Translate this page into:
Schiff base ligand L synthesis and its evaluation as anticancer and antidepressant agent
⁎Corresponding authors. zahoor@uom.edu.pk (Muhammad Zahoor), rullah@ksu.edu.sa (Riaz Ullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The current study focuses on the synthesis of Schiff base ligand L [1(pyridine-2-ylimino) methyl) naphthalene-2-ol], and its applications as anticancer agent against human lung (H-460) and breast (MCF-7) cell lines, and as antidepressant agent.
Methods
The Schiff base ligand L was synthesized by aldol condensation reaction by reacting commercially available 2-hydroxy-1-napthaldehyde with 2-amino-pyridine. The synthesized ligand was characterized by different spectroscopic techniques and evaluated for its anticancer potential against H-460 and MCF-7 cell lines. The antidepressant activity was evaluated by means of elevated plus maze model using Diazepam as reference drug.
Results
A dose dependent inhibitory response was recorded against both the cell line used. For highest tested dose (500 µg/ml), 89.20% of anticancer activity with IC50 = 23.99 µg/ml against H-460 was observed while an IC50 of 31.68 µg/ml against MCF-7 indicated the potential of this compound to be used as anticancer agent. A dose dependent response was also recorded for the synthesized ligand when tested as antidepressant in elevated plus maze model.
Conclusion
The synthesized Schiff base ligand L exhibited good anticancer and antidepressant activities that could be used as alternative drug to treat the related health complications. However, further toxicological and clinical studies are needed to confirm the safe use of this compound.
Keywords
Anticancer
Antidepressant
MCF-7 cell
Schiff base ligand
2-hydroxy-1-napthaldehyde
1 Introduction
Schiff bases are an important class of compounds with a general formula such as R-HC = N-R in which R is an alkyl group that are generally synthesized by reacting as amine with aldehyde or ketones. Through passage of reaction the carbonyl carbon is changed by an imine as shown in Fig. 1 and the resulting product is called a schiff base. Schiff base compounds of aliphatic aldehyde are not stable as they undergo free polymerisation while aromatic aldehyde based such compounds due to their conjugated structure do not suffers from such types of drawbacks. The essential part in these kind of compounds is azo-methine. These compounds have numerous applications in different fields and also used in inorganic synthesis (Santos et al., 2013). They have exhibited a number of biological potentials like anti‐inflammatory, anti-plasmodial, antioxidant, antibacterial (Dief et al., 2015), antifungal (Elzaher et al., 2016), anticancer, and anti-depressant activities (Elviyan et al., 2015). They are also used as optical sensors in chromatographic application for sensitive and selective detection.
General structure and scheme of synthesis of a Schiff base.
Cancer being a deadly disease is mostly caused by virus and out of the recorded death each year 20% deaths are due to cancer. Breast cancer is one of the most common cancer in female and is on rise each year. Annually world-wide almost 500,000 women passes away due to breast cancer. With the improvement of investigative and clinically beneficial methods, the proficiency of breast cancer therapies has been improved significantly over a past few years (Gou et al., 2017). Standard cancer cure procedures includes chemotherapy, surgical treatment, and radiotherapy. Unfortunately, chemotherapy is not operative in treating such kind of diseases due to associated inherent resistance to caspase-mediated cell death and assimilated resistance to medications during treatment. For tumours in the chest cavity, the suggested surgical procedure is a one-step en bloc esophagectomy and proximal gastrectomy through the abdomen and right pectoral routes while in upper chest cavity, a supplementary cervical method is recommended. In both circumstances, a two-field lymph-node surgical process is performed (Bosset et al., 1997). Radiotherapy is one of most useful procedure in treating breast cancer due to its success rate from last decade (Wang, et al., 2013; Kumar et al., 2007). A number of anticancer substances have been obtained from natural sources while some have been synthesized in the lab (Thomas et al., 2009) that have exhibited strong activities and have revolutionized the chemotherapeutic method of treating different types of cancers. Synthesis of new anticancer drugs is still is one of hot research area that needs to be investigated precisely.
Depression is serious health complication in human that adversely affect the feelings and thinking of a person. It is characterized by unhappy feelings and the person hurts every time even in normal circumstances. The person also feel difficulties in performing routine works (Alhamdani et al., 2013; Zhang et al., 2014). A number of therapies including drugs therapy and somatic cures like electroconvulsive analysis, vagus nerve prompt (Vahia, 2013), and transcranial compelling stimulation (Agelink et al., 2001) are being in use to treat depression. As drug therapy a number of Schiff bases substituted with nitro, halogen, and dimethoxy groups have exhibited antidepressant activities in in vivo (Holtzheimer and Mayberg, 2011; Valero et al., 2007; Thomas et al, 2016). Such type of compounds have exhibited significant anticancer and antidepressant activities even at micro molar concentrations as well and are nontoxic to normal cells in comparison with a number of similar reported compounds in the literature. Due to presence of amine moiety, Schiff bases are playing an essential role in perceiving the alteration phenomenon and racemization response in biotic systems Thomas et al., 2016.
The mentioned biological applications of schiff bases inspired us to synthesize Schiff base ligand L [1(pyridine-2-ylimino) methyl) naphthalene-2-ol] through aldol condensation reaction and evaluate its anticancer and antidepressant potentials. The ligand was characterized by different instrumental techniques as well.
2 Experimental
2.1 Chemicals and instruments
All the chemicals used were of analytical grade and used without any further refinement. The reagents like 2-hydroxy-1-napthaldehyde (Scharlau), acetic acid (BDH Chemicals), methanol, DMSO (Sigma Aldrich), 2-aminopyridine (Alfa Aesar), penicillin, and L-glutamine (Fisher Scientific) have been used as in this study. Melting point was measured on Stuart Scientific instrument model SMP 10. UV–vis spectra was recorded in the range of 200–800 nm. IR spectrum of synthesized ligand L in the frequency of 400–4000 cm−1 was recorded on 4300 Spectrophotometer (Shimadzu) and 1H NMR spectrum was measured with a 500 MHz Varian Gemini instrument.
2.2 Ligand L synthesis
The Schiff base ligand L was synthesized according to reported method in literature with slight modification (Vhanale et al., 2019). About 10 mmol (1.71 g) of 2-aminopyridine was treated with 10 mmol (0.93 g) of 2-hydroxy-1-napthaldehyde in 15 mL methanol in 100 mL round bottom flask. Aldehyde was initially dissolved in methanol, added four drops of acetic acid which work as catalyst and heated for 6 min on magnetic hot plate with continuous stirring. After 6 min, 2-aminopyridine were added to the reaction mixture and refluxed at 60 °C for 5 h. Formation of yellow colour precipitate confirmed the reaction completion which was further confirmed through TLC analysis (n- hexane and ethyl acetate at ratio of 7:3 were used as solvents). The spots on TLC plate were visualized with iodine vapours. Upon completion of reaction, product formed was filtered, washed with sufficient amount of solvent (methanol), and after drying were recrystallized in n-hexane. The yield of product was 80% while melting point was 67–70 °C. Fig. 2 represents the synthetic route of schiff base ligand L synthesis while Fig. 3.shows its tautomeric forms.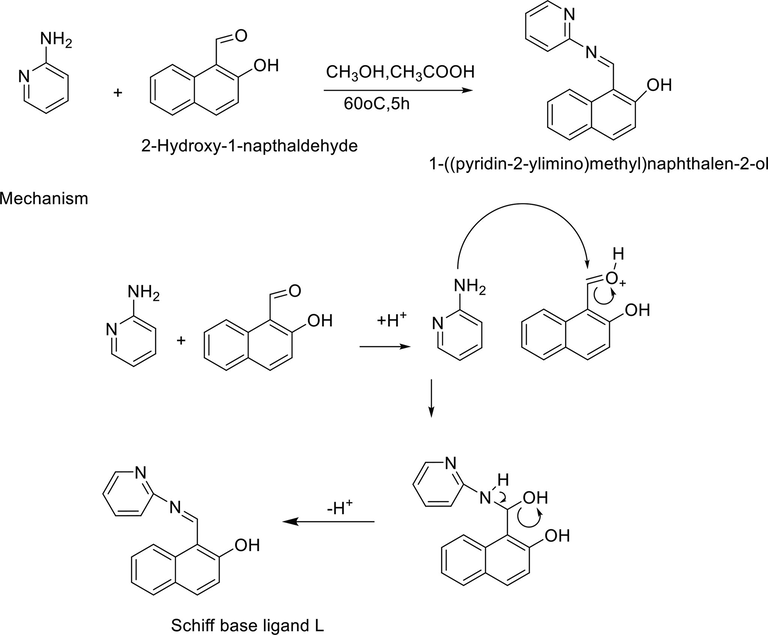
The overall reaction outline of ligand L synthesis.
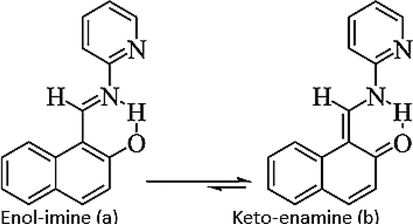
Tautomeric forms of ligand L.
IR spectra (KBr, cm−1): ν C-OH, 1294, 1205; ν C = N, 1612, ν aromatic C = C, 149 4, 1436; ν aromatic-C–H, 3036.
1H NMR in (500 MHz, DMSO‑d6): at δ = 6.83–8.51 (m, 10H, naphtha-H and pyridine-H), and 9.83 (d, J = 6.7 Hz, 1H, = CH-N), 15.18 (d, J = 6.7 Hz, 1H, NH).
2.3 Cytotoxicity test
Cytotoxic evaluation of ligand L were performed through a typical MTT (3-[4, 5-dimethylthiazole-2-yl]-2, 5-diphenyl-tetrazolium bromide) colorimetric test using a 96-well uniform-bottomed small micro sized plate. For such kind of analysis, H-460 (Human Lung) and MCF-7 (Breast Cancer) were developed in Lowest Crucial Medium Eagle, added with 5 percent of fetal bovine serum, penicillin (100 IU/ml) and streptomycin (100 µg/ml) in flasks of 75 cm2 area, retained in 5% carbon dioxide incubator 37 °C. Aggressively emergent cells remained reaped and haemocytometer was used for its counting. Dilution with a specific media was also applied. Culture of cell in concentration of 5x104 cells/ml were primed and put in (100 µL/well) into the mentioned plates. After an overnight incubation, medium were detached and were replaced by new medium (200 µL) having concentration of compound in ranges 31.5–500 µg/mL. After 48 h of incubation a 200 µL MTT (0.5 mg/mL) was introduced to each well and set on an incubator for additional 4 h. Then, to each well 100 µL of dimethyl sulfoxide were added. The decrease in MTT amount (from formazan inside cells) was noted at 570 nm with the help of Spectra Max plus (micro plate reader), USA. For calculation of % inhibition the given formula as presented in equation (1) was used and cytotoxicity were noted as concentration producing 50% growth inhibition (IC50) (Mosmann, 1983).
The % inhibition were calculated by Soft-Max Pro software (Molecular Device, USA).
2.4 Elevated plus maze (EPM) test
The EPM for the first time was presented by Handley and Mithani in an animal model study (Sharma et al., 2011). In this study Balb/C mice (19–23 g) were used. The apparatus comprises of four arms (among these arms two are opened while two are closed).The closed arms being 50 cm × 10 cm × 40 cm, and open arm 50 cm × 10 cm that are associated to a principal platform (10 cm × 10 cm). Maze was raised to an elevation of 50 cm overhead the ground. Initially every mouse was located in the centre section fronting one of open arms of the model for five min and their entry in close arm and open arm along with the time spend in each arms was noted. An entry was considered complete when all paws of each mouse were in an open or closed arm. A rise in an open arm entrance and intensification in time consumed within open arms were inferred as a file of prospective activity (Walf & Frye, 2007). All trial were recorded by means of digital camera as well.
3 Results and discussion
3.1 UV–Visible analysis
UV–Vis spectra of Schiff base ligand L displayed distinguishing absorption band in range from 280 to 330 nm with absorption maxima at λmax = 305 nm (Fig. 4). The analysis were performed in DMSO/water mixture (3:7) at room temperature. Absorption measurement were done using 6 μM of schiff base ligand L solution in a 1 cm quartz cell.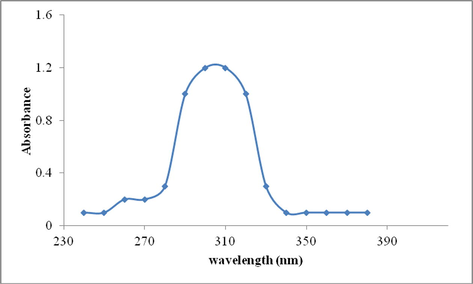
UV–Visible spectrum of ligand L (6 μM solution) showing max absorbance at 305 nm.
3.2 FTIR analysis
For determination of functional group in the synthesized Schiff base ligand L, IR analysis were performed in the range 400–4000 cm-1. The peaks identified are presented in the Table 1. The observed bands have been identified and assigned to specific functional group by comparison method with available standards in literature. As given in table 1 the appearance imine (-C = N) band at 1612 cm-1 confirms the synthesis of targeted ligand L. The bands at 1494, 1436 and 1294 1205 cm − 1 relates to aromatic C = C and C-OH stretching vibration. The spectrum exhibit absorption band at 3036 cm − 1 of aromatic -C–H stretching vibration as well. The aliphatic –C-O bands were noted at 1138 and 1038 cm − 1 (Saheb et al., 2011).
Band (C-OH)
Band aliphatic (C-O)
Band aromatic (C = C)
Band (C = N)
Band aromatic (C–H)
1294, 1205
1136, 1036
1494,1436
1612
3036
3.3 1HNMR analysis
The 1HNMR spectra of the ligand L displays a proton at δ 15.18 for NH. A singlet proton at δ 9–9.83 attributed to -N = CH denotes the imine moiety. Similarly the doublet protons at δ 6.83–8.51 represents the naphthalene and pyridine. Two singlet protons δ 4.4–5.2 can be assigned to the ArCH.
3.4 Anticancer activity
Cancer being the second major reason of death worldwide after heart disease, is a deadly disease. The researchers around the globe are trying to evaluate different chemical substances for their anticancer potentials. In this connection the synthesized Schiff base ligand L were screened for their in vitro cytotoxicity and growth restrictive activities against breast and lung cancer cell lines MCF-7 and H-460. Chemotherapy is the major approach of curing for both localized and metastasized cancer. Cell viability test were performed using trypan blue test. A dose-dependent ant proliferative activity in H-460 and MCF-7 cell cultures were observed.
For H-460 cells, at concentration of 500 µg/mL, 89.20 ± 1.12% of anticancer activity was observed followed by 78.80 ± 1.09, 72.39 ± 1.38, 69.75 ± 1.22, and 65.11 ± 1.36% for 250, 125, 62.5 and 31.25 µg/ml concentrations correspondingly. The IC50 were found to be 23.99 µg/mL. In case of the MCF-7 cell line similar type of dose response percent inhibit was observed and IC50 was found to be 31.68 µg/mL as shown in Table 2.
Concentration (µg/ml)
H-460
MCF-7
% response
IC50
% response
IC50
500
89.20 ± 1.12
23.99
84.9 ± 1.03
31.68
250
78.80 ± 1.09
76.92 ± 1.17
125
72.39 ± 1.38
61.54 ± 1.31
62.5
69.75 ± 1.22
57.60 ± 1.13
31.25
65.11 ± 1.36
49.31 ± 1.22
By comparing results of both cell lines, it was establish that the synthesized complex provide a better response to human lung cell (H-460) as compare to breast cell line (MCF-7). Cytotoxicity of xenobiotic might be described by the damage of either cellular regulation, intracellular production of macromolecular or biological processes signalling that is a frequent mean/analyses used for the cytotoxicity evaluations (Gutleb, Morrison & Murk, 2002). Studies on the growth inhibition caused by schiff bases have shown that the amine group in the molecular structure of these compounds is responsible for the inhibitory effect on cell lines (Yıldırım et al., 2014). The effect on cell proliferation is like that of anti-estrogens that can modulate the secretion of growth factor so that cell proliferation can be inhibited (Jaiswal & Mishra 2018).
3.5 Antidepressant activity of ligand L
Table 3 shows the results of elevated plus maze model. Diazepam (0.6 mg/kg) was used as reference drugs for comparison of the antidepressant activities. Schiff base ligand L at dose 6 mg/kg significantly enhanced the frequency of entries into open arm and time spend there from 6 ± 1.12 of control group to 8 ± 1.23 corresponding to time spend there 49.33 ± 3.85 sec to 75.09 ± 4.07 sec. At dose 12 mg/kg period spend in open arm were from 49.33 ± 3.85 sec (control) to 96 ± 1.83 sec with corresponding entries of 6.09 ± 1.12 to 9.36 ± 1.38.
Test sample
Dose (mg/kg)
Time (Sec) spend in open arm
Number of open arm entries
Control
3.5%
49.33 ± 3.85
6.09 ± 1.12
Diazepam
0.6
142.12 ± 3.98
16.22 ± 1.43
Schiff base ligand L
6.0
75.09 ± 4.07
8.12 ± 1.23
12.0
96.61 ± 3.83
9.36 ± 1.38
24.0
75.41 ± 3.13
8.5 ± 1.13
At dose of 24 mg/Kg time spend within open arm increased from 49.33 ± 3.85 to 75.41 ± 3.13 sec corresponding to entries 6 ± 1.12 and 8 ± 1.13. The Schiff base sensor L showed comparable antidepressant activities (Fig. 5a and b) which might be due to the presence of azomethine group (Gou et al., 2017).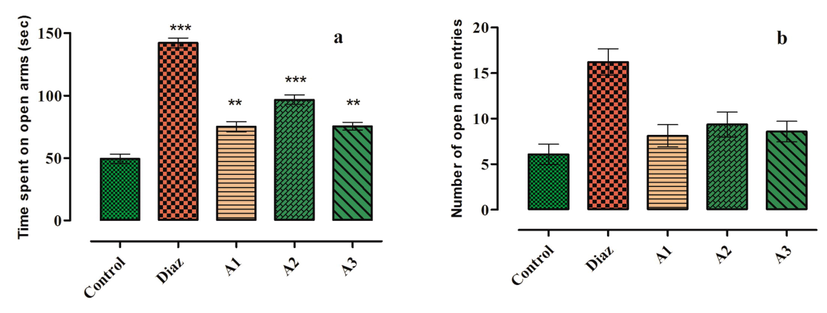
Results of elevated maize test (a): Time spend in open arm (b): Open arm entries.
3.6 Comparison of anticancer and antidepressant activities with reported studies
A comparison of anticancer activities of the ligand L with reported studies have been shown in Table 4 while that of the antidepressant studies in Table 5.
Compounds/ligand
Cell lines
IC50 µg/mL
References
Quinoline derivative
MCF-7
36
Broch et al., (2010)
Curcumin
MCF-7
42
Jaiswal & Mishra (2018)
Flavonol
MCF-7
50
Pham et al. (2018)
Coumarin-thiazole Schiff base
MCF-7
41
Omer et al. (2017)
Ferrite nanoparticles
MCF-7
37
Sarala et al. (2020)
Flavonol
H- 460
38
Pham et al. (2018)
1,2,4-triazole
H- 460
36
Hany et al. (2018)
Deguelin
H- 460
43
Hsu et al. (2017)
Ligand L
MCF-7
31.68
Current work
Ligand L
H- 460
23.99
Current work
Ligand/ compound
Dose (mg/Kg)
Time (Sec) spend in open arm
Number of open arm entries
References
Schiff base ligand L
6
75.09 ± 4.07
16.22 ± 1.43
Current work
Fatty acid analogue
10
70.01 ± 6.07
13.30 ± 2.1
Jubie et al. (2015)
Benzodiazepine like ligand
10
69.01 ± 5.07
10.10 ± 3.1
Prevot et al. (2019)
Pyrrolidine derivative
11
67.02 ± 5.03
11.12 ± 3.5
Wróbel et al. (2019)
The synthesized ligand have lower IC50 values against the same cell lines while other compounds have high values indicating its high growth inhibition potentials against cancer cell lines.
Table 5 shows that the synthesized ligand have good antidepressant activities even at small dose than other reported compounds.
4 Conclusion
A schiff base ligand L [(pyridine-2-ylimino) methyl) naphthalene-2-ol] was synthesized through an aldol condensation reaction and was characterized by various spectroscopic techniques. The synthesized ligand was evaluated for its anticancer and antidepressant activities using known literature methods. The synthesized ligand L exhibited comparable growth inhibition activities against cell lines MCF-7 and H-460 and a dose dependent response was observed. The ligand L also exhibited antidepressant potentials with a dose dependent response. The developed ligand L can be used as anticancer and antidepressant agent. However, further experiments are needed in this connection to evaluate its toxicological effects and confirm the observed potentials in in vivo in different animal models.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no RG-1440-100.
Declaration of Competing Interest
The authors declare no competing interest.
References
- Autonomic neurocardiac function in patients with major depression and effects of antidepressive treatment with nefazodone. J. Affective Disorders. 2001;62(3):187-198.
- [CrossRef] [Google Scholar]
- Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. New England J. Med.. 1997;337(3):161-167.
- [CrossRef] [Google Scholar]
- Synthesis and in vitro antiproliferative activities of quinoline derivatives. Eur. J. Med. Chem.. 2010;45(4):1657-1662.
- [Google Scholar]
- A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci.. 2015;4(2):119-133.
- [Google Scholar]
- Synthesis, characterization, and anticancer activity of new metal complexes derived from2-hydroxy-3-(hydroxyimino)-4-oxopentan 2ylidene)benzohydrazide. Bioinorganic Chem. Appl.. 2015;8(5):1236-1247.
- [CrossRef] [Google Scholar]
- synthesis, anticancer activity and molecular docking study of schiff base complexes containing thiazole moiety. Beni-Suef Univ. J. Basic Appl. Sci.. 2016;7(2):85-96.
- [CrossRef] [Google Scholar]
- Structure and biological properties of mixed-ligand Cu(II) Schiff base complexes as potential anticancer agents. Eur. J. Med. Chem.. 2017;134:207-217.
- [Google Scholar]
- Cytotoxicity assays for mycotoxins produced by Fusarium strains: A review. Environ. Toxicol. Pharmacol.. 2002;11(3-4):309-320.
- [Google Scholar]
- Stuck in a rut: rethinking depression and its treatment. Trends Neurosci.. 2011;34(1):1-9.
- [Google Scholar]
- Antitumor effects of deguelin on H 460 human lung cancer cells in vitro and in vivo: Roles of apoptotic cell death and H 460 tumor xenografts model. Environ. Toxicol.. 2017;32(1):84-98.
- [Google Scholar]
- Co-delivery of curcumin and serratiopeptidase in HeLa and MCF-7 cells through nanoparticles show improved anti-cancer activity. Mater. Sci. Eng. C. 2018;92:673-684.
- [Google Scholar]
- Design, synthesis and antidepressant activities of some novel fatty acid analogues. Med. Chem. Res.. 2015;24(4):1605-1616.
- [Google Scholar]
- Radiosensitization by diospyrin diethylether in MCF-7 breast carcinoma cell line. Mol. Cell Biochem.. 2007;304(1-2):287-296.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55-63.
- [CrossRef] [Google Scholar]
- New platinum (II) and palladium (II) complexes of coumarin-thiazole Schiff base with a fluorescent chemosensor properties: Synthesis, spectroscopic characterization, X-ray structure determination, in vitro anticancer activity on various human carcinoma cell lines and computational studies. J. Photochem. Photobiol. B. 2017;178:428-439.
- [CrossRef] [Google Scholar]
- Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Brief. 2018;21:1033-1036.
- [Google Scholar]
- Novel benzodiazepine-like ligands with various anxiolytic, antidepressant, or pro-cognitive profiles. Mol. Neuropsychiatry. 2019;5(2):84-97.
- [Google Scholar]
- A new Schiff base compound N,N′-(2,2-dimetylpropane)-bis(dihydroxylacetophenone): Synthesis, experimental and theoretical studies on its crystal structure, FTIR, UV–visible, 1H NMR and 13C NMR spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2011;81(1):144-150.
- [Google Scholar]
- Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011. Chem. Soc. Rev.. 2013;42(8):3489.
- [CrossRef] [Google Scholar]
- Green synthesis of Lawsonia inermis-mediated zinc ferrite nanoparticles for magnetic studies and anticancer activity against breast cancer (MCF-7) cell lines. J. Mater. Sci. Mater. Electron.. 2020;31:8589-8596.
- [CrossRef] [Google Scholar]
- Possible underlying influence of p38MAPK and NF-κB in the diminished anti-anxiety effect of diazepam in stressed mice. J. Pharmacol. Sci.. 2011;116(3):257-263.
- [Google Scholar]
- Synthesis and biological evaluation of Schiff’s bases and 2-azetidinones of isonocotinyl hydrazone as potential antidepressant and nootropic agents. Arabian J. Chem.. 2016;9:S79-S90.
- [Google Scholar]
- Green route synthesis of Schiff's bases of isonicotinic acid hydrazide. Green Chem. Lett. Rev.. 2009;2(1):23-27.
- [Google Scholar]
- Diagnostic and statistical manual of mental disorders 5: A quick glance. Indian J. Psychiatry. 2013;55(3):220-223.
- [CrossRef] [Google Scholar]
- Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp. Brain Res.. 2007;176(4):603-615.
- [Google Scholar]
- Synthesis, characterization, spectroscopic studies and biological evaluation of Schiff bases derived from 1–hydroxy-2-acetonapthanone. Heliyon. 2019;5(11):e02774.
- [CrossRef] [Google Scholar]
- The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc.. 2007;2(2):322-328.
- [Google Scholar]
- Inhibition of UBE2D3 expression attenuates radio sensitivity of MCF-7 human breast cancer cells by increasing TERT expression and activity. PloS One. 2013;8(5):64660.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of new multi-target 3-(1H-indol-3-yl)pyrrolidine-2,5-dione derivatives with potential antidepressant effect. Eur. J. Med. Chem.. 2019;183:111736.
- [CrossRef] [Google Scholar]
- A simple and effective fluorescent chemosensor for the cascade recognition of Zn2+ and H2PO4− ions in protic media. Tetrahedron. 2014;70(4):1011-1015.
- [Google Scholar]







