strains could be passed to humans via the food chain or by direct contact with infected birds. In addition, resistant E. coli may act as transporters for antimicrobial resistant genes to other pathogens (Petersen et al., 2006). Also, vertically transmission of E. coli strains (especially antibiotic resistant) were recorded (Giovanardi et al., 2005; Petersen et al., 2006). For these reasons, prevention of ExPEC through vaccination should be given high priority (Deb and Harrey, 1976). However, design of an effective vaccine is troubleshooting due to several reasons. No characteristic traits define the APEC phenotype or in other words a combination of virulence factors like level of expression and phylogenetic group determine its ability to cause disease (Rodriguez-Siek et al., 2005; Johnson et al., 2008). Another major limitation associated with potential vaccine candidates is the lack of cross-protection between E. coli serotypes displaying major diversity (Ewers et al., 2007). Furthermore, control of first week mortality in broilers and layers is more dependent on maternal immunity (Kariyawasam et al., 2004).
Herein, this study aimed to evaluate the spectrum of efficacy of a live vaccine (Poulvac E. coli) against 3 different pathogenic E. coli serotypes in broiler chickens in comparison to cefotaxime treatment as vaccination may be a valuable tool for controlling APEC infection rather than antibiotic usage avoiding antibiotic resistance as well as the drawbacks of antibiotic specially residues in poultry meat. Results obtained indicated that the significant high Bwt (p ≤ 0.05) and the significant better FCR (p ≤ 0.05) was recorded in both the vaccinated, non-treated chicken G2, which was challenged with E. coli serotype O27, and the vaccinated, non-infected, non-treated chicken G10 with a higher Bwt gain of 15.1% and 13.1%, respectively compared to control negative G11. Chicken G5 that was vaccinated, non-treated and challenged with E. coli serotype O8 had significantly higher Bwt (p ≤ 0.05) and better FCR than both G4 and G6 infected with the same serotype with higher Bwt gain of 3.1% in G5 and 12.9%, 18.8% Bwt gain loss in G4 and G6, respectively compared to control negative G11.
Vaccination in G2 and G5 were significantly minimize the clinical signs and PM lesions for 14 dpc. But, there were no significant differences in clinical signs, PM lesions and FCR between all chicken groups challenged with O115; however, chicken G8 had slightly higher Bwt than G7 and G9 and chicken G9 had slightly better FCR (non-significantly) than G7 and G8. These results indicate that the E. coli vaccine increased the Bwt and improved FCR for the chicken groups challenged with E. coli serotypes O8 and O27 much more than when treated with cefotaxime. This higher protection especially against the challenge with E. coli serotype O27 may be attributed to the similarity of serogroup antigens between the aroA mutant O78 strain in Poulvac® E. coli and the E. coli O27 isolate as they are serogrouped in polyvalent 4, while O8 and O115 are serogrouped in polyvalent 6.
La Ragione, et al. (2013) examined an aroA construct live attenuated E. coli (RML17 vaccine) and it was shown to be efficacious as a vaccine against colibacillosis in chickens and turkeys caused by a homologues APEC O78 and also against an un-typeable APEC strain in chickens which indicated a promising tool for wider cross-protection than other vaccines. Also similar results have been obtained by Cookson et al. (2009) where three vaccination strategies (at day 1, at day 18 and at days 1 & 18) with Poulvac® E. coli gave significant protection against the three serotypes tested, O1, O2 and O18, nevertheless there were at least subtle differences between them.
The vaccine in the present study completely prevented the mortality against E. coli serotype O27 (G2) and stopped the E. coli shedding in tracheal and cloacal swabs at 14 dpi. The mortality rate in chicken G5 (vaccinated, challenged with O8 and non-treated), was 10%, which means a 5% less than control positive G4 (challenged with O8, non-vaccinated and non-treated), while the mortality rate in G8 was 10% slightly higher than that of G7 nevertheless the vaccine decreased the E. coli shedding in G5 and G8 to 20% at 14 dpc. compared to 60% in non-vaccinated challenged non treated groups (G4& G7). Cefotaxime treatment decreased E. coli shedding in G3, G6 and G9 to 20% at 14 dpi. Frommer, et al. (1994) recorded similar results where they recorded 15, 55 and 60% mortalities in chickens experimentally challenged with E. coli serotypes O1, O8, and O78 respectively; however, these mortality percentages decreased down to 3–10% with the use of a BT-7 (piliated) vaccine strain.
Fibrinous pericarditis, perihepatitis, and airsacculitis were more severe in G1 and G4 than G7 and confirmed histopathologically with the presence of moderate to severe tracheitis, pulmonary lesions with severe interstitial exudation and heterophilic infiltration with noticeable parabronchial wall inflammation, focal necrotic hepatitis and a mild to moderate degree of enteritis in G1 and G4. While the examined chickens in G7 showed minimal degree of tracheal, pulmonary, hepatic, and enteric lesions. This may be a reflection for the systemic infection and septicemia associated with both O27 and O8 serotypes rather than O115 which is also an ExPEC but more associated with localized infections as synovitis and cellulitis. Dho-Moulin and Fairbrother (1999) and Zahid, et al. (2016) recorded the same changes in experimentally and naturally infected broiler chickens with different E. coli serotypes including O115.
Birds vaccinated with Poulvac® E. coli and challenged [specially with either E. coli serotype O27 (G2) or serotype O8 (G5)] showed marked amelioration of the respiratory lesions with normal ciliated tracheal epithelial lining and normal air capillaries with markedly decreased interstitial inflammatory reaction in lungs. Also, both liver and intestine were mostly within the normal limits while the Cefotaxime treated birds showed mainly noticeable respiratory and hepatic lesions especially in G3 and G6.
In the birds vaccinated, challenged and non-treated with E. coli serotype O115 (G8), the trachea and lungs were normal, but the liver showed mild focal leukocytic infiltration and the intestine showed mild epithelial hyperplasia. While the liver of birds in G9 showed moderate degree of diffuse periportal heterophillic infiltration. Antão et al. (2008) recorded the histopathological lesions of experimentally infected chickens with APEC strain IMT5155 where lung showed heterophilic infiltration and exudation within the air capillaries, thickening of septa due to the inflammatory reaction. Also, early microscopic changes were seen in the liver tissue as intravascular, perivascular as well as parenchymal lymphatic infiltrations. Inflammatory cell infiltration in the kidney was also characteristic of systemic infection.
5 Conclusions
The E. coli vaccine (Poulvac® E. coli) gave a significant protection against the challenge with the most common heterologous APEC serotypes in Egypt, O27 and O8, compared to cefotaxime treatment based on final Bwt, FCR, clinical signs, PM lesions, bacterial shedding and histopathological lesions. This vaccine also provided some protection against histopathological lesions associated with serotype O115 (the less prevalent) and numerically higher Bwt and Bwt gain % rather than control non vaccinated group.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for supporting the work through the research group project No.: (RG-1442-162).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The chicken as a natural model for extraintestinal infections caused by avian pathogenic Escherichia coli (APEC) Microb. Pathog.. 2008;45(5-6):361-369.
- [CrossRef] [Google Scholar]
- The hematoxylin and eosin. In: Suvarna S.K., Layton C., Bancroft J.D., eds. Theory Practice of Histological Techniques. Philadelphia: Churchill Livingstone of El Sevier; 2013.
- [Google Scholar]
- Serotypes of Escherichia coli isolated from septicaemic chickens in Galicia (Northwest Spain) Vet. Microbiol.. 1998;61:229-235.
- [Google Scholar]
- The in vitro antimicrobial sensitivity testing of Escherichia coli isolated from commercially reared chickens. Thai. J. Vet. Med.. 1995;25:275-283.
- [Google Scholar]
- In vitro and in vivo characterization of avian Escherichia coli. I. Serotypes, metabolic activity, and antibiotic sensitivity. Avian Dis.. 1985;29(4):1084.
- [CrossRef] [Google Scholar]
- E. coli challenge study in commercial broilers by either respiratory or skin route of exposure and the effect of prior vaccination with a live attenuated (aro-A) E. coli. Abstract 4457. In: 144th AVMA Annual Convention, Washington, D.C. July 2007. 2007.
- [Google Scholar]
- Crossprotection study of a modified live E. coli vaccine against three heterologous APEC serotypes in commercial broiller chickens. In: Proceedings of the 58th Western Poultry Disease Conference, Sacramento, California. March 2009. 2009.
- [Google Scholar]
- The efficacy of a novel live E. coli vaccine using a broiler skin challenge model. In: Abstract 1568. Proceedings of the 23rd World’s Poultry Congress. 2008.
- [Google Scholar]
- Laboratory trials with inactivated vaccines against Escherichia coli (078:K80) infection in fowls. Res. Vet. Sci.. 1976;20:131-138.
- [Google Scholar]
- Edwards and E wing’s Identification of Enterobacteriuceae (4th ed.). Elsevier Science Publishing Co., Inc.; 1986. p. :536.
- Isolation, serotyping, pathogenicity and antibiotic sensitivity testing of Escherichia coli from broiler chickens in Egypt. AJVS. 2019;61(2):45.
- [CrossRef] [Google Scholar]
- Committee for Medicinal Products for Veterinary Use CVMP assessment report for Poulvac E. coli (EMEA/V/C/002007) Veter. Med. Product Data Manage. 2013
- [Google Scholar]
- Avian pathogenic, uropathogenic, and newborn meningitis causing Escherichia coli: how closely related are they? Int. J. Med. Microbiol.. 2007;3:163-176.
- [Google Scholar]
- Experimental vaccination of young chickens with a live, non‐pathogenic strain of Escherichia coli. Avian Pathol.. 1994;23:425-433.
- [CrossRef] [Google Scholar]
- Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian Pathol.. 2005;34:313-318.
- [Google Scholar]
- The relationship between environmental contamination with septicemia strains of Escherichia coli. Vet. Rec.. 1965;77:241-245.
- [Google Scholar]
- Passive immunization of chicks to Escherichia coli. Avian Pathol.. 1990;19:345-354.
- [Google Scholar]
- Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Veterinary Res.. 2019;15:159-175.
- [CrossRef] [Google Scholar]
- Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. AEM. 2008;74(22):7043-7050.
- [CrossRef] [Google Scholar]
- Resistance of broiler chickens to respiratory tract infection induced by passively transferred egg-yolk antibodies. Vet. Microbiol.. 2004;98:273-284.
- [Google Scholar]
- Color Atlas and textbook of Diagnostic Microbiology (5th ed.). Lippincott; 1997. p. :55-73.
- Efficacy of a live attenuated Escherichia coli O78:K80 vaccine in chickens and turkeys. Avian Dis.. 2013;57:273-279.
- [CrossRef] [Google Scholar]
- A Vaccine against Avian Colibacillosis Based on Ultrasonic Inactivation of Escherichia coli. Avian Dis.. 1991;35(1):17.
- [CrossRef] [Google Scholar]
- Colibacillosis (13th ed.). Mosby-Wolf Publication Ltd.; 2013. p. :751-805.
- Vertical transmission of a fluoroquinolone-resistant Escherichia coli within an integrated broiler operation. Vet. Microbiol.. 2006;116(1-3):120-128.
- [CrossRef] [Google Scholar]
- Veterinary Microbiology And Microbial Disease. Black Well Science; 2002. Ch. 26-36
- An outbreak of visceral coligranuloma in a backyard chicken flock. Comp. Clin. Pathol.. 2012;23(2):381-384.
- [CrossRef] [Google Scholar]
- The efficacy of escherichia coli AroA-live vaccine in broilers against avian E. coli Serotype O78 Infection. Thai J. Veter. Med.. 2009;39:337-342.
- [Google Scholar]
- Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology. 2005;151:2097-2110.
- [Google Scholar]
- Identification of antimicrobial resistance and class 1 integrons in Shiga toxin-producing Escherichia coli recovered from humans and food animals. J. Antimicrob. Chemother.. 2005;56:216-219.
- [CrossRef] [Google Scholar]
- Characterization of Escherichia coli isolates from peritonitis lesions in commercial laying hens. Avian Dis.. 2007;51(4):840-844.
- [CrossRef] [Google Scholar]
- In vitro and In vivo Pathogenicity tests of Local Isolates APEC from Naturally Infected Broiler in Baghdad. Int. J. Adv. Res. Biol. Sci.. 2016;3:89-100.
- [Google Scholar]







