Translate this page into:
In-vitro antimicrobial activities of organic solvent extracts obtained from Dipcadi viride (L.) Moench
⁎Corresponding author. adilqau5@gmail.com (Adeel Mehmood)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In a pharmacological screening methanol, ethanol and chloroform extracts of Dipcadi viride (L.) Moench, were assayed for antibacterial and antifungal activities. Pathogenic bacteria and fugai were used as test strain to validate their anti-microbial potential. Disc diffusion and agar tube dilution methods were used to assess antibacterial and antifungal activities, respectively. Crude plant extracts exhibited inhibitory effect against maximum of pathogenic microbes. Ethanol and methanol proved better solvents compared with the chloroform. Results of crude plant extracts and standard antibiotic doxycycline (DOX) showed comparable effect against pathogenic bacteria and fungi (A. niger, 124 mm; A. fumigates, 109 mm). Results of the current study were interesting as inhibition zones were observed even, when extracts were used in lower concentrations. There should be detailed pharmacological screening of the crude extracts of this plant for exploration of effective and natural drug.
Keywords
Antibacterial
Antifungal
Bioassay
Antibiotic resistance
- SDA
Sabouraud dextrose agars
- DMSO
dimethyl sulfoxide
- MIC
minimum inhibitory concentration
- DOX
doxycycline
Abbreviations
1 Introduction
Plants produce secondary metabolites, which constitute a main source of biologically active substances (Antonisamy et al., 2015; Balamurugan, 2015; Al-Dhabi et al., 2015; Valsalam et al., 2019a; Rajkumari et al., 2019). Today, the increasing resistance pattern of pathogenic microbes to commonly used antimicrobial drugs has increased the interest of scientists in secondary metabolites for discovery of new therapeutic agents from plants (Mahmood et al., 2013; Glorybai et al., 2015; Barathikannan et al., 2016; Al-Dhabi and Arasu, 2016; Haritha et al., 2016; Cuong et al., 2017; Park et al., 2016a,b; Elango et al., 2017; Gurusamy et al., 2019). Antibiotic resistance is one of the major issues of WHO for present era (Mahmood et al., 2012; Arokiyaraj et al., 2015; Elango et al., 2016a;). However, in last few years, an increase in screening of plants as a source of disease management has been observed (Prashanth et al., 2001; Elango et al., 2016b; Fowsiya et al., 2016). More ever, it is estimated that two-third population of the world dependent on traditional plants in healthcare system due to expensive pharmaceutical products (Tagboto and Townson, 2001; Helan et al., 2016; Ilavenil et al., 2017; Park et al., 2016a,b; Park et al., 2017)
Dipcadi is a diverse genus of family Hyacinthaceae, having a number of medicinally important species, bearing essential oil and secondary metabolites over 400 plant species (Rathi et al., 2015; Valsalam et al., 2019b). Secondary metabolites and essential oils of this genus have been used since many years for different medicinal purposes. Dipcadi viride is a commonly known plant with wide distributions. D. viride has been reported ethno-medicinally as effective in jaundice, ulcer, diuretic and purgative; plant extract also exhibit effect for cardiac diseases along with the insecticidal properties (Mahmood et al., 2011b; Akhtar et al., 2013; Surendra et al., 2016a; Surendra et al., 2016b). A number of species of Dipcadi have been studied for their biological activities. This genus is considered to have the majority of medicinally valued metabolites (Akhtar et al., 2013). A variety of secondary metabolites such as flavonoids, alkaloids and steroids etc. from different species of Dipcadi have been detected by HPLC, GC–MS and NMR (Kutacek et al., 1981; Araghiniknam et al., 1996; Surendra et al., 2016c). The screening of latent antimicrobial activities of D. viride extracts could be useful to treat diseases as natural antimicrobial agents.
Presenting research work was aimed to screen the anti-microbial activities of bio-active compounds against infectious and resistant pathogens. D. viride was investigated for antibacterial and antifungal activities against different pathogens, food poisoning bacteria and fungi. D. viride is glabrous plant, varied size, one to several leaves per shoot, lenear-lanceolate and some time crinkly or spirally twisted margins.
2 Materials and methods
2.1 Plant materials
Fresh plants D. viride was collected in May 2018 from Taif region, which is located at main foothills of western mountains at 2500 m a.s.l. (above sea level) and identified as Dipcadi viride (L.) Moench. Herbarium specimen was prepared and research work was done at Botany and Microbiology Department, College of Sciences, King Saud University, Saudi Arabia.
2.2 Test organisms
Five bacteria i.e. two Gram-positive Bacillus subtilis, Staphylococcus aureus and three Gram negative, Enterobacter aerogenes, Klebsiella pneumonia and Escherichia coli and two fungus i.e. Aspergillus niger and Aspergillus fumigates were used as test organisms. These test organism’s cultures were obtained from the Botany and Microbiology Department, College of Sciences, King Saud University, Saudi Arabia. Bacterial cultures were sub-cultured on nutrient agar (MERCK; Germany) and fungus was sub-cultured on SDA (MERCK; Germany).
2.3 Preparation of plant extract
Whole plant of D. viride was shade dried and pulverized by grinder into powder form. A 100 g of powdered plant material was soacked in 1L of methanol, ethanol and chloroform solvents for 7 days at room temperature. This mixture was filtered by Whatman 41 filter paper and then concentrated under reduced pressure by using rotary evaporator at 40 °C. Before performing the antimicrobial activity stock solution of each extract were made by dissolving 15 mg of plant extract in 10 ml of DMSO. Furthermore, eight dilutions of 15 mg/ml, 12.50 mg/ml, 10 mg/ml, 7.5 mg/ml, 5 mg/ml and 3 mg/ml, 2 mg/ml and 1 mg/ml concentration were made by this stock solution.
2.4 Antibacterial activities
Antimicrobial activities of methanol, ethanol and chloroform extracts was determined by using Agar well diffusion method followed by Kivack et al. (2001). Culture plates were allowed to solidify and then seeded with bacterial strains. After that plates were punched with sterile cork borer and these open wells were inoculated by 80 µl test solution. Standard antibiotic doxycycline was used as positive control. Finally, these plates were incubated at 37 °C for 24 h.
2.5 Antifungal activities
Antifungal activity was performed by agar tube dilution method (Fatima et al., 2009). Test plant material was prepared by dissolving 20 mg/ml in DMSO of each extract i.e. methanol, ethanol and chloroform. 100 μl Test plant material was added to the autoclaved media. Terbinafin was used as positive control and DMSO was used as negative control. 83 μl of terbinafin and 100 μl of pure DMSO was added to media. Test tubes were allowed to solidify in slanting positions. Each tube was inoculated with 3 mm piece of fungus from one week old culture. Linear growth of fungus was measured in mm and percentage inhibition was calculated by formula:
3 Results
3.1 Antibacterial activities
Results showed that crude methanolic, ethanolic and chloroform extract of D. viride has potential against all the bacterial strains screened (Figs. 1, 2 & 3). Crude extract of D. viride in ethanol was more effective compared with the methanol and chloroform extract. Maximum activity of ethanolic extract was observed against Escherichia coli and Bacillus subtilis. The rank of antibacterial activity of ethanolic extract against each bacterial strain is; Escherichia coli > Bacillus subtilis > Enterobacter aerogenes > Klebsiella pneumonia > Staphylococcus aureus.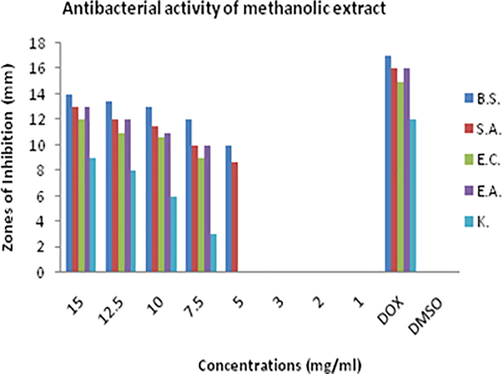
Zones of inhibitions (mm) showing antimicrobial activity of methanolic extract of D. viride against Bacillus subtilis (B.S.), Staphylococcus aureus (S.A.), Escherichia coli (E.C.), Enterobacter aerogenes (E.A.) and Klebsiella pneumonia (K).
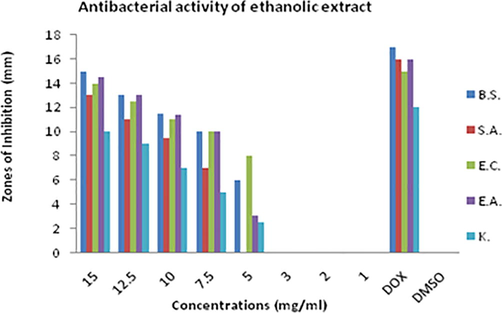
Zones of inhibitions (mm) showing antimicrobial activity of ethanolic extract of D. viride against Bacillus subtilis (B.S.), Staphylococcus aureus (S.A.), Escherichia coli (E.C.), Enterobacter aerogenes (E.A.) and Klebsiella pneumonia (K).
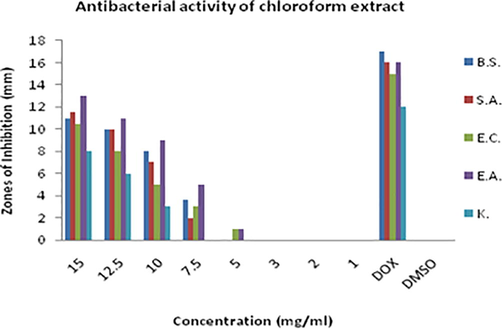
Zones of inhibitions (mm) showing antimicrobial activity of chloroform extract of D. viride against Bacillus subtilis (B.S.), Staphylococcus aureus (S.A.), Escherichia coli (E.C.), Enterobacter aerogenes (E.A.) and Klebsiella pneumonia (K).
Methanolic extract of D. viride showed considerable activity. The rank of its activity against each strain is; Bacillus subtilis > Staphylococcus aureus > Escherichia coli = Enterobacter aerogenes = Klebsiella pneumonia. MIC of methanolic plant extract was found 5 mg/ml. Chloroform extract also showed moderate activity against all the bacterial strains. But its activity was low compared with the other two extracts. It showed maximum activity against Enterobacter aerogenes i.e. 13 mm inhibition zone at the concentration of 15 mg/ml. MIC was found 5 mg/ml. The rank of crude chloroform extract of D. viride is; Enterobacter aerogenes > Klebsiella pneumonia > Escherichia coli = Staphylococcus aureus > Bacillus subtilis.
The results showed that ethanol is better solvent for the extraction of antibacterial agents compared with the methanol and chloroform. Antibacterial activity of methanolic extract was also prominent and was almost equal or little bit less than ethanolic extract. Rank of solvents based on antibacterial activity of D. viride is; ethanol > methanol > chloroform.
3.2 Antifungal activities
Results showed that the methanolic and ethanolic extract of D. viride exhibited best antifungal activities against Aspergillus niger and Aspergillus fumigates. Maximum activity was shown by methanolic extract against A. fumigates i.e. 77.1% and ethanolic extract against A. niger i.e. 76.8%. Crude extract of chloroform also showed average antifungal activity against both the tested strains. Results are presented in Table 1. *Aspergillus niger – A. niger and Aspergillus fumigates – A. fumigates. L.G.C. (mm) = Linear Growth in Control (millimeter), L.G.T. (mm) = Linear Growth in Test (millimeter).
Test plant extract
*Fungal strains used
*L.G.C. (mm)
*L.G.T. (mm)
% Inhibition
Methanolic extract (D. viride)
A.niger
124
35
71.2%
A. fumigates
109
25
77.1%
Ethanolic extract (D. viride)
A.niger
125
29
76.8%
A. fumigates
109
30
72.5%
Chloroform extract (D. viride)
A.niger
125
51
59.2%
A. fumigates
109
49
55.1%
4 Discussion
D. viride is indigenous medicinal plant which is being used to treat various diseases for many years such as bronchitis, cough, fever, jaundice, cardiac problems and skin problems. It is diuretic, emetic, antidote and purgative (Mahmood et al., 2011b). Present study reported the antimicrobial activity of different extracts of D. viride in an attempt to verify the reported beneficial uses. Antibacterial and antifungal activities were investigated in this research work as antibiotic resistance is a burning issue of ongoing time.
Pathogenic microorganisms are being resistance to the available antibiotics resulting, a number of clinical infections (Priscila et al., 2007). Regarding antibiotic resistance, Asia Pacific is at the top, compared with the whole world. This infection is the result of frequent use of antibiotics even against a minor health problem. Thus, it is required to establish the understanding of this budding issue to reduce this problem in healthcare (Anderson and Keye, 2009). This issue forced the pharmacists and botanists to focus on discovering new plant originated drugs (Mahmood et al., 2011a). Now, pharmaceutical companies are also paying their attentions to discover plant based drugs (Kannan and Agastian, 2015).
D. viride is an effective plant against pathogenic microbes. Antibacterial and antifungal activity of D. viride was strongly dependent on the solvent, which was used for the extraction. Agar well diffusion method is most convenient to evaluate the maximum number of crude extract concentration tested against bacterial strains. Ethanol was the best solvent for extraction, because it extracts active compounds that exhibit maximum antimicrobial activities. Methanolic extract also showed better results. It is confirmed through this research work that all the solvents used for extraction of bio-active compounds for biological activities of D. viride, were best. Our results showed that there was a consistent behavior of antibacterial and anti-fungal activities. In antibacterial activity MIC was found 5 mg/ml against 90% of the tested strains. Antifungal activity of each crude extract was above than 50%. Maximum antifungal activity was exhibited by the methanolic and ethanolic extract that was 77.1% and 76.8% against A. fumigates and A. niger respectively.
5 Conclusion
It is concluded that all extracted compound has potential to treat superficial fungal and bacterial infections, when used properly after modifying in crude drug. Actually, these finding justifies the use of D. viride in traditional healthcare system for the treatment of various ailments, whose symptoms might engage fungal and bacterial infections. This study also proves that ethno-medicinal approaches are useful for the discovery of new biological active agents to synthesize new drugs. Further, detail research is required to isolate the bio-active agents responsible for antibacterial and antifungal activity of D. viride.
Acknowledgement
The authors extend their appreciation to The Researchers Supporting Project number (RSP-2019/78) King Saud University, Riyadh, Saudi Arabia.
Conflict of Interest statement
The authors of the manuscript entitled “In-vitro antimicrobial activities of Dipcadiviride (L.) Moench” declared no conflict in this manuscript and publications.
References
- Diversity and use of ethno-medicinal plants in the region of Swat. J. Ethnobiol. Ethnomed. North Pakistan 2013
- [CrossRef] [Google Scholar]
- In vitro antibacterial, antifungal, antibiofilm, antioxidant, and anticancer properties of isosteviol isolated from endangered medicinal plant Pittosporum tetraspermum. Evidence-Based Complement. Altern. Med.. 2015;2015
- [Google Scholar]
- Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evidence-Based Complement. Altern. Med.. 2016;2016
- [Google Scholar]
- Controlling antimicrobial resistance in the hospital. Infect. Dis. Clin. North Am.. 2009;23(4):847-864.
- [Google Scholar]
- Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha Lam. in Wistar rats. South Ind. J. Biol. Sci.. 2015;1:34-37.
- [Google Scholar]
- Antioxidant activity of Dioscorea and Dehydroepiandrosterone (DHEA) in older humans. Pharmacol. Let. Acc. Comm.. 1996;59(11):147-157.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Ind. J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci.. 2015;1:47-51.
- [Google Scholar]
- Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Compl. Altern. Med.. 2016;16:264.
- [Google Scholar]
- Medically important carotenoids from Momordica charantia and their gene expressions in different organs. Saudi J. Biol. Sci.. 2017;24:1913-1919.
- [Google Scholar]
- Cocos nucifera coir-mediated green synthesis of Pd NPs and its investigation against larvae and agricultural pest. Artificial Cells. Nanomed. Biotechnol. 2017
- [Google Scholar]
- Coir mediated instant synthesis of Ni-Pd nanoparticles and its significance over larvicidal, pesticidal and ovicidal activities. J. Mol. Liquids. 2016;223:1249-1255.
- [Google Scholar]
- Spectroscopic investigation of biosynthesized nickel nanoparticles and its larvicidal, pesticidal activities. J. Photochem. Photobiol. B: Biol.. 2016;162:162-167.
- [Google Scholar]
- Biological activities of Rumexdentatus L.: Evaluation of methanol and hexane extracts. Afr. J. Biotech.. 2009;8(24):6945-6951.
- [Google Scholar]
- Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B: Biol.. 2016;162:395-401.
- [Google Scholar]
- Some biological activities of Epaltes divaricata L. - an in vitro study. Ann. Clin. Microbiol. Antimicrob.. 2015;2015(14):18.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol. B: Biol.. 2019;193:118-130.
- [Google Scholar]
- Green chemical approach towards the synthesis of SnO2 NPs in argument with photocatalytic degradation of diazo dye and its kinetic studies. J. Photochem. Photobiol. B: Biol.. 2016;162:441-447.
- [Google Scholar]
- Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results Phys.. 2016;6:712-718.
- [Google Scholar]
- Ferulic acid in Lolium multiflorum inhibits adipogenesis in 3T3-L1 cells and reduced high-fat-diet-induced obesity in Swiss albino mice via regulating p38MAPK and p44/42 signal pathways. J. Funct. Foods. 2017;37:293-302.
- [Google Scholar]
- In vitro regeneration of a rare antidiabetic plant Epaltes divaricata L. South Ind. J. Biol. Sci.. 2015;1:52-59.
- [Google Scholar]
- Antimicrobial and cytotoxic activities of Ceratoniasiliqua L. extracts. Turk J. Biol.. 2001;26:197-200.
- [Google Scholar]
- Comparison of anthranilate synthase activity and lAA content in normal and auxin-habituated Dioscorea viride tissue cultures. Biochem. Physiol. Pflanzen. 1981;176(3):244-250.
- [Google Scholar]
- Ethnomedicinal survey of plants from District Sialkot, Pakistan. J. App. Pharm.. 2011;02(03):212-220.
- [Google Scholar]
- Indigenous knowledge of medicinal plants from Leepa valley, Azad Jammu and Kashmir, Pakistan. J. Ethnopharmacol.. 2012;143:338-346.
- [Google Scholar]
- Ethno medicinal survey of plants from district Bhimber Azad Jammu and Kashmir, Pakistan. JMPR. 2011;5(11):2348-2360.
- [Google Scholar]
- Indigenous knowledge of medicinal plants from Gujranwala district, Pakistan. J. Ethnopharmacol.. 2013;148:714-723.
- [Google Scholar]
- Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus) Molecules. 2016;21:157.
- [Google Scholar]
- Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus. 2016;5:1628.
- [Google Scholar]
- Accumulation of carotenoids and metabolic profiling in different cultivars of Tagetes flowers. Molecules. 2017;22:313.
- [Google Scholar]
- Antibacterial activity of medicinal plant extracts. Braz. J. Microbiol. 2007;38:717-719.
- [Google Scholar]
- Kaviyarasu K. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill andevaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B: Biol. 2019
- [Google Scholar]
- Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci.. 2015;1:60-65.
- [Google Scholar]
- Vegetable peel waste for the production of ZnO nanoparticles and its toxicological efficiency, antifungal, hemolytic, and antibacterial activities. Nanoscale Res. Lett.. 2016;11:546.
- [Google Scholar]
- RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol., B: Biol.. 2016;162:550-557.
- [Google Scholar]
- Phenolic compounds in drumstick peel for the evaluation of antibacterial, hemolytic and photocatalytic activities. J. Photochem. Photobiol. B: Biol.. 2016;161:463-471.
- [Google Scholar]
- Antiparasitic properties of medicinal plants and other natural occurring products. Adv. Parasitol.. 2001;50:199-295.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B: Biol.. 2019;191:65-74.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol., B: Biol. 2019
- [Google Scholar]







