Translate this page into:
The cytotoxic and anti-inflammatory potential of Tetragonula sapiens propolis from Sulawesi on raw 264.7 cell lines
⁎Corresponding author at: Department of Chemical Engineering, Faculty of Engineering, Universitas Indonesia, Kampus UI, Depok 16424, Indonesia. sahlan@che.ui.ac.id (Muhamad Sahlan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Propolis is a naturally produced resinous mixture the bees are secreted through collecting resin of plants, buds, and exudates. According to several studies, propolis possessed various properties, one of which known as immunomodulatory property. Immunomodulatory property can be assessed and observed through the anti-inflammatory and cytotoxicity properties of propolis. In Indonesia, Tetragonula sapiens (T. sapiens) from Sulawesi is considered as one of the most commercial and productive bees. Despite of their productivity and commerciality, they have never been tested for their immunomodulatory property. In order to explore their potential even further, this present study was aimed to observe the anti-inflammatory and cytotoxicity properties of T. sapiens propolis from Sulawesi, Indonesia against RAW 264.7 cell lines. The cytotoxic assay of propolis against RAW264.7 cell lines was conducted using MTS assay. The anti-inflammatory properties were determined using LPS-induced RAW 264.7 macrophages cells to measure inhibitory activity of propolis on production of tumor necrosis factor-alpha (TNF-α), inducible nitric oxide synthase (iNOS), and nitric oxide (NO). The cytotoxic test results showed that propolis extract concentration affected the cell in terms of increasing or decreasing the cell viability. The results of the anti-inflammatory test disclosed that propolis at 120 µg/mL had an anti-inflammatory property which was evidenced by TNF-α levels of 304.28 ± 30.25 pg/mL, iNOS of 5.42 ± 0.82 ng/mL, and NO 101.09 ± 1.49 µmol/L. It can be concluded that the Indonesian propolis from T. sapiens possess anti-inflammatory properties related to the inhibition of NO production by macrophages.
Keywords
Immunomodulatory
Cytotoxicity
Anti-inflammatory
Tetragonula sapiens
Propolis
1 Introduction
Propolis is a naturally produced resinous mixture the bees are secreted through collecting resin of plants, buds, and exudates (Wagh, 2013). Till date, around 300 different compounds have been identified in propolis which compositions strongly depend on geographical regions and botanical sources (De Castro, 2001; Savka et al., 2015). In general, propolis comprise of resin (50%–70%), oil and wax (30%–50%), pollen (5%–10%) and other chemical compounds such as, amino acids, minerals, sugars, vitamins, flavonoids, phenol, and aromatic compounds (Bankova, 2005; Kuropatnicki et al., 2013). Some of those compounds are responsible for developing propolis anti-inflammatory and cytotoxicity properties (Diva et al., 2019; Onbas et al., 2016; Sahlan et al., 2019).
Stingless bees play an important role in producing not only honey but also propolis. Till date, a total of 89 stingless bee species from 15 genus have identified native to the Indo-Australian region (Rasmussen, 2008; Sayusti et al., 2020). Among all genus, Tetragonula govern the highest percentage of biodiversity with 31 species originate from Indonesia. Tetragonula sapiens (T. sapiens) from Sulawesi is considered as one of the most commercial and productive bees.
According to work propolis have various properties, one of which known as immunomodulatory property and it can be assessed and observed through the anti-inflammatory and cytotoxicity properties of propolis (dos Santos Thomazelli et al., 2017; Medjeber et al., 2018; Santiago et al., 2016; Shang et al., 2020; Wolska et al., 2019). Indonesian propolis compounds from North Luwu region, South Sulawesi province have been identified by several researches (Miyata et al., 2020, 2019). The cytotoxicity and anti-inflammatory activities of Indonesian propolis, which also related to immunomodulatory activity has reported by our previous research (Iqbal et al., 2019; Sabir, 2019; Sabir and Sumidarti, 2017; Muhamad Sahlan et al., 2019). Sulawesi propolis are produced by Tetragonula sapiens stingless bee (Sayusti et al., 2020). Other study showed that Indonesian propolis from South Sulawesi has potential antioxidant agent (Christina et al., 2018; Pratami et al., 2018).
To explore the potential of propolis from T. sapiens even further, this present study was aimed to observe the anti-inflammatory property of T. sapiens propolis from Sulawesi, Indonesia. This study also examined the cytotoxicity of Indonesian propolis extract against RAW 264.7 cell lines, a monocyte or macrophage-like cells. This was a preliminary study that might lead to the exploration of cytotoxicity and anti-inflammatory mechanisms of propolis produced by T. sapiens.
2 Materials and methods
2.1 Material
The raw material of propolis from Tetragonula sapiens were taken from Masamba, North of the Luwu district, in the South Sulawesi Province of Indonesia. The extract ethanol propolis (EEP) was extracted with 96% ethanol using the method described by Pratami et al. (2018). The EEP was microencapsulated using spray drying with maltodextrin and gum arabic as described by Pratami et al (2020) (Pratami et al., 2019). The microencapsulation process was using industrial spray dryer at Phytochemindo Reksa company. The propolis sample was dry extract. Its appearance was brown powder with characteristic odor and taste.
2.2 Cell culture
RAW264.7 macrophages cell line (ATCC ® TIB- 71TM) were grown in Dulbecco’s Modified Eagle Medium (DMEM, Biowest L0060) supplemented with contain 10% fetal bovine serum (FBS) (Biowest, S1810) and 1% Antibiotic and Antimycotic (Biowest, L0010-100) and then incubated at 37 °C and 5% CO2 until the cells were confluent around 70–80%. The growth medium was frequently replaced after 2–3 days. The cells were then washed and harvested using trypsin-EDTA (Biowest, L0931-500). The cell culture has done according to the methods describe by Novilla et al. (2017).
2.3 Cytotoxicity test
The cytotoxicity test has done according to methods described by Widowati 2018 (Laksmitawati et al., 2017; Rusmana et al., 2015; Sandhiutami et al., 2017; Widowati et al., 2018). Cell viability was measured using MTS (3- (4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy- phenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay (Promega, ab197010). Briefly, 5x103 cells were seeded into each well of a 96-well plate then incubated for 24 h at 37 °C and 5% CO2. the medium was the discarded and changed with 180 µl of fresh growth medium and 20 µl of sample (200; 150; 100; 50; 25;12,5; 6,25 µg/mL) then the cells were re-incubated for 24 h at 37 °C and 5% CO2. Next, into each well, 20 µl of MTS was added and incubated for 3 h at 37 °C and 5% CO2. The absorbance was measured using spectrophotometer (Multiskan GO Thermo Scientific 51119300, Thermo Fisher Scientific, USA) at 490 nm. Cells without treatment were considered as control.
2.4 The anti-inflammatory test with LPS-induced RAW264.7
There were six treatments required for the anti-inflammatory test: (1) The negative control: RAW 264.7 cells without being induced by lipopolysaccharide; (2) The positive control: RAW 264.7 cells that were induced by 1 μg/mL of lipopolysaccharide (LPS from Escherichia coli); (3) The mixture of positive control and 1% DMSO; (4) The mixture of 120 μg/mL of propolis extract, LPS, and RAW 264.7 cells; (5) The mixture of 30 μg/mL of propolis extract, LPS, and RAW 264.7 cells; and (6) The mixture of 7.5 μg/mL of propolis extract, LPS, and RAW 264.7 cells. The cells were then incubated for 24 h. The following day, the medium was collected and centrifuged at 2000 × g for 10 min to get cell-free supernatant which were then used for TNFα, iNOS, and NO ELISA assay. The methods of in vitro anti-inflammatory assay has done according to the methods describe by Widowati et al. (2018) (Laksmitawati et al., 2017; Rusmana et al., 2015; Sandhiutami et al., 2017; Widowati et al., 2018).
2.4.1 Total protein content using Bradford assay
The Bradford assay has done using the method described by Lister et al (Lister et al., 2020). The BSA stock served as the standard and was prepared by dissolving 2 mg of BSA in 1000 µl ddH2O. BSA underwent serial dilutions from 200 to 0. The standard solutions (BSA) and sample (CM) were added separately into each well plate followed by propolis sample and Quick Start Dye Reagents (Bio Rad, 500-0205). As the mixture mixed, the solution turned blue. The mixtures were incubated at room temperature for 5 min and were measured for their absorbances at 595 nm using spectrophotometer (Multiskan GO Thermo Scientific 51119300, Thermo Fisher Scientific, USA).
2.4.2 TNF–α ELISA assay
TNF-α level was quantified using Mouse TNF-α ELISA Kit (E-EL-M0049, ElabSci) and the absorbance was measured at 450 nm based on maufacturer protocol. The activity inhibition toward TNF-α was calculated between the percentage (%) of TNF-α concentration in each treatment compared to the positive control. The TNF–α ELISA assay has done using the methods described by Widowati et al. (2020).
2.4.3 NOS2/iNOS ELISA assay
NOS2/iNOS level was measured using Mouse NOS2/iNOS ELISA Kit (E-EL-M0696, ElabSci) and the absorbance was measured at 450 nm based on maufacturer protocol. The activity level of NOS2/iNOS was obtained from the percentage (%) of NOS2/iNOS concentration in each treatment compared to the positive control. The NOS2/iNOS ELISA assay has done using the methods described by Sandhiutami et al. (2017).
2.4.4 NO colorimetric test
NO level was performed using NO assay Kit (E-BC-K035-M, ElabSci) and the absorbance was measured using spectrophotometer (MultiScan Go, Thermo Scientific) at 550 nm. The inhibition activity toward NO was calculated between the percentage (%) of NO concentration in each treatment compared to the positive control. The NO Colorimetric Test has done using the methods described by Widowati et al. (2018).
2.5 Statistical analysis
SPSS software was used to statistically analyze all the data. A one-way ANOVA was used to find significant difference between treatments, P < 0.05 was considered to be significant and further significance between groups was analyzed using a Tukey post hoc test. Results are presented as the mean ± standard deviation of 3 independent experiments.
3 Result and discussion
3.1 Cytotoxicity test
Cell viability of previously cultured RAW 264.7 cells was evaluated in the presence of propolis extract in vitro. The propolis concentration was set at 200, 150, 100, 50, 25, 12.5, and 6.25 μg/mL. Data were presented in mean ± standard deviation. Different superscript signs (a, ab, bc, cd, cde, de, e) indicated a significant difference (p < 0.05) in the Tukey HSD post hoc test.
Fig. 1 revealed the ability of propolis at 150 μg/mL and 200 μg/mL to substantially reduce the RAW 264.7 viability to 88.14% ± 8.43%ab and 75.99% ± 2.44%a respectively relative to the negative control. Meanwhile, propolis at concentrations of 6.25–50 μg/mL were not toxic to RAW 264.7 cells as they did not suppress the cell viability, instead they escalated the viability of RAW 264.7 cells with respect to negative control. Our study substantiated the fact that propolis concentration affected the cell in terms of increasing or decreasing the cell viability which can be useful for finding the optimal dose of propolis.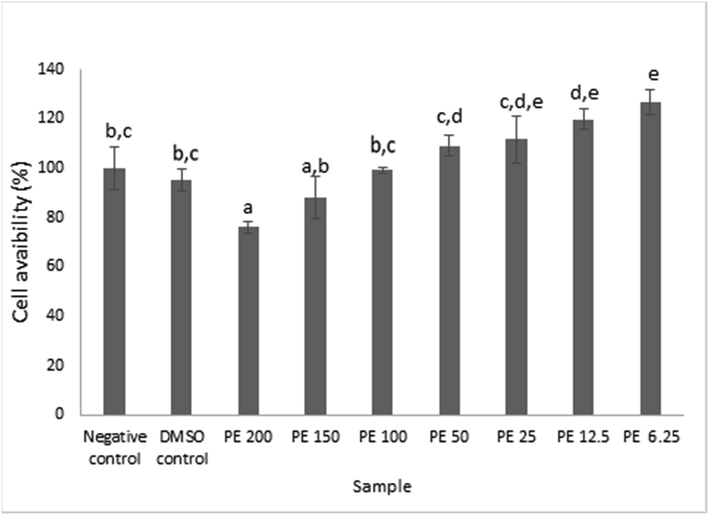
The viability of RAW 264.7 cell after treatment with propolis extract at various concentrations. Data was presented as Mean±Standard Deviation (n = 3), Different superscript letters (a,ab,bc,cd,cde,de,e) on the bars showed significant different at p < 0.05, Tukey HSD post hoc test.
3.2 Anti-inflammatory test
The inflammatory test consists of several key parts such as protein quantification using Bradford assay, the evaluation of TNF–α and iNOS using ELISA, and NO colorimetric test. The results were elaborated separately.
3.2.1 Total protein content using Bradford assay
The measurement of total protein content using Bradford in Table 1 showed that propolis extract at different concentration had an average protein content ranging from 203.08–208.30 μg/mL. Note: The data were given in mean ± SD, n = 3
Sample
Protein Content (µg/mL)
Negative Control
208.29 ± 1.93
Positive Control
204.24 ± 4.38
Positive Control +DMSO
207.39 ± 8.18
Propolis Extract 120 μg/mL
203.08 ± 5.21
Propolis Extract 30 μg/mL
208.30 ± 7.77
Propolis Extract 7.5 μg/mL
207.65 ± 6.89
3.2.2 TNF–α ELISA assay
Fig. 2 summarized the concentrations of TNF-α protein on RAW 264.7 cells after propolis extract induction. Data were presented in mean ± SD. Different superscript signs (a, b, c, bc) in TNF-α concentration (pg/mL) showed a significant difference (p < 0.05) Tukey HSD post hoc test. After measuring the optical density, the results proved that propolis significantly stimulated TNF-α production in RAW 264.7 cells at all propolis extract concentration, which was aligned with Bufalo et al. (2014) findings, and the inhibitory effect was absent relative to the negative control (Bufalo et al., 2014). TNF-α is a strong pro-inflammatory cytokine which plays an important role in the immune system during inflammation (Baud and Karin, 2001). Propolis at lower concentration triggered more TNF-α productions whilst, at higher concentration propolis lowered the TNF-α productions. In other words, higher propolis concentration resulted in less pro-inflammatory property as it depressed the TNF-α productions.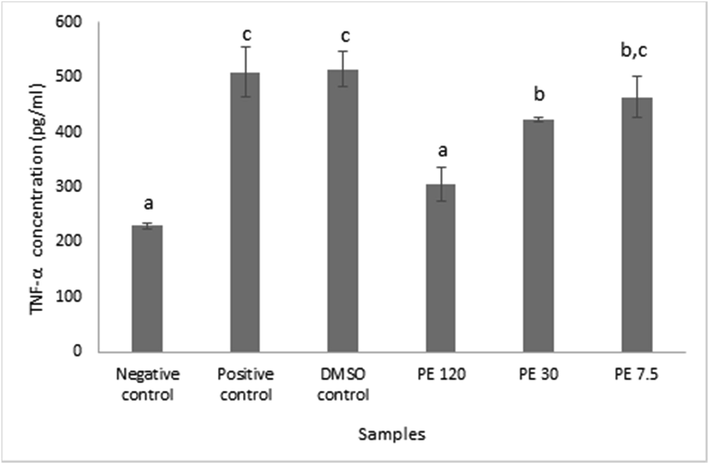
TNF-α protein concentration in RAW 264.7 cells after being induced with propolis extract. Data was presented as Mean±Standard Deviation, Different superscript letters (a,b,c.bc) on the bars showed significant different at p < 0.05, Tukey HSD post hoc test.
When comparing the result with the positive control, different view arose. The positive control was basically the combination between RAW 264.7 cells and LPS. LPS was notable as one of the main stimulants that triggers TNF-a production (Zelová and Hošek, 2013). Propolis addition was proven to be able to suppress TNF-α production at certain level depend on its concentration. Hence, it was appropriate to say that TNF-α involved in the pro-inflammatory property of Indonesian propolis from T sapiens.
3.2.3 iNOS (inducible nitric oxide synthase) ELISA assay
ELISA iNOS was performed in the principle of color changes in the solution when enzyme – substrate reaction occurs. The Optical Density (OD) was measured at nm ± 2 nm and the OD was proportional to the concentration of Mouse NOS2/iNOS.
Fig. 3 presented the iNOS protein concentration in RAW 264.7 cells after propolis extract induction. Data were presented in mean ± SD. Different superscript signs (a, b, c, d) showed a significant difference (p < 0.05) in the Tukey HSD post hoc test. iNOS functionated as a factory of NO production in macrophages, hence the observation results affected one another. The concentrations of iNOS were declined as the propolis concentration elevated, indicating that propolis extract samples contributed to the inhibition of iNOS production. Since iNOS and NO were strongly related, the iNOS downregulation in response to propolis extract treatment would degrade the NO production.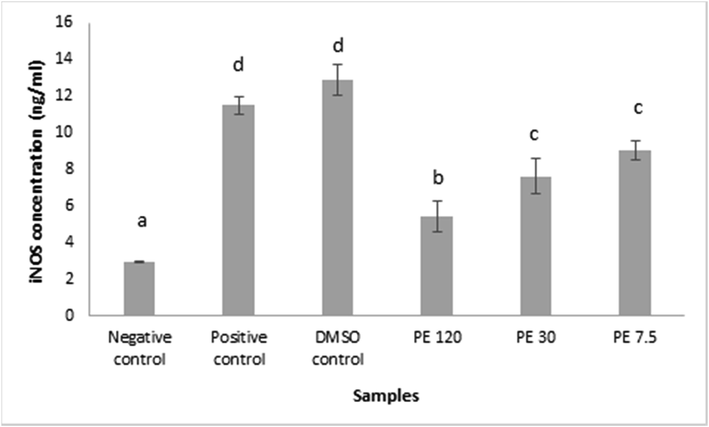
iNOS protein concentration in RAW 264.7 cells after propolis extract induction. Data was presented as Mean±Standard Deviation, Different superscript letters (a,b,c,d) on the bars showed significant different at p < 0.05, Tukey HSD post hoc test.
3.2.4 NO colorimetric test
NO colorimetric test was performed in order to determine the concentration of NO in RAW 264.7 cells. The principal of the measurement was the ability of NO to reacts with oxygen and water to form nitrates or nitrites which form red azo compounds when they encounter chromogenic reagents. The absorbance of this compound was used to measure the NO level directly.
Depicted NO colorimetric test result on Fig. 4. The data were presented in mean ± SD. Different superscript signs (a, b, c, d, e) showed a significant difference (p < 0.05) in the Tukey HSD post hoc test. LPS was used to activate the macrophages. As shown in Fig. 4, the NO production in the LPS stimulated RAW 264.7 cell (positive control) successfully increased relative to the negative control by the help of iNOS. The propolis extract administration also affected the result by stimulating the NO production. The raise was considered significant compared to the negative control. The trend revealed that when the propolis extract concentration mounted, the production of NO declined. 120 μg/mL of propolis extract was able to downregulated the NO production at the lowest level. These results suggested that the anti-inflammatory property of Indonesian propolis from T. sapiens, may function, at least in part, related to the inhibition of NO production by macrophages (Nagaoka et al., 2003).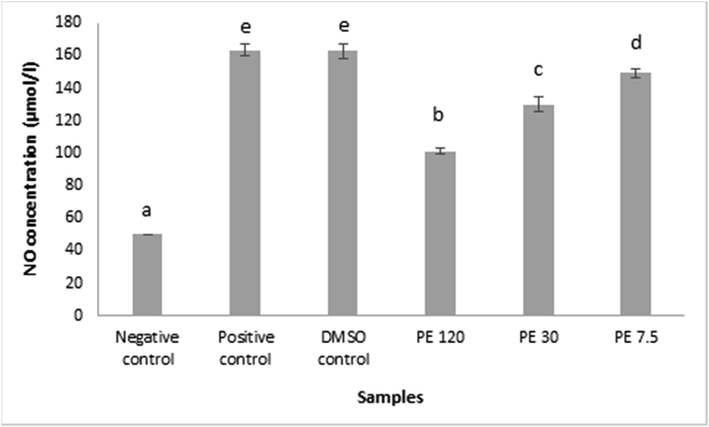
The concentration of NO compounds in RAW 264.7 cells after being induced with propolis extract. Data was presented as Mean±Standard Deviation, Different superscript letters (a,b,c,d,e) on the bars showed significant different at p < 0.05, Tukey HSD post hoc test.
4 Conclusions
Our study confirmed the fact that cell viability was dose-dependent to some extent. At higher concentration the viability decreased and at lower concentration the viability increased. The cytotoxic test results also showed that propolis extract from T. sapiens was safe in the concentration range of 6.25–200 µg/mL. The study may useful for finding the optimal concentration of propolis to modulate cell viability.
Anti-inflammatory properties of propolis were determined by observing the concentration of TNF-α, iNOS, and NO using ELISA. Propolis extract at concentration of 120 µg/mL disclosed the anti-inflammatory properties. In details, the reduction of NO production in response to propolis extract treatment was caused by iNOS downregulation. It can be concluded that the Indonesian propolis from T. sapiens possess anti-inflammatory properties related to the inhibition of NO production by macrophages. This was a preliminary study that might lead to the exploration of cytotoxicity and anti-inflammatory mechanisms of propolis produced by T. sapiens, hence increasing the use of that propolis as a constituent in pharmaceutical industry, specifically for anti-inflammation drug development.
Acknowledgments
The research was financially supported by DRPM Universitas Indonesia through Grant Publikasi Terindeks Internasional (PUTI) Q1 2020 (Grant No. NKB-1403/UN2.RST/HKP.05.00/2020). The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP‑ 2020/154), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol.. 2005;100(1-2):114-117.
- [CrossRef] [Google Scholar]
- Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol.. 2001;11(9):372-377.
- [CrossRef] [Google Scholar]
- The immunomodulatory effect of propolis on receptors expression, cytokine production and fungicidal activity of human monocytes: Propolis effect on human monocytes. J. Pharm. Pharmacol.. 2014;66(10):1497-1504.
- [CrossRef] [Google Scholar]
- Christina, D., Hermansyah, H., Wijanarko, A., Rohmatin, E., Sahlan, M., Pratami, D.K., Mun’Im, A. 2018. Selection of propolis Tetragonula sp. extract solvent with flavonoids and polyphenols concentration and antioxidant activity parameters, in: AIP Conference Proceedings. https://doi.org/10.1063/1.5023967.
- Propolis: biological and pharmacological activities. Therapeutic uses of this bee-product. Annu. Rev. Biomed. Sci.. 2001;3:49-83.
- [Google Scholar]
- Effect of ethanolic propolis extract from Tetragonula biroi bees on the growth of human cancer cell lines HeLa and MCF-7. In: AIP Conference Proceedings. AIP Publishing. 2019. p. :30002.
- [CrossRef] [Google Scholar]
- Brazilian propolis promotes immunomodulation on human cells from American Tegumentar Leishmaniasis patients and healthy donors infected with L. braziliensis. Cell. Immunol.. 2017;311:22-27.
- [CrossRef] [Google Scholar]
- Heliyon preliminary studies: The potential anti-angiogenic activities of two Sulawesi Island (Indonesia) propolis and their chemical characterization. Heliyon. 2019;5:e01978
- [CrossRef] [Google Scholar]
- Historical aspects of propolis research in modern times. Evid. Based Complement. Alternative Med.. 2013;2013:1-11.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of Anredera cordifolia and Piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line. Bangladesh J. Pharmacol.. 2017;12:35-40.
- [CrossRef] [Google Scholar]
- Hepatoprotective properties of red betel (Piper crocatum Ruiz and Pav) leaves extract towards H2O2-induced HepG2 cells via anti-inflammatory, antinecrotic, antioxidant potency. Saudi Pharmaceutical J.. 2020;28(10):1182-1189.
- [CrossRef] [Google Scholar]
- Ex vivo immunomodulatory effect of ethanolic extract of propolis during Celiac Disease: Involvement of nitric oxide pathway. Inflammopharmacology. 2018;26(6):1469-1481.
- [CrossRef] [Google Scholar]
- Erratum: propolis components from stingless bees collected on South Sulawesi, Indonesia, and their xanthine oxidase inhibitory activity. J. Nat. Prod.. 2020;83:1356.
- [CrossRef] [Google Scholar]
- Propolis components from stingless bees collected on South Sulawesi, Indonesia, and their xanthine oxidase inhibitory activity. J. Nat. Prod.. 2019;82(2):205-210.
- [CrossRef] [Google Scholar]
- Caffeic acid phenethyl ester (CAPE) analogues: Potent nitric oxide inhibitors from the Netherlands propolis. Biol. Pharm. Bull.. 2003;26(4):487-491.
- [CrossRef] [Google Scholar]
- Anti-inflammatory properties of oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pacific J. Trop. Biomedi.. 2017;7(11):1005-1009.
- [CrossRef] [Google Scholar]
- Cytotoxic and nitric oxide inhibition activities of propolis extract along with microencapsulation by complex coacervation. Plant Foods Hum. Nutr.. 2016;71(3):286-293.
- [CrossRef] [Google Scholar]
- Microencapsulation optimization of propolis ethanolic extract from Tetragonula spp using response surface methodology. Int. J. App. Pharm. 2020;12
- [Google Scholar]
- Pratami, D.K., Mun’Im, A., Yohda, M., Hermansyah, H., Gozan, M., Putri, Y.R.P., Sahlan, M. 2019. Total phenolic content and antioxidant activity of spray-dried microcapsules propolis from Tetragonula species. AIP Conf. Proc. 2085. https://doi.org/10.1063/1.5095018.
- Phytochemical profile and antioxidant activity of propolis ethanolic extract from Tetragonula Bee. Pharmacogn. J.. 2018;10(1):128-135.
- [CrossRef] [Google Scholar]
- Catalog of the Indo-Malayan/Australasian stingless bees (Hymenoptera: Apidae: Meliponini) Zootaxa. 2008;1935:1-80.
- [CrossRef] [Google Scholar]
- Inhibition of Inflammatory Agent Production by Ethanol Extract and Eugenol of Syzygium aromaticum (L.) Flower Bud (Clove) in LPS-Stimulated Raw 264.7 Cells. Res. J. Med. Plant. 2015;9(6):264-274.
- [CrossRef] [Google Scholar]
- Indonesian propolis suppressed the expression of COX-2 in inflamed rat dental pulp in direct capping treatment. J. Dentomaxillofacial Sci.. 2019;4:109-113.
- [Google Scholar]
- Interleukin-6 expression on inflamed rat dental pulp tissue after capped with Trigona sp. propolis from south Sulawesi, Indonesia. Saudi J. Biol. Sci.. 2017;24(5):1034-1037.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of Tetragronula species from Indonesia. Saudi J. Biol. Sci.. 2019;26(7):1531-1538.
- [CrossRef] [Google Scholar]
- In vitro assesment of anti-inflammatory activities of coumarin and Indonesian cassia extract in RAW264. 7 murine macrophage cell line. Iran. J. Basic Med. Sci.. 2017;20:99.
- [Google Scholar]
- Immunomodulatory/anti-inflammatory effects of a propolis-containing mouthwash on human monocytes. Pathogens Dis.. 2016;74(8)
- [CrossRef] [Google Scholar]
- Chemical composition and disruption of quorum sensing signaling in geographically diverse United States propolis. Evid. Based Complement. Alternative Med.. 2015;2015:1-10.
- [CrossRef] [Google Scholar]
- Stingless bees (Hymenoptera: Apidae) in South and West Sulawesi, Indonesia: Morphology, nest structure, and molecular characteristics. J. Apicultural Res.. 2020;60(1):143-156.
- [CrossRef] [Google Scholar]
- Effect of propolis supplementation on C-reactive protein levels and other inflammatory factors: A systematic review and meta-analysis of randomized controlled trials. J. King Saud Univ. Sci.. 2020;32(2):1694-1701.
- [CrossRef] [Google Scholar]
- Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci.. 2013;2013:1-11.
- [CrossRef] [Google Scholar]
- Widowati, W., Darsono, L., Suherman, J., Afifah, E., Rizal, R., Arinta, Y. 2018. Mangosteen Peel Extract (Garcinia mangostana L.) and its Constituents to Lower Lipid Content on Adipogenesis Cells Model (3T3-L1). https://doi.org/10.18311/jnr/2018/18654.
- Effect of interleukins (IL-2, IL-15, IL-18) on receptors activation and cytotoxic activity of natural killer cells in breast cancer cell. Afr. Health Sci.. 2020;20:822-832.
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of Propolis and its components on basic immune cell functions. Indian J. Pharm. Sci.. 2019;81:575-588.
- [CrossRef] [Google Scholar]
- TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res.. 2013;62(7):641-651.
- [CrossRef] [Google Scholar]







