Translate this page into:
Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In these years a variety of polymeric nanocarriers such as dendrimers, polymeric micelles, nanoparticles, nanogels, nanocapsules and vesicles are widely investigated as potential drug delivery systems. In addition to the different morphologies and sizes, these carriers may have on their surfaces specific functionalizations to improve the drug loading and controlled release and specific ligands for cell receptors, in order to achieve a precise targeting. This review focuses on recent functionalized polymeric nanomaterials used as drug delivery systems, with an emphasis on morphology and surface modifications of polymeric nanocarriers to improve controlled drug delivery. Moreover, this work offers a number of suggestions on how to achieve the systematization of data on the most relevant physico-chemical parameters, which govern and control the interaction between carrier and drug, with the aim to give the reader an overview of the most significant advances in this field.
Keywords
Nanostructured polymers
Drug delivery systems
Dendrimers
polymeric micelles
Polymeric nanoparticles
Nanogels
Polymeric nanocapsules
Vesicles
1 Introduction
In the last decade the fabrication of nanostructures has allowed to obtain materials, organic, inorganic and composites, with better performance in huge range of different applications, such as sensors (Scognamiglio, 2013; Fratoddi et al., 2016; Potyrailo et al., 2011; Prosposito et al., 2016), optoelectronics (Zhao et al., 2010; Venditti et al., 2010a,b; Venditti, 2017; Beshkar et al., 2017a,b), catalysis (Safardoust-Hojaghan et al., 2017a,b; Valian et al., 2017) energy (Zhang et al., 2013; Persano et al., 2015; Venditti et al., 2014; Shi et al., 2015), biotechnology (Safardoust-Hojaghan et al., 2017a,b; Beshkar et al., 2017a,b; Fratoddi et al., 2012a,b), and medicine (Ho et al., 2015; Venkataraman et al., 2011; Fratoddi et al., 2015; Sangsefidi et al., 2017). In particular polymers can be used in the fabrication of several nanostructures, such as polymeric micelles, dendrimers, nanopartilces, nanogels, nanocapsules and vesicles, that are widely used as drug delivery systems. These nanostructures show the properties of the carries and often chosen polymers respond to a stimulus or their environment, such as changes in temperature, pH, light, redox potential, inducing dynamic and reversible changes useful to release the drugs (Sagadevan and Periasamy, 2014; Hruby et al., 2015; Rossi et al., 2016). Moreover, many scientific researchers are moving towards the realization of efficient vectors from the point of view of load and controlled release and, at the same time, aimed to a specific action site (Chithrani et al., 2006; Jiang et al., 2008; Bessar et al., 2016; Porcaro et al., 2016).
This review seeks to give a wide view of the recent developments in drug delivery systems based on nanostructured polymers, in particular on dendrimers, micelles, nanoparticles, nanogels, nanocapsules, vesicles, stressing that the surface chemistry and the introduction of specific functionalization likely to be crucial in drug delivery applications. Let us now see specifically the advantages and limitations of the various nanostructured polymeric systems.
2 Polymeric nanocarries
Most polymeric materials have been adopted as a preferred method for drug delivery because they show a good potential for surface modification via chemical transformations, provide excellent pharmacokinetic control, and are suitable for the entrapment and delivery of a wide range of therapeutic agents (Couvreur, 2013; Jia et al., 2013). In particular, several morphologies, including dendrimers (Newkome and Shreiner, 2008; Kim et al., 2007; Murugan et al., 2014; Cong et al., 2016), micelles (Hong et al., 2017; Cho et al., 2012; Otsuka et al., 2003; Matsuno and Ishihara, 2011; Feng et al., 2006; Zhang et al., 2009; Yang et al., 2009), polymeric nanoparticles (Fratoddi et al., 2012a,b; Laganà et al., 2011; Kumari et al., 2010; Fratoddi et al., 2011) nanogels (Joung et al., 2013; Koehler et al., 2013), nanocapsules (El-Gogary et al., 2014; Chen et al., 2014; De Koker et al., 2012) and vesicles (Carafa et al., 2010; Coviello et al., 2015) schematized in Fig. 1, are used for their easy surface modulation in terms of charges and functionalities.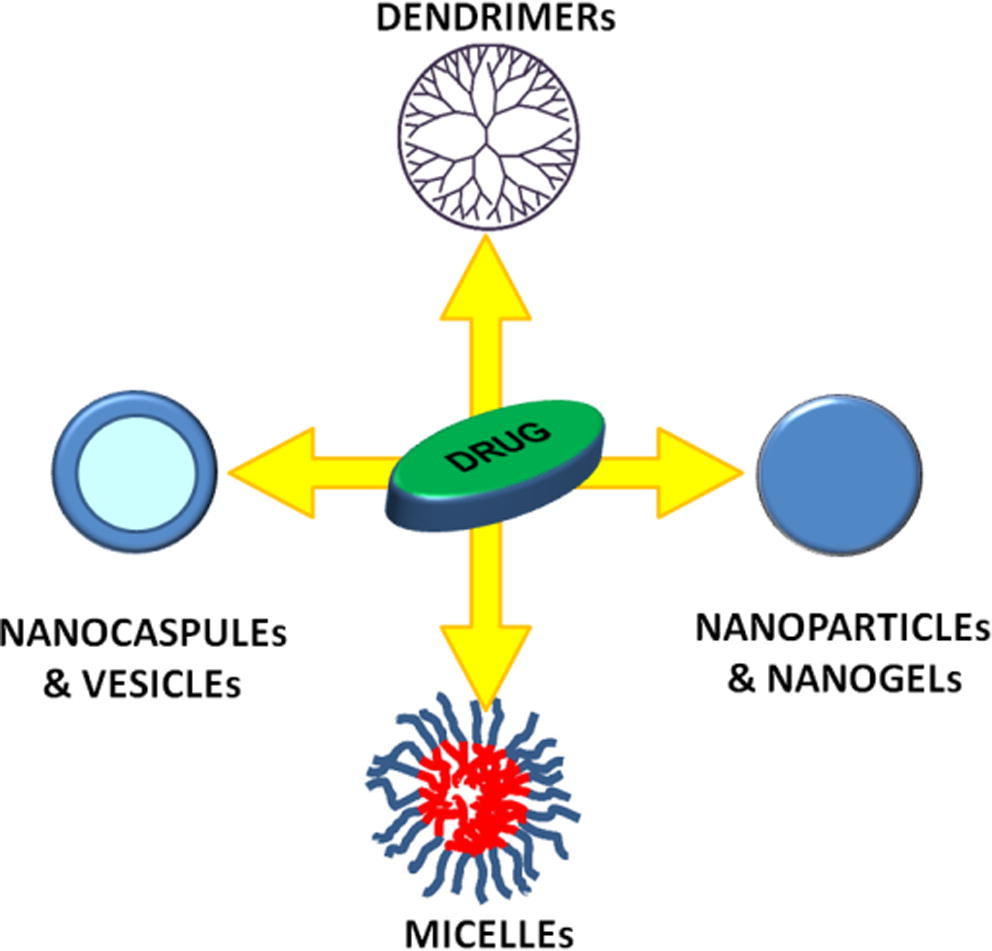
Schematic structures of various representative polymeric drug delivery systems.
In fact, several properties can be achieved by opportune choice of superficial functionalization of polymeric nanomaterials, such as loading optimization, best bioavailability and controlled-targeted release to specific site. For this reason, research is focused on the introduction of specific surface functionalization, often amine and acid, on the nanostructured drug delivery systems.
In Table 1 common functionalities were reported with several morphologies that highlight the importance of surface chemistry in nanomaterials for biomedical application.
Monomer
Polymer Acronym
Functionality
Morphology
References
Amidoamine
PAMAM
-RCONR1-NH2
Dendrimers
Newkome and Shreiner (2008)
Propyleneimine
PPI
-[N-(CH2)3-]nNH2
Dendrimers
Newkome and Shreiner (2008), Kim et al. (2007), Murugan et al. (2014), Cong et al. (2016)
Ethylenglycol
PEG
-OH
Micelles
Hong et al. (2017), Cho et al. (2012), Otsuka et al. (2003)
2-methacryloyloxyethyl phosphorylcholine
pMPC
-PO3−-CH2-N+R3
Micelles
Matsuno and Ishihara (2011), Feng et al. (2006)
Carboxybetainemethacrylate
pCBMA
RCOOR1
Micelles
Zhang et al. (2009), Yang et al. (2009)
Methylmethacrylate
PMMA
-COOMe
Spherical NPs
Fratoddi et al. (2012a,b), Laganà et al. (2011)
Lactic acid
PLA
-COOR
Spherical NPs
Kumari et al. (2010)
Dimethyl propargyl amine
PDMPA
-N(Me)2
Core-shell NPs
Fratoddi et al. (2011)
Heparin-Pluronic nanogel
Hep-Pr
-SO3- - COOH
Nanogel
Joung et al. (2013)
Ethylenglycol
PEG
-OH
Nanogel
Koehler et al. (2013)
Lactide-co-glycolide
PLGA
-OH
Capsules
El-Gogary et al. (2014), Chen et al. (2014)
Styrenesulfonate- allylamine
PSS-PAH
SO3− -CH2=CH-CH2-NH2
Capsules
De Koker et al. (2012)
Alginate-N-isopropylacrylamide-N,N′-dimethylacrylamide
Alg-PNIPA- PDMAA
-CONHCHMe2
-CONMe2
Multilayer capsules
Zarket and Raghavan (2017)
Liposomes
Vesicles
Carafa et al. (2010)
Niosomes
Vesicles
Coviello et al. (2015)
In fact, the polymer matrix prevents drug degradation and may provide management of drug release from these soft nanoparticles. Varying the drug-to-polymer ratio and molecular weight and composition of the polymer can modify the extent and level of drug release (Prabha and Labhasetwar, 2004). Surface properties of these materials are also the main component of their targeting characteristics: when the cellular membranes come into direct contact with soft nanoparticles surface and their properties, this can determine the mechanism of internalization and intracellular localization: the polymeric surface can be conjugated with peptides, aptamers, or antibodies to enable specific targeting (Ernsting et al., 2012; Gan and Feng, 2010; Kim et al., 2012).
2.1 Dendrimers
Dendrimers are highly branched nanostructures with an inner core. The drugs are incorporated both in the interior core both attached on the branched surface, covalently or by electrostatic mode, as schematically reported in Fig. 2.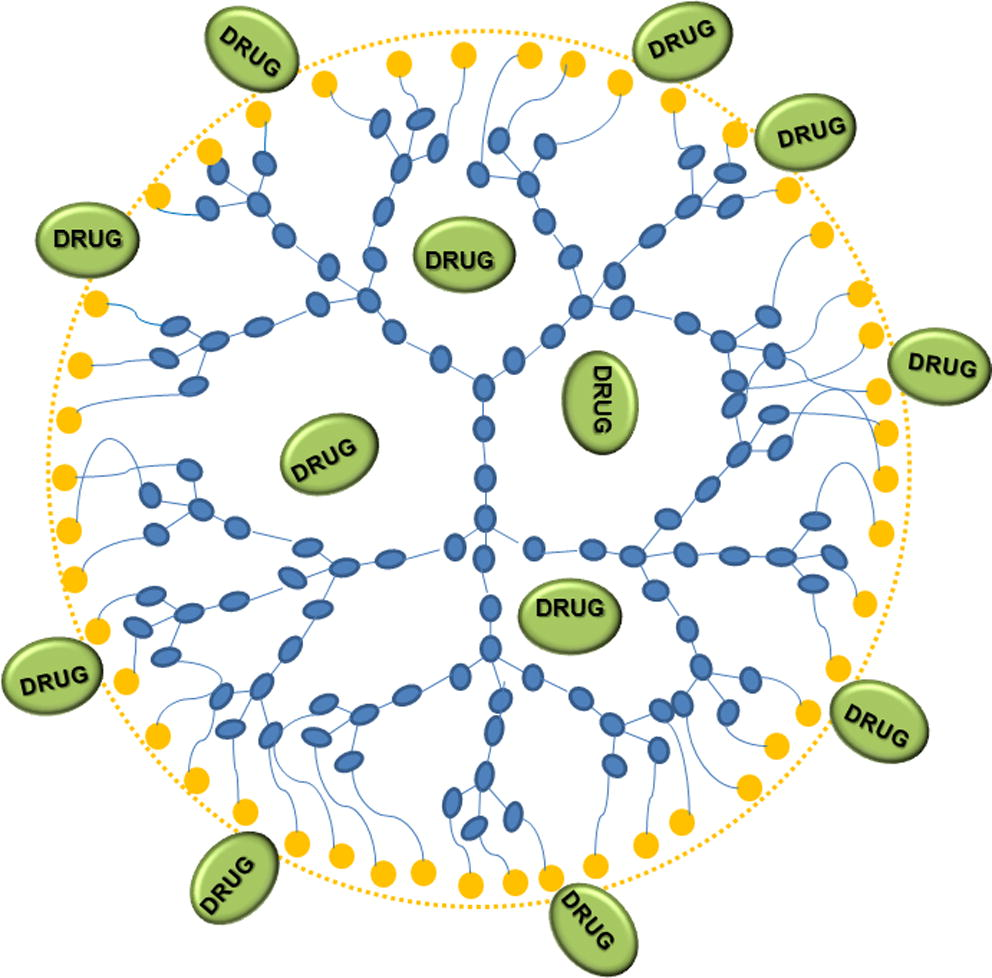
Scheme of drug loading in dendrimeric structure.
In general the most used macromolecules in this field are produced from macromolecules such as polyamidoamine (PAMAM), polypropyleneimine and polyaryl ether (Zhao et al., 2009; Eichman et al., 2001; Newkome and Shreiner, 2008; Kim et al., 2007; Murugan et al., 2014; Ponnapati et al., 2011). The particle size range is between 1 and 100 nm although, in general, their sizes are mostly less than 10 nm (Tomalia et al., 2012; Sadekar and Ghandehari, 2012). The uniqueness of dendrimers is based on their series of branches, multivalency, well defined molecular weight and globular structure with controlled surface functionality, which enhances their potential as carriers for drug delivery (Imae, 2012; Gupta et al., 2006). Due to their versatility, both hydrophilic and hydrophobic drugs can be incorporated into dendrimers (Imae, 2012; Wolinsky and Grinstaff, 2008; Nowacek and Gendelman, 2009). Their advantages, which include increased half-life, increased solubility, stability, and permeability of drugs, the capability to deliver a variety of drugs, reduced macrophage uptake, targeting ability, facile passage across biological barriers, rapid cellular entry, improved de-livery efficiency, and reduced side effects by targeted delivery (Nowacek and Gendelman, 2009; Najlah et al., 2007; Najlah and D’Emanuele, 2006;Wong et al., 2012; Menjoge et al., 2010). However, dendrimers suffer from several limitations such as poor/unstable hydrophobic drug loadings, inefficient release of drug at targeting (Bugno et al., 2015). New class of molecules called dendronized polymers, which are linear polymers that bear dendrons at each repeat unit, are recent development to answer at this problem (Tomalia et al., 2012). Another approach is to use dendrimers incorporating a degradable link that can be further used to control the release of the drug. For example Chang et al. prepare drug release system based on folic acid (FA) conjugated to poly(ethylene glycol) (PEG)-modified dendrimers (PAMAM) with doxorubicin (DOX) and superparamagnetic iron oxide (Fe3O4) (FA-PEG-PAMAM-DOX@IONPs) (Chang et al., 2012). This conjugates have pH-responsive drug release systems, which enabled pH-controlled activation of DOX in buffers that model the environment within endosomes/lysosomes of tumor cells. Further limitation of dendrimers is their toxicity due to their size and to the existence of positively charged surface functionalities, in case of cationic dendrimers, in particular amino groups. In fact, their characteristic size and functionalizations guide to non-specific interaction with biological entities, such as mitochondria, enzymes and cell membrane. Recent research has focused on the biocompatibility improvement of dendrimers: surface engineering masks the cationic charge of dendrimer surface either by neutralization of charge, for example PEGylation, acetylation, carbohydrate, peptide conjugation and complexation with DNA (Jain et al., 2010; Janaszewska et al., 2013; Caminade et al., 2015a,b; Kesharwani et al., 2015). Although the good outlook, dendrimers based drug delivery systems application in therapies with defined dosage regimen is still not acceptable, above all due to the difficulty of synthesizing the desired systems in large quantities at clinical grade purity, for clinical trials (Yeo, 2013). Recently, Cong et al. focused the study upon the bioconjugation of glycyrrhetinic acid (GA) onto PPI dendrimers to enhance their liver cell targeting capacity and minimize cytotoxicity: one step synthesis of GA-PPI dendrimers was developed through introduction of GA to the backbone of the PPI dendrimer by EDC chemistry with fine tuning substitution, that influenced particle size and zeta potentials and consequently the DNA binding and protection capability of GA-PPI carriers, as reported in Fig. 3 (Cong et al., 2016).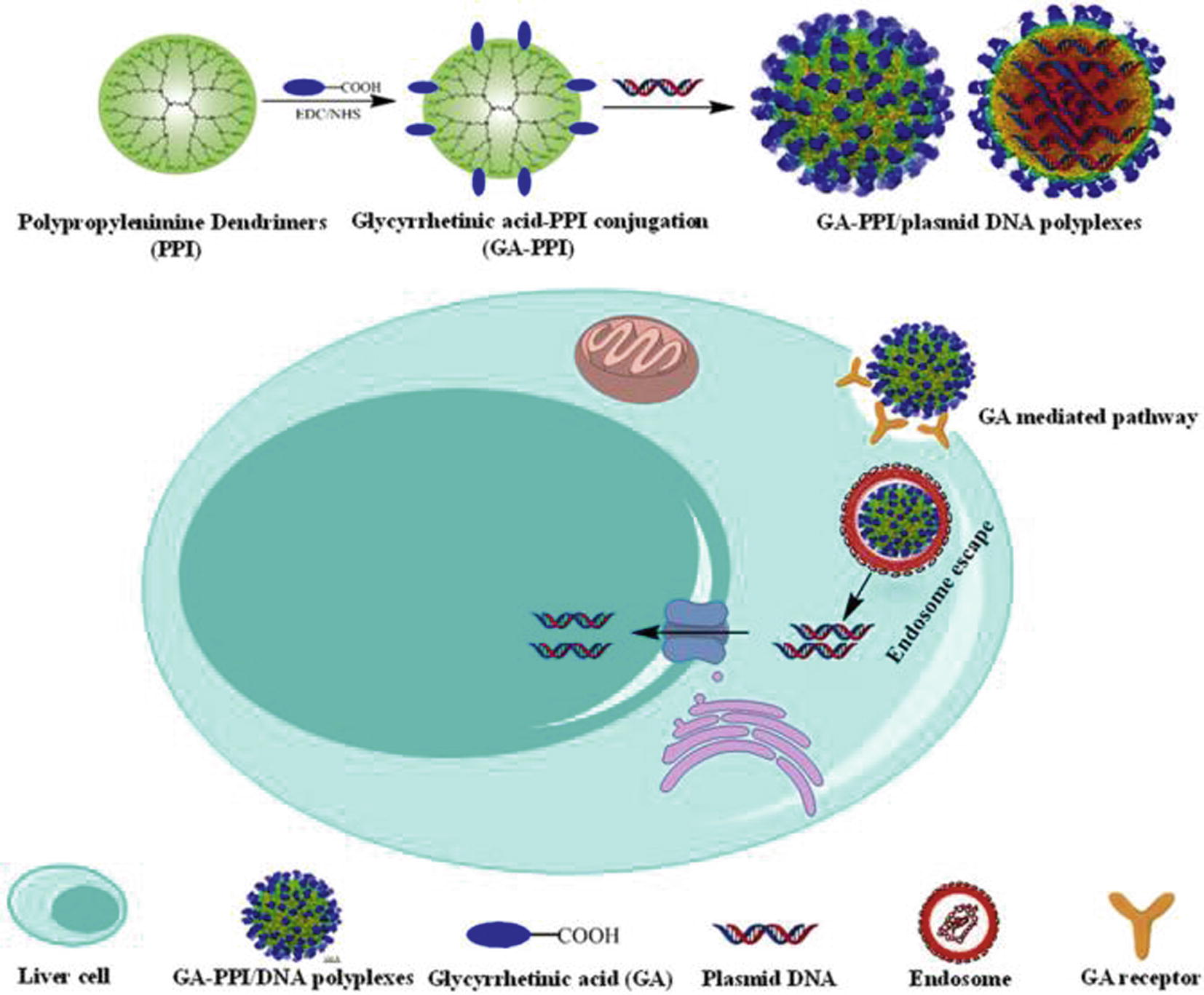
Schematic illustration for the targeted gene delivery of GA equipped PPI dendrimers (GA-PPI) (Cong et al., 2016).
In this work it is demonstrated how to develop high-performance gene carriers based on PPI dendrimers via one step conjugation. Moreover authors the authors show how it is necessary to test in vitro and in vivo systems, thus opening the prospect of subsequent clinical trials.
2.2 Micelles
Amphiphilic polymeric molecules, associated in aqueous medium to form core-shell structures or vesicles, form polymeric micelles (see Fig. 4). Hydrophobic drugs or contrast agents can be encapsulated in the core of the polymeric micelle (Liang et al., 2006) and its multifunctionality should lead to more developments regarding biomedical applications.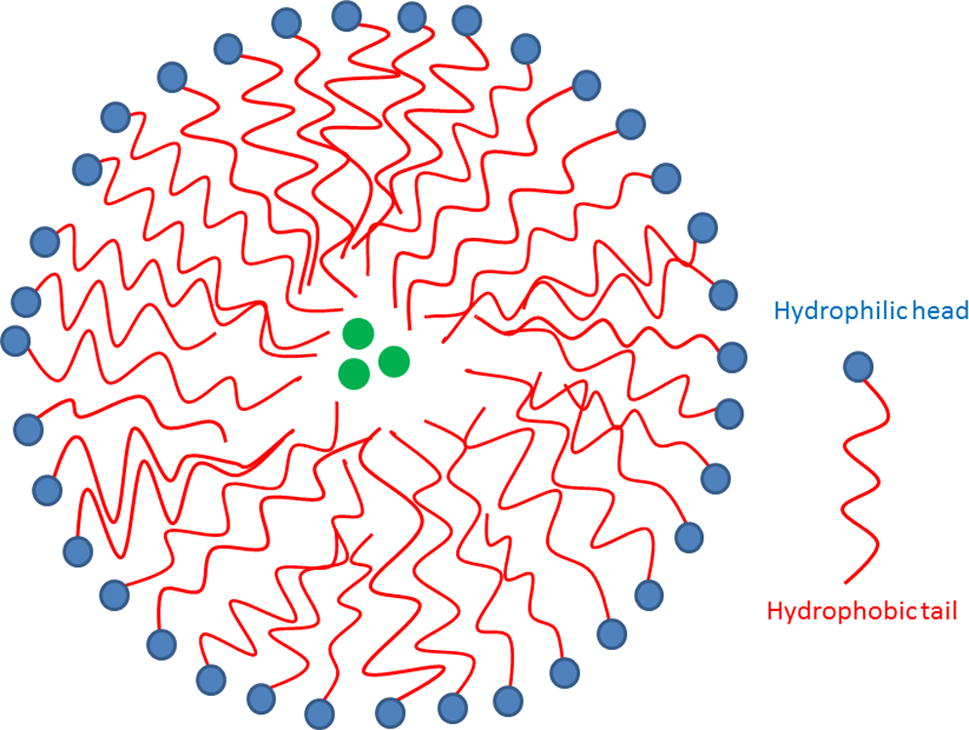
Scheme of drug loaded micelle.
In general polymeric micelles are formed from amphiphilic block copolymers and are more stable than surfactant micelles in physiological solutions: the inner core of a micelle is hydrophobic while the surface corona is hydrophilic as a result of conjugating with commonly utilized polymers such as polyethylene glycol (PEG) and oligo(ethylene glycol) (OEG) (Hong et al., 2017; Cho et al., 2012; Almgren et al., 1995). The hydrophilic shell and size (<100 nm) of polymeric micelle naturally gives a surface-smoothing effect, reducing its interaction with serum proteins and prolonging their circulation time in the blood (Jiang et al., 2011; Lai et al., 2012). Lipid moieties, such as cholesterol and fatty acyl carnitines, can also be employed to impart good stability to the polymeric micelles. Polymeric micelles have been extensively used for passive targeting, i.e. by exploiting the enhanced permeability and retention (EPR) effect of tumor tissues (Bae and Kataoka, 2009; Tyrrell et al., 2010). However, one of the significant disadvantages of normal self-assembled polymeric micelles is that micelles are not stable and they may dissociate upon dilution. Besides, sometimes, the targeting ability of polymeric micelles is limited due to low drug loading and low drug incorporation stability which cause the drug release before getting to the action site (Yamamoto et al., 2007; Seow et al., 2007). Cross-linking approaches have been shown to be an effective way to improve the stability of micelles (Read and Armes, 2007; Li et al., 2006), but most techniques resulted in overly stable micelles, which are not desirable due to the extremely slow drug release after the micelles arrive at the target sites. In this sense, the application of degradable linkages for cross-linking would facilitate the drug release. Stimuli responsive polymeric micelles are very promising because they can achieve sudden drug release with environmental stimulus such as temperature (Fujimori et al., 2005), pH (Chan et al., 2008), light (Patnaik et al., 2007; Dai et al., 2011), and redox (Song et al., 2011; Li et al., 2011)., With the investigation on the mechanism of nonfouling materials, polyzwitterionic materials, such as poly(2-methacryloyloxyethyl phosphorylcholine) (pMPC) (Matsuno and Ishihara, 2011; Feng et al., 2006), poly(sulfobetaine methacrylate) (pSBMA) (Chien et al., 2013), poly(carboxybetaine methacrylate) (pCBMA) (Zhang et al., 2009; Yang et al., 2009), and simply mixed-charge materials (Tah and Bernards, 2012; Li et al., 2013) have been recognized as effective nonfouling materials which can maintain the stability of micelles in complex media such as serum. Therefore, polyzwitterionic materials might be good alternatives of PEGs for excellent stability in blood.
A promising approach to reverse multidrug resistance (MDR) is intracellular co-delivery of different MDR-modulating agents. Hong et al. report the synergistic MDR reversal effect induced by curcumin and the Pluronic L61 unimers that was evacuate using a system designed for intracellular co-delivery with pH-sensitive micelles: a micellar delivery system, including a copolymer of PHis-PLA-PEG-PLA-PHis and Pluronic F127, was partially conjugated with folate (see Fig. 5). Folate is used to ensure intracellular co-delivery via endosomal pH-triggered drug release, copolymer facilitates endosomal escape and both the Pluronic L61 unimers and curcumin were selectively accumulated in the mitochondria (Hong et al., 2017).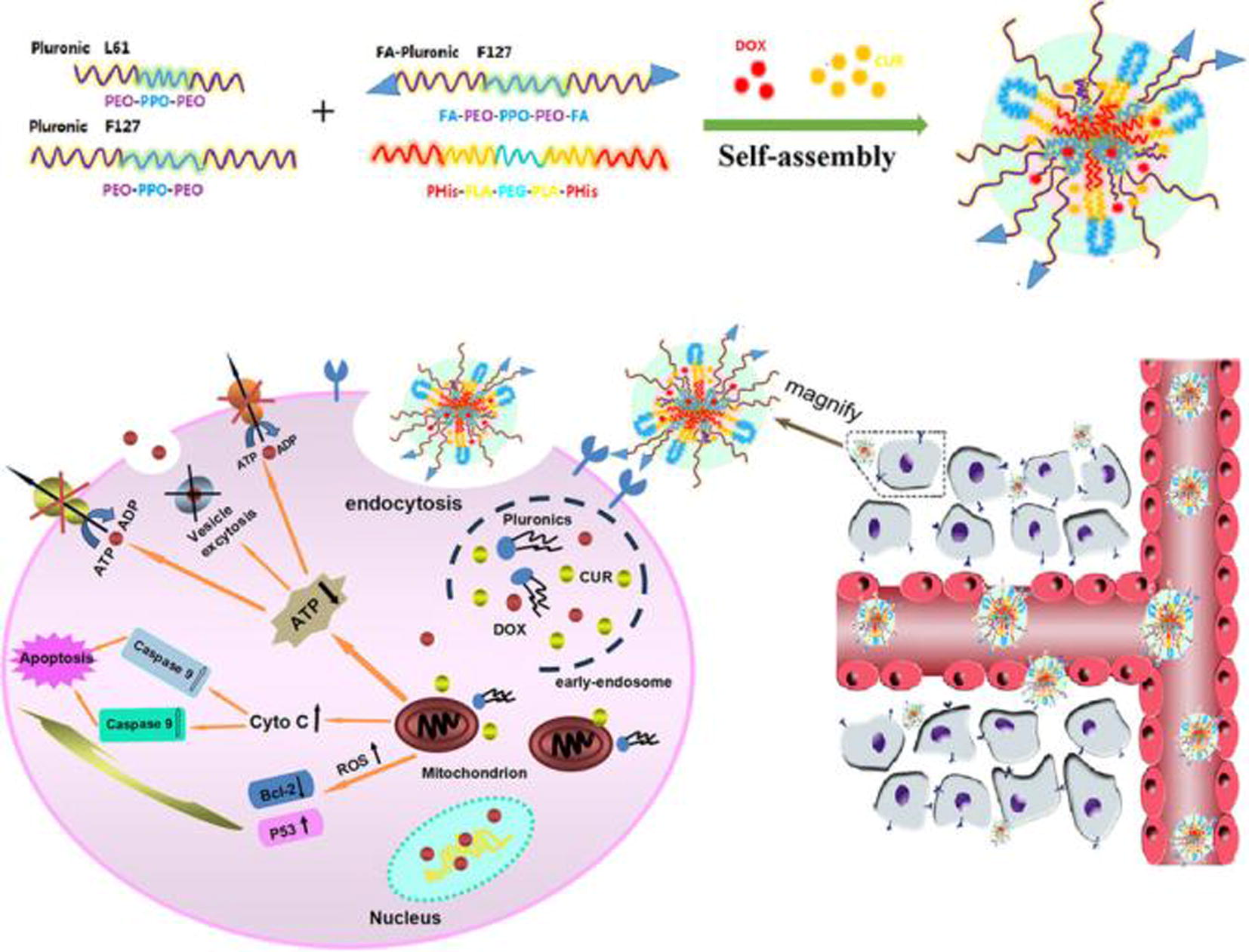
Scheme of the design and proposed mechanism of F-pHSM-L61/CUR/DOX (Hong et al., 2017).
2.3 Polymeric nanoparticles and nanogels
Nanoparticles are known as hard/inorganic nanoparticles, referring those particles made by inorganic materials that keep their original shape and size, or soft/polymeric nanoparticles, made by organic materials that are subject to size and shape change in specific conditions of temperature, pH, pressure and ionic strength (Chen et al., 2016; Sangtani et al., 2017).
In particular the polymeric nanoparticles are colloidal soft particles with a size range of 10 to 1000 nm (Yih and Al-Fandi, 2006; D’Amato et al., 2006; De Angelis et al., 2014; Pantalei et al., 2007) and they can be spherical, branched or shell structures (Venditti et al., 2011; Bearzotti et al., 2008; Fratoddi et al., 2011). They are developed from non-biodegradable and biodegradable polymers (Venditti et al., 2015; Venditti et al., 2007; Chronopoulou et al., 2009; Kumari et al., 2010; Venditti et al., 2010a,b). Their small sizes enable them to penetrate and to be taken up by cells, thereby increasing the accumulation of drugs at target sites. Several method can be used to incorporate drug into polymeric nanoparticles such as dissolution, precipitation, adsorption or attachment, as schematically shown in Fig. 6 (Kumari et al., 2010; Tang et al., 2012; Reis et al., 2006).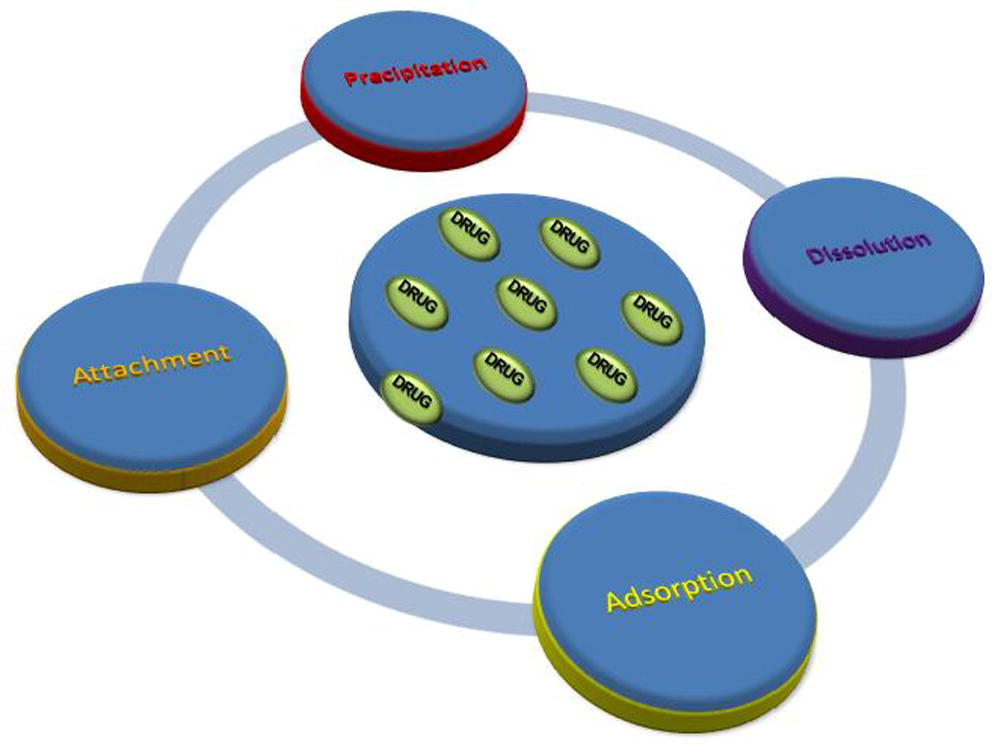
Scheme of methods for drug loading in polymeric nanoparticles and nanogels.
The polymeric nanoparticles can provide sustained release of the drugs for longer periods, e.g., days and weeks. (Arias et al., 2008; Fratoddi et al., 2012a,b) and they can enhance immunization by prevention of degradation of the vaccine and increased uptake by immune cells (Singh et al., 2006). To target drugs to site of action, the drug can be conjugated to a tissue or cell specific ligand or coupled to macromolecules that reach the target organs (Guicun et al., 2012; Laganà et al., 2011).
In general nanoparticles used for drug delivery have at least three components: the constituent material, the therapeutic molecules and the biological surface modifiers, which enhance the biodistribution and tumour targeting of the nanoparticles, as reported by M. Ferrari and schematically represented in Fig. 7 (Ferrari, 2005).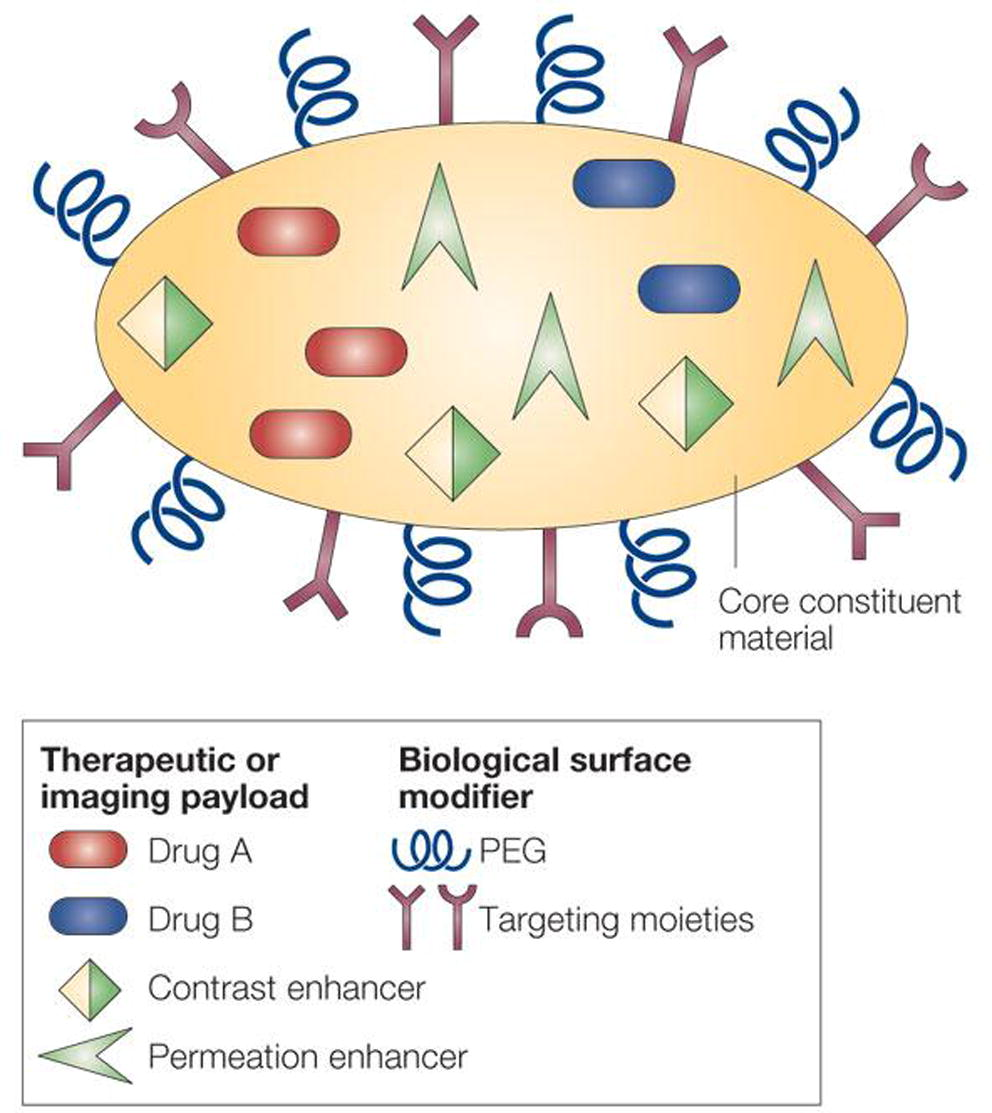
Scheme of multifunctional nanoparticles (Ferrari, 2005).
Some applications of polymeric nanoparticles include brain drug targeting for neurodegenerative disorders such as Alzheimer’s disease(Mittal et al., 2011; Masserini, 2013), topical administration to enhance penetration and distribution in and across the skin barrier (Alvarez-Román et al., 2004; Schneider et al., 2009) and pH-sensitive polymeric nanoparticles to improve oral bioavailability of drugs (Dai et al., 2004; Vijay and Wagh, 2014). Most of the polymers used in the fabrication of nanoparticles are biodegradable, such as chitosan, alginate, albumin, gelatin, polyacrylates, polycaprolactones, poly(d,l-lactide-co-glycolide) and poly (d,l-lactide) (Venditti et al., 2008; Venditti et al., 2011; Kumari et al., 2010). However, there are concerns about their scalability in biomedicine applications, due to some disadvantageous aspects such as: a) degradable polymers can exhibit substantial dose dumping at some point following implantations; b) “burst effect” or high initial drug release soon after administration is typical of most system; c) degradable systems which are administered by polymeric nanoparticles injection are non-retrievable (Phale et al. 2013; Shen and Burges, 2012). A new class of polymeric nanocarriers showing amazing properties are the nanogels (Jiang et al., 2014). These materials show excellent biocompatibility, a fine structure with modificable porosity being flexible and feasible platform for targeted drug delivery (Joung et al., 2013; Hu et al., 2015). In the past decade, various physical and chemical cross-linking strategies have been developed Nanogels can be to fabricated by two main ways, the physical approach and the chemical approach. The physical approach produces thermosensitive, stereocomplexed, and ionically crosslinked hydrogels, under particularly mild conditions, but these materials show poor long-term stability in tissues (Hoare and Kohane, 2008). On the other hand, chemical approach forms nanogels generally characterized by better stability, durability, and mechanical properties (Censi et al., 2010), but they can bring in potential toxicity concerns, such as the presence of copper catalyst in some cases. In recent years, a new strategy based on the click reactions is emerging to produce nanogels for biomedical applications. This approach allow to obtain materials with high coupling efficiency and specificity, bioorthogonality, compatible with live cells, proteins and therapeutics. Thiol-ene click reaction, DielseAlder and inverse electron demand Diels-Alder reaction, oxime reaction, and tetrazole-alkene photo-click reaction are used as click reaction to produces nanogels. As example, Diels Alder click reaction to produce hydrogels could be reversed at high temperature through the retro-DA reaction, which opens a way to controller drug release (Koehler et al., 2013).
2.4 Nanocapsules and vesicles
Nanocapsules are spherical hollow structures in which the drug is confined in the cavity and is surrounded by a polymer membrane (Landfester et al., 2010; Couvreur, 2013). Biodegradable polymers are used for preparing nanocapsules, which include both natural polymers and synthetic polymers. The main preparation methods are: nanoprecipitation, emulsion-diffusion, double emulsification, emulsion-coacervation, layer-by-layer assembly (Mora-Huertas et al., 2010) The selection of appropriate components for nanocapsule preparation is crucial for achieving a long term stability and biocompatibility of the functional cargo as well as its improved internalization by target tumor cells: the crucial requisite to construction of long-term container is formation of stable interfacial complex between an appropriate ionic surfactant and the first layer of oppositely charged PE. Bazylińska et al. report two types of multifunctional core-shell nanocarriers obtainable by self assembly approaches (Fig. 8) (Bazylińska, et al., 2016). The strategy applied for fabrication of the NaYF4:Tm3+,Yb3+ NPs-loaded nanocapsules via a two-step process is presented in Fig. 5: first evaporation method (Stage I), followed by layer-by-layer (LbL) saturation technique (Stage II). Furthermore, LbL assembly allows for engineering their shells on the nano-level, leading to the construction of biocompatible nanocontainers with desired biospecific properties including (i) improved biodistribution via pegylation process; (ii) tumor targeting via functionalization of the top PE layer with ligand of a specific cell receptor overexpressed on tumor cells; (iii) reduced immunogenicity via application of protein- or polysaccharide-based materials (del Mercato et al., 2014).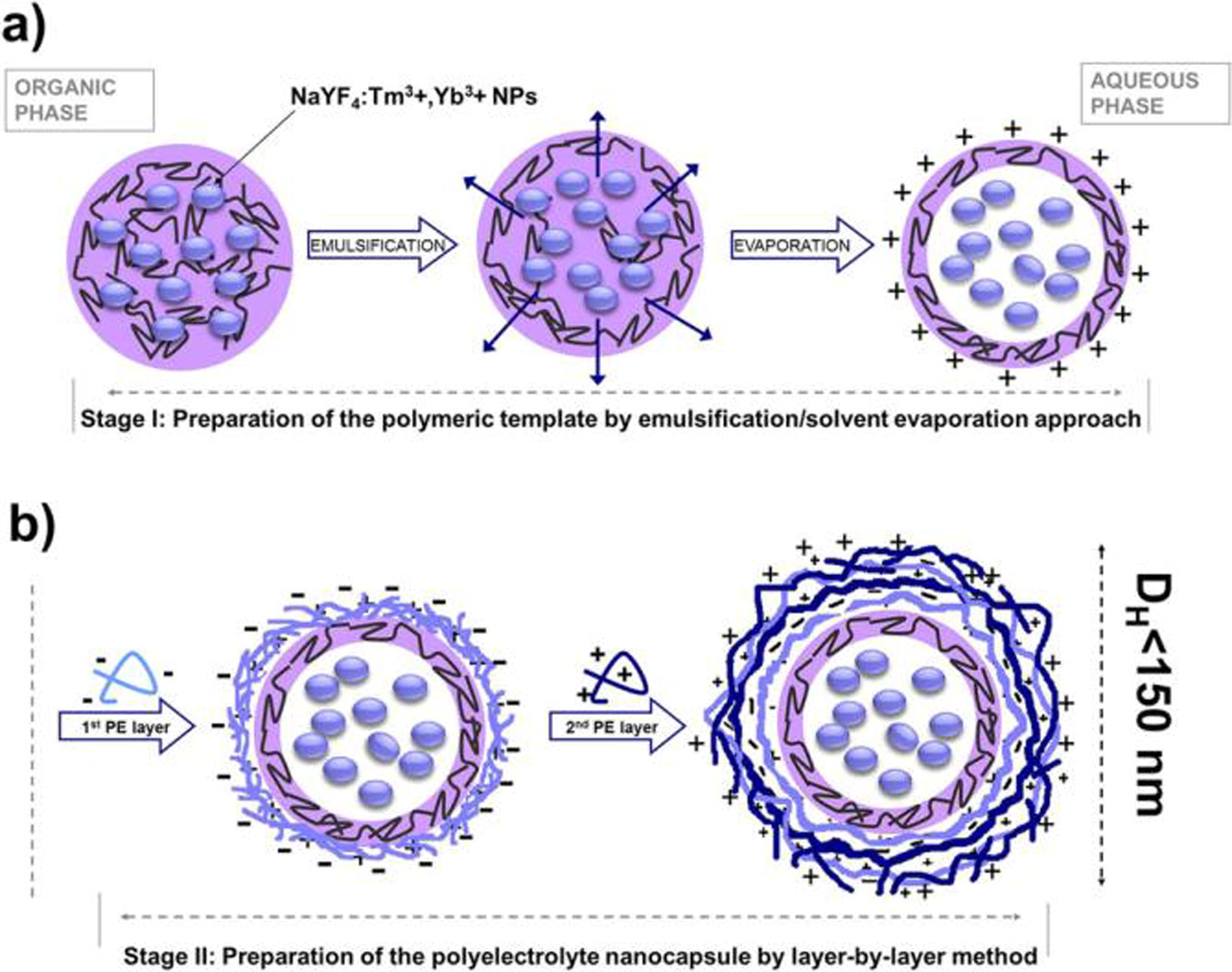
NaYF4:Tm3+,Yb3+NPs loaded oil-core polyelectrolyte nanocapsules preparation by two-steps: emulsification/solvent evaporation (a) and LbL (b) approach (Bazylińska, et al., 2016).
Nanocapsules use in drug delivery systems involves targeting drug delivery, controlled/sustained release drug delivery systems, transdermal drug delivery systems and improving stability and bioavailability of drugs (Rong et al., 2011; El-Gogary et al., 2014). Sizes between 50 and 400 nm are preferred for drug delivery and they can be employed as confined reaction vessels, protective shell for cells or enzymes, transfection vectors in gene therapy, dye dispersants, carriers in heterogeneous catalysis, imaging and drug carriers (Baier et al., 2012; Chen et al., 2014). Indiscriminate drug distribution and severe toxicity of systemic administration of chemotherapeutic agents can be overcome through encapsulation (MacDiarmid et al., 2007; Hervella et al., 2008). An interesting example of a new smart materials for drug delivery are the polymeric multilayer capsules (PMLCs), that are generated by sequential deposition of polymer layers from aqueous solutions onto a sacrificial template (De Koker et al., 2012; Peyratout and Dahne, 2004) or by a strategy involving successive free-radical polymerizations around an initial gel core (Zarket and Raghavan, 2017) (see Fig. 9). This latest approach allows to modulate both thickness and composition of each layer and in particular, the polymeric layers can be responsive to different stimuli, such as temperature and pH. PMLCs have attracted attention for drug-delivery applications because they are now being engineered to encapsulate various classes of drug molecules, by using polymers that are biodegradable (Sukhorukov et al., 2007; De Geest et al., 2009) and because they can respond and release their payload in response to well-defined stimuli following step-like profiles.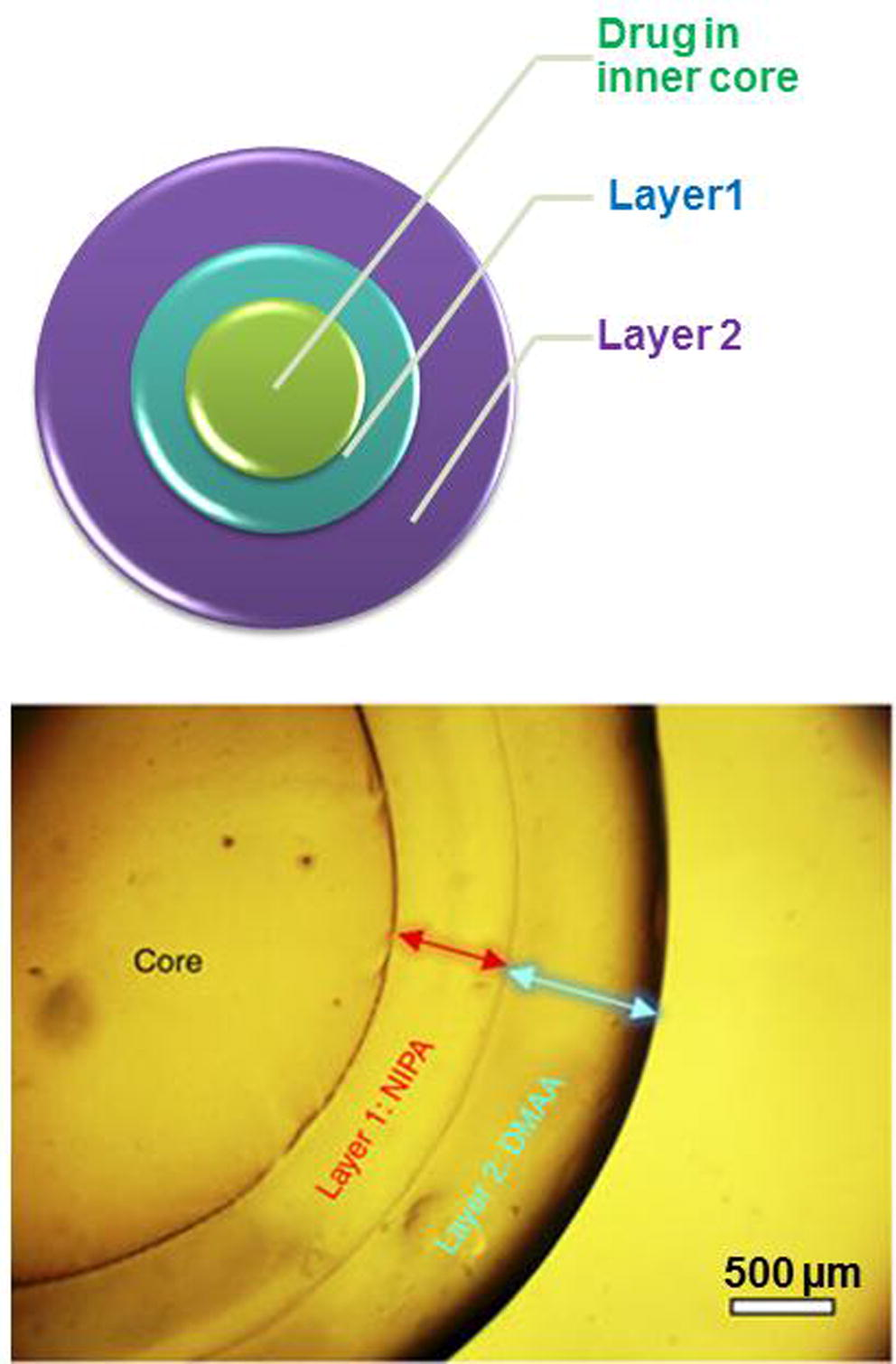
a) Scheme of drug loaded polymeric multilayer capsules (PLMCs); b) Example of real PLMCs: optical micrograph of a capsule with an alginate (Alg) core, a layer 1 of N-isopropylacrylamide (NIPA) and layer 2 of N,N′-dimethylacrylamide (DMAA). (Zarket and Raghavan, 2017).
The major benefit of PMLCs is their flexibility: they can be fabricated using various templates, with sizes varying from a few nanometers to hundreds of micrometers, and their chemical and mechanical properties can be precisely tailored by modulating the thickness and constitution of the shell (Parakhonskiy et al., 2014).
Among other Cyclodextrins (CDs) have been studied and widely used as pharmaceutical excipient for being capable to incorporate or adsorb the guest molecules into their central cavity. The arrangement of D-glucopyranose monomers in chair confirmation gives cyclodextrin a specific truncated cone shape structure with hydrophobic inner cavity and hydrophilic outer surface. The inner central cavity is lined by skeletal C H groups and ethereal oxygen of the glucose residue imparting lipophilic property. The hydroxyl functions of sugar moieties of CDs are oriented to the exterior of the cone where the secondary hydroxyl group are located at the wider edge and the primary ones are positioned on the narrow edges, which make the outside surface hydrophilic. Cavity is used for encapsulation of hydrophobic drug of suitable size. Recently, new CDs based nanomaterials were proposed, such as cyclodextrin nanosponges: drug is loaded into nanocavities of cyclodextrin by suspending nanosponges within drug dispersion followed by freeze drying with drug. Solvent evaporation is another technique to load the drug into nanosponges in which suitable organic solvent is used to dissolve drug. Nanosponges are added to this drug dispersion and triturated till the solvent gets evaporated (Gurusalkar et al., 2013). Moreover, to control drug release in response to exogenous or endogenous stimulations, stimuli sensitive nanosponges were developed. Other class of these materials are the molecularly imprinted nanoponges, in which the drug could be included in the cross-linked structure during the synthesis, thereby leading to an increased payload and much slower drug release (Swaminathan et al., 2016; Caldera et al., 2017).
In these years, vesicles are extensively studied as drug nanocarrier, due to their chemico-physico properties useful to obtain a multi-functional devices. In fact, these materials have amazing properties such as nanoscale size, high surface-to-volume ratio, and they have the potential to modulate both the pharmacokinetic and pharmacodynamic profiles of loaded drug (Marianecci et al., 2016). Among others liposomes and niosomes have attracted great attention (Tavano and Muzzalupo, 2016). Liposomes are nanovesicles that contain amphipathic phospholipids arranged in one or more concentric bilayers, which enclose an equal number of aqueous compartments. Niosomes are vesicles composed mainly of hydrated non-ionic surfactants in addition to, in many cases, cholesterol (CHOL) or its derivatives: this tructures make niosomes capable of encapsulating both hydrophilic and lipophilic substances. The main advantages of these nanomaterials are the ability to respond to external stimuli, prolonged blood circulation, the capability to penetrate across peptide channels inside the cell (Carafa, et al., 2010; Coviello et al., 2015).The drawback of liposomes and niosomes is a physical instability because during dispersion there is possibility of aggregation, fusion, drug leakage, or hydrolysis of encapsulated drugs (Moghassemi and Hadjizadeh, 2014). Moreover the challenge is open regarding the understanding of the mechanisms by which these nanocarrier reach the target site and exercise drug action at cellular level.
3 Critical properties of nanostructured polymers
Currently materials science is promoting the study and development of stimuli-responsive materials, not only in the construction of model systems to understand the response of biological materials to trigger, but also in designing and implementing new “smart” materials with stimuli-responsive structures and functionalities (Girard et al., 2007; Mitragotri and Lahann, 2009; O’Reilly et al., 2006; Moughton and O’Reilly, 2008). Polymer-based systems are promising in this field because it can be produced with a variety of chemical functionalities, post synthetically modified easily, in large scale and processed in different forms such as films, solutions, solids. In fact, external stimuli such as pH, temperature, redox potential, light and magnetic field, can induce variation of density, transparency and conductivity, volume (or degree of swelling), or solvent absorption capacity of the polymeric materials (Hirst et al., 2008; Esser-Kahn et al., 2011).
The preparation of polymeric nanostructured materials, with controlled dimensions, morphology and surface features, properties that directly affect the “smart” behavior, require specific methods of preparation. Synthesis of dendrimers include the use of Tomalia’s divergent growth approach, convergent growth approach, and orthogonal coupling strategy, while methods of preparing polymeric micelles include dialysis, solution-casting, direct dissolution (Gilles and Fréchet, 2005; Gaucher et al., 2005; Chen et al., 2018). The polymeric nanoparticles can be prepared by ionic gelation, coacervation, solvent evaporation, spontaneous emulsification/solvent diffusion, salting out/emulsification-diffusion, supercritical fluid technology and emulsion polymerization, and nanocapsules are produced by microemulsion, miniemulsion polymerization and interfacial polymerization (D’Amato et al., 2003; Vauthier and Bouchemal, 2009).
When a NP enters a biological environment its surface is rapidly covered by various biomolecules (typically proteins), leading to the formation of a ‘corona’: these adsorption of proteins alters the particle size, stability and surface properties and, more importantly, provides the NPs with a biological identity and NP–protein interactions are dependent on the NP physicochemical properties, exposure time as well as protein source and concentration (Fig. 10) (Shi et al., 2017). While ligand-functionalized NPs might lose targeting capability when a protein corona forms on their surface, decoration of NPs with some particular plasma proteins could improve delivery to specific organs. In contrast, NP–protein interactions in clinical settings can also trigger hypersensitivity reactions in patients by activating the complement system (Karczewski et al., 2012; Fytianos et al., 2016).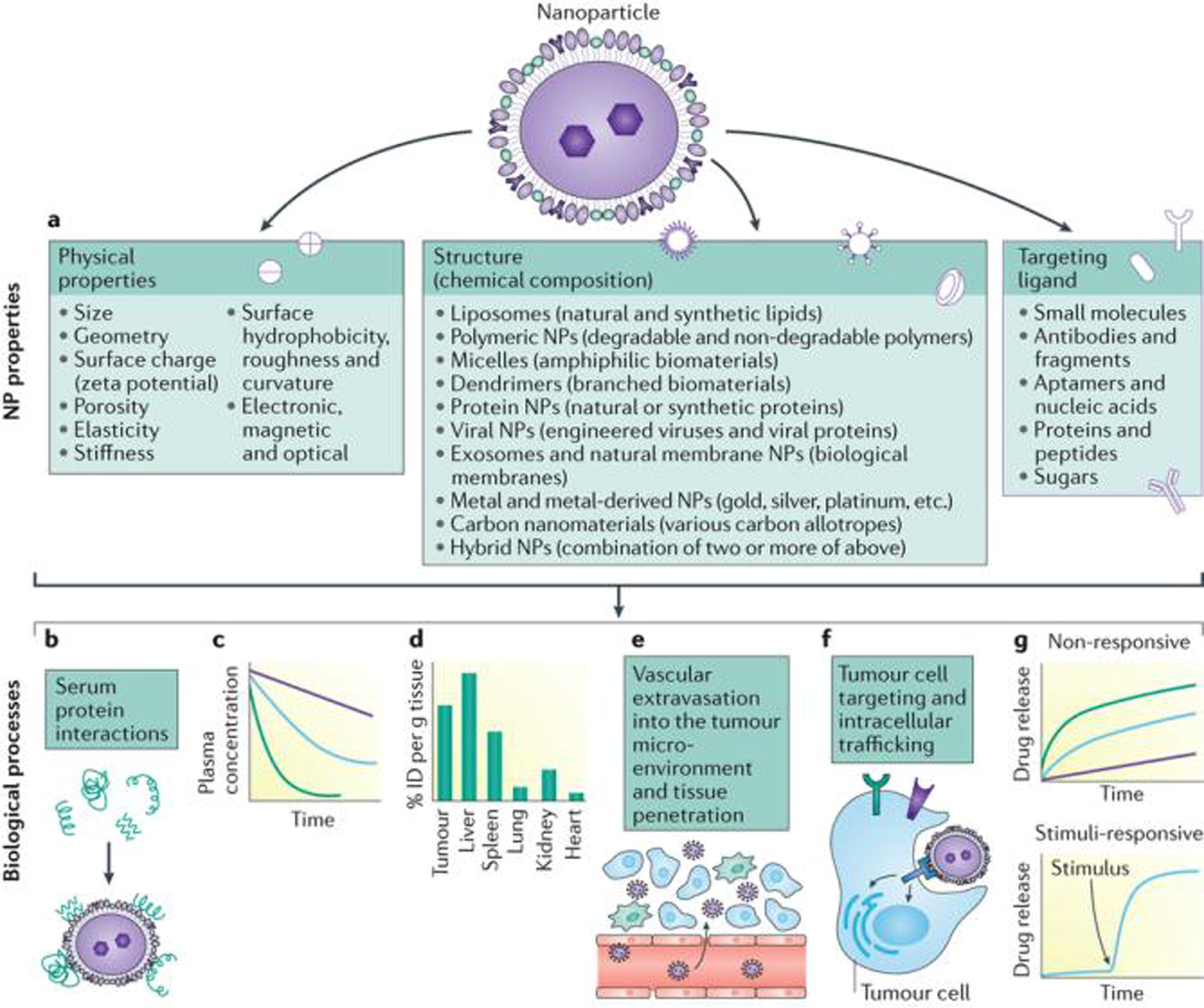
Nanoparticles (NPs) from different materials can have different physicochemical properties and can be modified with ligands of different surface density (part a). NP properties affect the biological processes involved in the delivery to tumour tissues, including interactions with serum proteins (part b), blood circulation (part c), biodistribution (part d), extravasation to perivascular tumour microenvironment through the leaky tumour vessels and penetration within the tumour tissue (part e), and tumour cell targeting and intracellular trafficking (part f). NPs can also be designed to control the release profile of payloads (part g). ID, injected dose (Shi et al., 2017) .
The size of the nanoparticle carrier, which also can be used as passive targeting mechanism, alters the biological distribution profile. The small nanomaterials, 1–20 nm, have long circulatory residence times and slower extravasation from the vasculature into interstitial spaces (Winter et al., 2003). Local injections require an engineering of polymeric nanoparticles of slightly larger sizes, 30–100 nm, sufficient to avoid leakage into capillaries, but also small enough to avoid reticulo endothelial clearance (Moghimi et al., 2001). Polymeric nanoparticles greater than approximately 100–150 nm in diameter will tend to accumulate in tumors due to their poor extravasation from normal vasculature (Kohane, 2007).
The presence of disturbed, porous vascular beds at the tumor allows for selective targeting by this passive mechanism. In general, the cancer drug delivery process can be divided into three steps, as reported by Sun et al. (2012)). A) Initially, the drug-loaded nanocarriers circulate in the blood compartments, including the liver and the spleen. When passing through tumor blood vessels, some carriers may fall into the pores in the blood vessel wall and diffuse into the tumor tissue (EPR effect) (Torchilin, 2000; Maeda et al., 2000). B) Next, they may further penetrate the tumor tissue, which is non-trivial because of the high cell density and high interstitial osmotic pressure (Wong et al., 2011). C) Upon sticking to the surrounding cancer cell membrane the carrier is expected to enter the cells via one or several possible pathways, and finally traverse the crowded intracellular structures and viscous cytosol to the targeted subcellular sites and release the carried drug cargo.
Thus, to achieve efficient drug delivery from the injection site to the target in the tumor cells, the nanocarrier must simultaneously meet two pairs of challenges: (a) the nanocarrier retain the drug very tightly, but it must be able to efficiently release the drug once reaching the intracellular target to exert its pharmaceutical action; (b) the nanocarrier must evade the reticulo endothelial system (RES) screening, particularly the capture by liver and spleen, for a long blood circulation time: with the blood circulation time of the nanocarrier increases so does its opportunity passing the hyperpermeable tumor blood vessel and extravasation into the tumor. Only a nanocarrier capable of simultaneously satisfying the opposite 2R2S requirements at the right places, that is, “drug Retention in blood circulation versus Release in tumor cells (2R)” and “Stealthy in blood versus Sticky in tumor (2S)” will deliver the drug specifically to the tumor, giving rise to high therapeutic efficacy and few side effects.
Other important aspects are the clearance and the excretion. In fact, following systemic administration, the body allocate nutrients, clears waste, and deliver drugs via the vascular and lymphatic systems. Intravenously injected particles are scavenged and cleared from circulation by the reticulo endothelial system with a process that involves the deposition of opsonic factors and complement proteins on the nanoparticles themselves (Singh et al., 2011). Both clearance and opsonization are influenced by the size and surface characteristics of injected nanoparticles: particles greater than 200 nm in diameter activate the complement system more efficiently and are cleared more rapidly than very small nanoparticles. This may be a result of the geometry, charge, and functional groups on the surface of these particles that mediate binding to proteins and blood opsonins (Emerich and Thanos, 2007).
4 Strategies for drug loading and release
The interaction between nanocarrier and drug molecules has attracted great interest during these years. Different interaction mechanisms have been explored, and they can be broadly subdivided into three types: electrostatic interaction, covalent conjugation, encapsulations.
Electrostatic Interaction. The high density of functional groups (such as amine groups and carboxyl groups) on nanocarrier surface have potential applications in enhancing the solubility of hydrophobic drugs by electrostatic interaction: nonsteroidal antiinflammatory drugs with carboxyl groups, including ibuprofen, ketoprofen, diflunisal, naproxen, and indomethacin, have been widely been complexed with dendrimers by electrostatic interactions (Imae, 2012; Gupta et al., 2006). Studies on many drug delivery systems based on electrostatic interaction between nanocarrier and other drugs, such as some anti-cancer drugs and anti-bacterial drugs, have also been reported. Often a common property of these drug molecules is that they are weakly acidic drugs with carboxyl groups in the molecules, such as for example, the well know drugs, aspirin, methotrexate and furosemide (Manallack et al., 2013).
Covalent Conjugation. The presence of large numbers of functional groups on carrier surface allows covalent conjugation with drugs using relevant functional groups (Chang et al., 2012). In this case, the drug is covalently bound to carrier, and its release occurs via chemical or enzymatic cleavage of hydrolytically labile bonds. Moreover covalent conjugation allows tissue targeting and controlled delivery as the drug–carrier conjugates diffuse slower than the free drug in the body and might be absorbed in specific interfaces (Alvarez-Román et al., 2004).
Encapsulation. The ellipsoidal or spheroidal shape, empty internal cavities, and open nature of the architecture of dendrimers and nanocapsules make it possible to directly encapsulate guest molecules into the macromolecule interior (Arpicco et al., 2015; Patil et al., 2016; El-Gogary et al., 2014; Tomalia et al., 2012; Rong et al., 2011). These empty internal cavities usually have hydrophobic properties, which make it suitable to interact with poorly-soluble drugs through hydrophobic interactions: in view of these specific properties, the relationship between the internal cavities of carrier and drug molecules may involve physical encapsulation, hydrophobic interaction, or hydrogen bonding.
The strategies for release involving the use of designed carriers to bond, encapsulate, or mask the therapeutic agent. The delivery of the drug to a tissue whereby penetration and distribution may not otherwise occur is possible with these ‘Trojan Horse’ strategies. In fact, during the course of evolution, cells have developed various mechanisms to prevent the entry of xenobiotics. Some of these mechanisms include the presence of a lipophilic cell membrane; existence of P-glycoproteins which efflux the drugs out; the occurrence of degradative enzymes and the development of endosomes which are highly acidic and these degrade xenobiotics which are endocytised into the cells. A succession of several membrane layers provides an obstacle for therapeutic agents attempting to target intracellular structures. During this process, the compound is lost due to ineffective partitioning across biological membranes. The extent of partition across a membrane is related directly to the polarity of a molecule; nonpolar or lipophilic molecules easily bypass this obstacle with greater membrane penetration, generally via diffusion. However, the situation is much more complicated, as a myriad of other cellular processes directly affect the intracellular concentrations and effectiveness of the therapeutic agent. In fact, as reported by Faraji and Wipf (2009) variable efficiencies of endocytosis mechanisms, intracellular trafficking, release of the therapeutic agent into the cytoplasm, diffusion and translocation of the therapeutic agent to its susceptible target, and partition into the nucleus or other organelles alter the actual activity of the therapeutic agent.
Polymeric nanoparticles present an interesting opportunity for eliminating much of this ‘waste’ due to masking of the therapeutic agent from its biological environment; this effectively limits the influence of a compound’s physical properties on intracellular drug concentrations. Instead, the properties and surface characteristics of the nanoparticle play a greater role in compound delivery and resulting intracellular drug concentrations. Nanoparticles may be ingested by endocytosis process that includes three subtypes: phagocytosis, pinocytosis, and receptor mediated endocytosis. Phagocytosis involves the ingestion of materials up to 10 μm in diameter and can be accomplished by few cell types of the reticulo endothelial system, such as macrophages, neutrophils, and dendritic cells. Pinocytosis is an uptake mechanism that can be conducted by virtually all cell types, and normally involves ingestion of sub-micron material and substances in solution. Larger microparticles provide selective access to phagocytic cells, while smaller nanoparticles provide access to virtually all cell types. This distinct capability of nanoparticles may be utilized for the delivery of therapeutic agents to a wide array of cellular types and targets.
Cross-linking of receptors by ligands attached to the polymeric nanoparticles results in a more pronounced crater leading to membrane enfolding and reunification of the cell membrane to form an endosome: the size of the nanoparticles between 25 and 50 nm is a requirement for optimal endocytosis and intracellular localization (Chithrani et al., 2006; Jiang et al., 2008). In addition, the selective active targeting of polymeric nanoparticles to specific tissues can take advantage of the differential receptor expression between cell types. For example, the attachment of multiple herceptin molecules on the surface of the nanoparticles induced higher cross-linking of the receptors over expressed on human breast cancer cells as ErbB2, with variable internalization depending on size of the nanoparticles (Jiang et al., 2008). Switchable polymeric nanoparticles can be classified based on the type of stimulus as internally and externally controllable materials. Internal stimuli (e.g. activation by pH, redox potential, enzymes) might be controlled by a molecular mechanism highly specific for a disease and therefore excel in targeting properties (Lee et al., 2008; Yoo et al., 2011). However, absolutely disease-specific internal molecular triggers are difficult to find for certain diseases. External stimuli like light, ultrasound, electromagnetic fields or ionizing radiation have the advantage of being focusable on certain body areas (Sun et al., 2011; Fomina et al., 2011). This may be a significant advantage where a target cell is strongly involved in pathogenesis at one location (e.g., cancer stem cells in a cancer tissue), but of vital importance in other locations (e.g., stem cells in the bone marrow). A schematic overview of a cancer cell, presenting internal (glutathione) and external stimuli (e.g. magnetic field, ultrasound, light, radiation) used for imaging, drug release and therapeutical treatment is reported by Lehner et al. (2012).
Other interesting results are presented by Li et al. (2014). They demonstrated that DOX-loaded, dextran-based reversible crosslinked micellar nanoparticles can efficiently deliver DOX into cancer cells in vitro, and reduce A549 xenograft tumor size in vivo. Importantly, in situ crosslinking of the DOX-loaded polysaccharide nanoparticles by introducing a small amount of cisplatin as the crosslinker, could significantly increase the surface charge and stability, which would further improve the tolerability, in vivo pharmacokinetics, biodistribution, and antitumor efficacy, and reduce drug-related multiorgan toxicity side-effect. This study demonstrated that pH responsive polysaccharide-based cisplatin crosslinked nanoparticles held great potential for achieving an optimal therapeutic effect of the transported drugs in cancer therapy.
Much effort has focused on NP-mediated selective drug delivery to the tumor vasculature (Fig. 11), which is crucial to tumor growth and metastasis (Shi et al., 2017). This is commonly achieved by coating NPs with ligands that bind specifically to overexpressed receptors such as αvβ3 integrin on the surface of tumor endothelial cells. Targeting stromal cells such as tumor-associated fibroblasts and macrophages has also been proposed for cancer treatment. Comparatively little effort has been devoted to exploiting nanotechnology to modify the premetastatic microenvironmental niche and suppress tumors growth. In a recent study, a bone-homing polymeric NP platform was engineered for spatiotemporally controlled delivery of therapeutic agents (Fig. 11b) (Shi et al., 2017).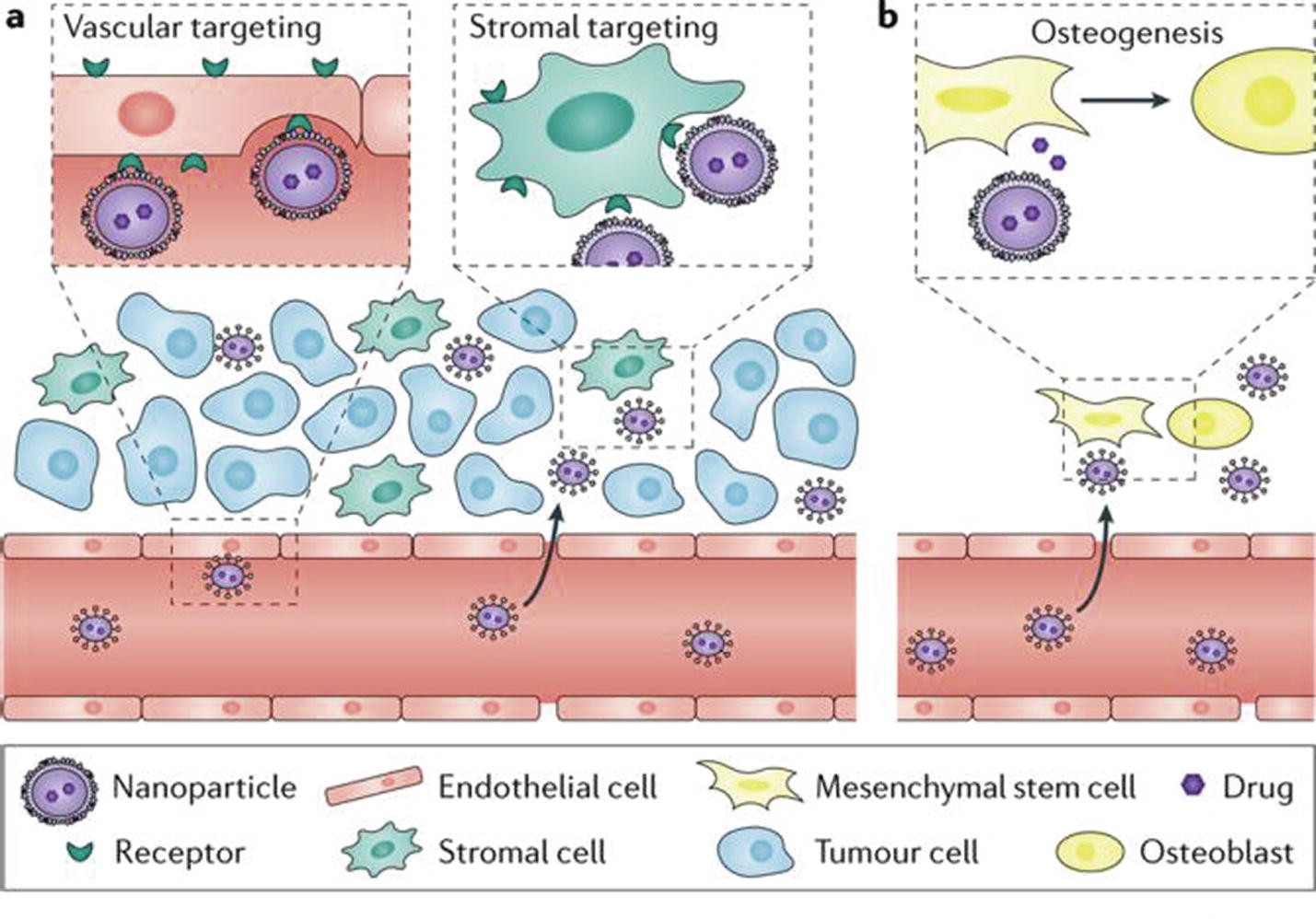
Targeting of the tumour vasculature or stromal cells in the tumour microenvironment (part a) and the premetastatic microenvironments such as the bone marrow niche, where induction of the osteogenic differentiation of mesenchymal stem cells enhances bone strength and volume (part b). Modification of NPs by ligands that bind to specific receptors allows Cell-specific targeting (Shi et al., 2017).
5 Conclusions
Polymer based nanostructures used as drug delivery systems hold great potential to efficiently target drugs to several cell types, overcoming some of the main problems, such as problems of drug resistance and to facilitate the movement of drugs across barriers. The challenge, however, remains open and regards the precise characterization of molecular targets and ensuring that these molecules only affect on targeted organs. In fact, one of the major problems that contribute to a low efficiency in drug delivery, we can mention the low drug concentrations to the active site and the very short drug residence time in the cellular and anatomical sites. The challenges associated with the optimization of drug therapy require research in the field of novel delivery systems. In recent years, smart polymeric nanodelivery systems have shown remarkable ability to overcome many of the anatomical and physiological barriers and deliver drugs locally to sites of interest thus improving therapy. The most successful approaches have used a combination of passive and active targeting. The current focus in the pharmaceutical industry is moving towards a ‘smart drug’, which increases the effectiveness and decreases the toxicity.
Acknowledgement
The author gratefully acknowledges the Sapienza University of Rome (Ateneo Sapienza 2016 projects) for financial support.
Conflict of interest
Author declare no conflict of interest.
References
- Self-aggregation and phase behavior of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymers in aqueous solution. Colloid Polym. Sci.. 1995;273:2-15.
- [Google Scholar]
- Skin penetration and distribution of polymeric nanoparticles. J. Cont. Release. 2004;99:53-62.
- [Google Scholar]
- Poly(alkylcyanoacrylate) colloidal particles as vehicles for antitumour drug delivery: a comparative study. Colloids Surfaces B. 2008;62:64-70.
- [Google Scholar]
- Recent studies on the delivery of hydrophilic drugs in nanoparticulate systems. J. Drug Delivery Sci. Technol.. 2015;2015:1-15.
- [Google Scholar]
- Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev.. 2009;61:768-784.
- [Google Scholar]
- Surface Click Reactions on Polymeric Nanocapsules for Versatile Functionalization. Macromolecules. 2012;45:3419-3427.
- [Google Scholar]
- Polymeric nanocapsules with up-converting nanocrystals cargo make ideal fluorescent bioprobes. Scientific Reports. 2016;6:29746.
- [Google Scholar]
- Alcohol vapors sensory properties of nanostructured conjugated polymer. J. Phys.: Condens. Matter. 2008;20:6.
- [Google Scholar]
- Recyclable magnetic superhydrophobic straw soot sponge for highly efficient oil/water separation. J. Colloid Inter Sci.. 2017;497:57-65.
- [Google Scholar]
- Flower-like CuO/ZnO hybrid hierarchical nanostructures grown on copper substrate: glycothermal synthesis, characterization, hydrophobic and anticorrosion properties. Materials. 2017;10:697.
- [Google Scholar]
- Functionalized gold nanoparticles for topical delivery of Methotrexate for the possible treatment of psoriasis. Colloids Surfaces B: Biointerfaces. 2016;141:141-147.
- [Google Scholar]
- Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: potential nanocarriers for improved cancer targeting. J. Drug Target.. 2015;23:642-650.
- [Google Scholar]
- Evolution of Cyclodextrin Nanosponges. International Journal of Pharmaceutics. 2017;531:470-479.
- [Google Scholar]
- The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nature Communications. 2015;6:7722.
- [Google Scholar]
- The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev.. 2015;2015(44):3890-3899.
- [Google Scholar]
- Potential dopamine prodrug-loaded liposomes: preparation, characterization, and in vitro stability studies. J. Liposome Res.. 2010;20:250-257.
- [Google Scholar]
- Photopolymerized thermosensitive poly(HPMAlactate)-PEG-based hydrogels: effect of network design on mechanical properties, degradation, and release behavior. Biomacromolecules. 2010;11:2143-2151.
- [Google Scholar]
- Acid-labile core cross-linked micelles for pH-triggered release of antitumor drugs. Biomacromolecules. 2008;9:1826-1836.
- [Google Scholar]
- Synthesis and characterization of DOX-conjugated dendrimer-modified magnetic iron oxide conjugates for magnetic resonance imaging, targeting, and drug delivery. J. Mater. Chem.. 2012;22:9594-9601.
- [Google Scholar]
- Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug-gene co-delivery to cancer cells. Nanoscale. 2014;6:1567-1572.
- [Google Scholar]
- Shape control of soft nanoparticles and their assemblies. Chem. Mater.. 2016;29:1918-1945.
- [Google Scholar]
- Folic acid grafted and tertiary amino based pH-responsive pentablock polymeric micelles for targeting anticancer drug delivery. Mater. Sci. Eng. C. 2018;82:1-9.
- [Google Scholar]
- Surface conjugation of zwitterionic polymers to inhibit cell adhesion and protein adsorption. Colloids Surf. B. 2013;107:152-159.
- [Google Scholar]
- Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett.. 2006;6:662.
- [Google Scholar]
- Polyethylene glycol-conjugated hyaluronic acid-ceramide self-assembled nanoparticles for targeted delivery of doxorubicin. Biomaterials. 2012;33:1190-1200.
- [Google Scholar]
- Osmosis based method drives the self-assembly of polymeric chains into micro and nanostructures. Langmuir. 2009;25:1940-1946.
- [Google Scholar]
- One-step conjugation of glycyrrhetinic acid to cationic polymers for high-performance gene delivery to cultured liver cell. Scientific Reports. 2016;6:21891.
- [Google Scholar]
- Nanoparticles in drug delivery: Past, present and future. Adv. Drug Delivery Rev.. 2013;65:21-23.
- [Google Scholar]
- Gel-embedded niosomes: preparation, characterization and release studies of a new system for topical drug delivery. Colloids Surf. B Biointerfaces. 2015;125:291-299.
- [Google Scholar]
- Chemical synthesis of polyphenylacetylene nanospheres with controlled dimensions for photonic crystals. Mater. Sci. Eng. C. 2003;23:861-865.
- [Google Scholar]
- Growth control and long range self-assembly of polymethylmethacrylate nanospheres. J. Appl. Polymer Sci.. 2006;102:4493-4499.
- [Google Scholar]
- pH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine A. Int. J, Pharmaceutics. 2004;280:229-240.
- [Google Scholar]
- Interlayer-crosslinked micelle with partially hydrated core showing reduction and pH dual sensitivity for pinpointed intracellular drug release. Angew. Chem. Int. Ed.. 2011;50:9404-9408.
- [Google Scholar]
- From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interf. Sci.. 2014;414:24-32.
- [Google Scholar]
- The pros and cons of polyelectrolyte capsules in drug delivery. Expert Opin. Drug Delivery. 2009;6:613-624.
- [Google Scholar]
- Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev.. 2012;41:2867-2884.
- [Google Scholar]
- Biological applications of LbL multilayer capsules: from drug delivery to sensing. Adv. Colloid Interface Sci.. 2014;207:139-154.
- [Google Scholar]
- Dendrimers and other dendritic polymers. Chichester: Wiley; 2001. p. :441-462.
- Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano. 2014;8:1384-1401.
- [Google Scholar]
- Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target.. 2007;15:163-183.
- [Google Scholar]
- Tumor-targeted drug delivery using MR-contrasted docetaxel-carboxymethylcellulose nanoparticles. Biomaterials. 2012;33:3931-3941.
- [Google Scholar]
- Non-biofouling materials prepared by atom transfer radical polymerization grafting of 2-methacryloloxyethyl phosphorylcholine: separate effects of graft density and chain length on protein repulsion. Biomaterials. 2006;27:847-855.
- [Google Scholar]
- Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5:161-171.
- [Google Scholar]
- UV and Near-IR triggered release from polymeric nanoparticles. J. Am. Chem. Soc.. 2011;132:9540-9542.
- [Google Scholar]
- Core shell hybrids based on noble metal nanoparticles and conjugated polymers: synthesis and characterization. Nanoscale Res. Lett.. 2011;6(98):8.
- [Google Scholar]
- Functional polymeric nanoparticles for dexamethasone loading and release. Colloids Surf. B. 2012;93:59-66.
- [Google Scholar]
- Structural Changes of conjugated Pt-containing polymetallaynes exposed to gamma-ray radiation doses. J. Phys. Chem. A. 2012;116:8768-8774.
- [Google Scholar]
- The puzzle of toxicity of gold nanoparticles. The case-study of HeLa cells. Toxicol. Res.. 2015;4:796-800.
- [Google Scholar]
- Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensors. Sensors Actuators B. 2016;225:96-108.
- [Google Scholar]
- Application of Eudragit RS to thermo-sensitive drug delivery systems: II. Effect of temperature on drug permeability through membrane consisting of Eudragit RS/PEG 400 blend polymers. J. Control. Release. 2005;102:49-57.
- [Google Scholar]
- Current in vitro approaches to assess nanoparticle interactions with lung cells. Nanomedicine (Lond.). 2016;11(18):2457-2469.
- [Google Scholar]
- Transferrin-conjugated nanoparticles of poly(lactide)-D-alpha-tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood-brain barrier. Biomaterials. 2010;31:7748-7757.
- [Google Scholar]
- Block copolymer micelles: preparation, characterization and application in drug delivery. J. Controlled Release. 2005;109:169-188.
- [Google Scholar]
- Dendrimers and dendritic polymers in drug delivery. Drug Discovery Today. 2005;10:35-43.
- [Google Scholar]
- Cellular chemomechanics at interfaces: sensing, integration and response. Soft Matter. 2007;3:307-326.
- [Google Scholar]
- Novel Mannan-PEG-PE Modified Bioadhesive PLGA nanoparticles for targeted gene delivery. J. Nanomaterials 2012:9.
- [Google Scholar]
- A review of in vitro-in vivo investigations on dendrimers: the novel nanoscopic drug carriers, Nanomedicine K. NBM. 2006;2:66-73.
- [Google Scholar]
- Cyclodextrin based nanosponges for pharmaceutical use: a review. Acta Pharmaceutica. 2013;63:335-358.
- [Google Scholar]
- Nanomedicine: new challenges and opportunities in cancer therapy. J. Biomedical. Nanotechnol.. 2008;4:276-292.
- [Google Scholar]
- High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: from regenerative medicine to electronic devices. Angew. Chem. Int. Ed.. 2008;47:8002-8018.
- [Google Scholar]
- Unibody core-shell smart polymer as a theranostic nanoparticle for drug delivery and MR imaging. Biomaterials. 2015;37:436-446.
- [Google Scholar]
- pH-sensitive micelles for the intracellular co-delivery of curcumin and Pluronic L61 unimers for synergistic reversal effect of multidrug resistance. Sci. Rep.. 2017;7:42465.
- [Google Scholar]
- Smart polymers in drug delivery systems On crossroads: Which way deserves following? Eur. Polym. J.. 2015;65:82-97.
- [Google Scholar]
- Synergistic combination chemotherapy of camptothecin and floxuridine through self-assembly of amphiphilic drug−drug conjugate. Bioconjugate Chem.. 2015;26:2497-2506.
- [Google Scholar]
- Physicochemical properties of dendrimers and dendrimer complexes. In: Cheng Y., ed. Dendrimer-Based Drug Delivery Systems. Hoboken, NJ: John Wiley & Sons Inc; 2012. p. :55-92.
- [Google Scholar]
- Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomedicine. 2013;9:461-464.
- [Google Scholar]
- Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. J. Controlled Release. 2013;172:1020-1034.
- [Google Scholar]
- Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969-4985.
- [Google Scholar]
- Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol.. 2008;3:145-150.
- [Google Scholar]
- Self-aggregated pegylated poly (trimethylene carbonate) nanoparticles decorated with c(RGDyK) peptide for targeted paclitaxel delivery to integrin-rich tumors. Biomaterials. 2011;32:9457-9469.
- [Google Scholar]
- Heparin-conjugated pluronic nanogels as multi-drug nanocarriers for combination chemotherapy. Mol. Pharmaceutics. 2013;10:685-693.
- [Google Scholar]
- PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. MaterialsToDay. 2015;108:565-572.
- [Google Scholar]
- Arginine-conjugated polypropylenimine dendrimer as a non-toxic and efficient gene delivery carrier biomaterials. Biomaterials. 2007;28:2061-2067.
- [Google Scholar]
- The targeted intracellular delivery of cytochrome C protein to tumors using lipid-apolipoprotein nanoparticles. Biomaterials. 2012;33:3959-3966.
- [Google Scholar]
- Diels-Alder mediated controlled release from a poly(ethylene glycol) based hydrogel. Biomacromolecules. 2013;14:538-547.
- [Google Scholar]
- Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng.. 2007;96:203-209.
- [Google Scholar]
- Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B. 2010;75:1-18.
- [Google Scholar]
- Nanostructured functional copolymers bioconjugate integrin inhibitors. J. Colloids Interf. Sci.. 2011;361:465-471.
- [Google Scholar]
- A novel micelle of coumarin derivative monoend-functionalized PEG for anti-tumor drug delivery: in vitro and in vivo study. J. Drug Target. 2012;20:246-254.
- [Google Scholar]
- From polymeric particles to multifunctional nanocapsules for biomedical applications using the miniemulsion process. J. Polymer Sci. A: Polymer Chemistry. 2010;48:493-515.
- [Google Scholar]
- Recent progress in tumor pH targeting nanotechnology. J. Control. Release. 2008;132:164-170.
- [Google Scholar]
- Designing switchable nanosystems for medical application. J. Controlled Release. 2012;161:307-316.
- [Google Scholar]
- Hemocompatibility and anti-biofouling property improvement of poly(ethylene terephthalate) via self-polymerization of dopamine and covalent graft of zwitterionic cysteine. Colloids Surf. B. 2013;110:327-332.
- [Google Scholar]
- Synthesis of reversible shell cross-linked micelles for controlled release of bioactive agents. Macromol.. 2006;39:2726-2728.
- [Google Scholar]
- Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery. of doxorubicin. Biomaterials. 2014;35:3851-3864.
- [Google Scholar]
- Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32:6633-6645.
- [Google Scholar]
- Paclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials. 2006;27:2051-2059.
- [Google Scholar]
- Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell. 2007;11:431-445.
- [Google Scholar]
- Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271-284.
- [Google Scholar]
- The significance of acid/base properties in drug discovery. Chem Soc Rev.. 2013;21:485-496.
- [Google Scholar]
- Some recent advances on liposomal and niosomal vesicular carriers. J. Drug Delivery Sci. Technol.. 2016;32:256-269.
- [Google Scholar]
- Integrated functional nanocolloids covered with artificial cell membranes for biomedical applications. Nano Today. 2011;6:61-74.
- [Google Scholar]
- Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discovery Today. 2010;15:171-185.
- [Google Scholar]
- Development and evaluation of polymer nanoparticles for oral delivery of estradiol to rat brain in a model of Alzheimer’s pathology. J. Control Release. 2011;10:220-228.
- [Google Scholar]
- Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J. Controlled Release. 2014;185:22-36.
- [Google Scholar]
- Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev.. 2001;53:283-318.
- [Google Scholar]
- Polymer-based nanocapsules for drug delivery. Int. J. Pharmaceutics. 2010;385:113-142.
- [Google Scholar]
- Noncovalently connected micelles, nanoparticles, and metal-functionalized nanocages using supramolecular self-assembly. J. Am. Chem. Soc.. 2008;130:8714-8725.
- [Google Scholar]
- Drug delivery investigations of quaternised poly(propylene imine) dendrimer using nimesulide as a model drug. Colloids Surf. B. 2014;114:121-129.
- [Google Scholar]
- Crossing cellular barriers using dendrimer nanotechnologies. Curr. Opinion Pharmacol.. 2006;6:522-527.
- [Google Scholar]
- In vitro evaluation of dendrimer prodrugs for oral drug delivery. Int. J. Pharm.. 2007;336:183-190.
- [Google Scholar]
- Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1 → 2 branching motifs: An overview of the divergent procedures. Polymer. 2008;49:1-173.
- [Google Scholar]
- Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chem. Soc. Rev.. 2006;35:1068-1083.
- [Google Scholar]
- PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev.. 2003;55:403-419.
- [Google Scholar]
- Enhanced sensory properties of a multichannel quartz crystal microbalance coated with polymeric nanobeads. Sensors. 2007;7:2920-2928.
- [Google Scholar]
- Colloidal micro- and nano-particles as templates for polyelectrolyte multilayer capsules. Adv. Colloid Interface Sci.. 2014;207:253-264.
- [Google Scholar]
- Recent advancements in mucoadhesive floating drug delivery systems: a mini-review. J. Drug Delivery Sci. Technol.. 2016;31:65-71.
- [Google Scholar]
- Photoregulation of drug release in azo-dextran nanogels. Int. J. Pharm.. 2007;342:184-193.
- [Google Scholar]
- Active polymer nanofibers for photonics, electronics, energy generation and micromechanics. Progress Polym. Sci.. 2015;43:48-95.
- [Google Scholar]
- Tailor-made polyelectrolyte microcapsules: from multilayers to smart containers. Angew. Chem.. 2004;43:3762-3783.
- [Google Scholar]
- Technical advancement in Biodegradable polymers and their recent patents. Int. J. Pharm. Sci. Res.. 2013;5:37-44.
- [Google Scholar]
- Electropolymerizable terthiophene-terminated poly(aryl ether) dendrimers with naphthalene and perylene cores. Macromolecules. 2011;44:7530-7537.
- [Google Scholar]
- Synthesis of functionalized gold nanoparticles capped with 3-mercapto-1-propansulfonate and 1-thiolglucose mixed thiols and in vitro bioresponse. Colloids Surf. B: Biointerfaces. 2016;142:408-416.
- [Google Scholar]
- Materials and transducers toward selective wireless gas sensing. Chem. Rev.. 2011;111:7315-7354.
- [Google Scholar]
- Critical determinants in PLGA/PLA nanoparticle-mediated gene expression. Pharm. Res.. 2004;21:354-364.
- [Google Scholar]
- Hydrophilic silver nanoparticles with tunable optical properties: application for the detection of heavy metals in water. Beilstein J. Nanotechnol.. 2016;7:1654-1661.
- [Google Scholar]
- Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Pharmacol. Nanomed.: Nanotechnol. Biol. Med.. 2006;2:8-21.
- [Google Scholar]
- Applications of polymeric nanocapsules in field of drug delivery systems. Curr. Drug Discov. Technol.. 2011;8:173-187.
- [Google Scholar]
- Negatively charged gold nanoparticles as dexamethasone carrier: stability and citotoxic activity. RCS Adv.. 2016;6:99016-99022.
- [Google Scholar]
- Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv. Drug Delivery Rev.. 2012;64:571-588.
- [Google Scholar]
- Preparation of highly luminescent nitrogen doped graphene quantum dots and their application as a probe for detection of Staphylococcus aureus and E. coli. J. Mol. Liq.. 2017;241:1114-1119.
- [Google Scholar]
- Degradation of methylene blue as a pollutant with N-doped graphene quantum dot/titanium dioxide nanocomposite. J. Cleaner Prod.. 2017;148:31-36.
- [Google Scholar]
- A review on role of nanostructures in drug delivery systems. Rev. Adv. Mater. Sci.. 2014;36:112-117.
- [Google Scholar]
- Green synthesis and characterization of cerium oxide nanostructures in the presence carbohydrate sugars as a capping agent and investigation of their cytotoxicity on the mesenchymal stem cell. J. Cleaner Prod.. 2017;156:741-749.
- [Google Scholar]
- Multifunctional nanoparticle composites: progress in the use of soft and hard nanoparticles for drug delivery and imaging. WIREs Nanomed. Nanobiotechnol. 2017:e1466.
- [CrossRef] [Google Scholar]
- Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol.. 2009;1:197-206.
- [Google Scholar]
- Nanotechnology in glucose monitoring: advances and challenges in the last 10 years. Biosensors Bioelectron.. 2013;47:12-25.
- [Google Scholar]
- Targeted and intracellular delivery of paclitaxel using multi-functional polymeric micelles. Biomaterials. 2007;28:1730-1740.
- [Google Scholar]
- Accelerated in vitro release testing methods for extended release parenteral dosage forms. J Pharm Pharmacol. 2012;64:986-996.
- [Google Scholar]
- Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17:20-37.
- [Google Scholar]
- Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev.. 2015;44:6684-6696.
- [Google Scholar]
- Diphtheria toxoid loaded poly-(epsilon-caprolactone) nanoparticles as mucosal vaccine delivery systems. Methods. 2006;38:96-105.
- [Google Scholar]
- Nanoparticle in the cellcular drug delivery. Proc. Natl. Acad. Sci. U.S.A.. 2011;103:3357-3362.
- [Google Scholar]
- Preparation and in vitro properties of redox-responsive polymeric nanoparticles for paclitaxel delivery. J. Colloids Surf. B. 2011;87:454-563.
- [Google Scholar]
- Multifunctionalized polymer microcapsules: novel tools for biological and pharmacological applications. Small. 2007;3:944-955.
- [Google Scholar]
- Challenges in design of translational nanocarriers. J. Controlled Release. 2012;164:156-169.
- [Google Scholar]
- Two-photon-sensitive and sugar-targeted nanocarriers from degradable and dendritic amphiphiles. Small. 2011;7:401-406.
- [Google Scholar]
- Cyclodextrin-based nanosponges: a versatile platform for cancer nanotherapeutics development. WIREs Nanomed. Nanobiotechnol.. 2016;8:579-601.
- [Google Scholar]
- Nonfouling polyampholyte polymer brushes with protein conjugation capacity. Colloids Surf. B. 2012;93:195-201.
- [Google Scholar]
- Immunosuppressive activity of size-controlled PEG-PLGA nanoparticles containing encapsulated cyclosporine A. J. Transplantation. 2012;2012:896141.
- [Google Scholar]
- Multi-functional vesicles for cancer therapy: the ultimate magic bullet. Colloids Surf. B: Biointerfaces. 2016;147:161-171.
- [Google Scholar]
- Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future. Cambridge, UK: Cambridge University Press; 2012.
- Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci.. 2010;35:1128-1143.
- [Google Scholar]
- Two facile methods to produce the cobalt manganite nanostructures and evaluation of their photocatalytic performance. J. Mater. Sci.: Mater. Electron.. 2017;28:6292-6300.
- [Google Scholar]
- Methods for the preparation and manufacture of polymeric nanoparticles. Pharmaceutical Res.. 2009;26:1025-1058.
- [Google Scholar]
- Gold nanoparticles in photonic crystals applications: a review. Materials. 2017;10:97.
- [Google Scholar]
- Synthesis of conjugated polymeric nanobeads for photonic bandgap materials. Sensors and Actuetors B. 2007;126:35-40.
- [Google Scholar]
- Alcohol vapors sensory properties of nanostructured conjugated polymer. J. Phys.: Condens. Matter. 2008;20:474202.
- [Google Scholar]
- Self-assembled copolymeric nanoparticles as chemical interactive materials for humidity sensors. Nanotechnology. 2010;21:8.
- [Google Scholar]
- Self-assembled nanoparticles of functional copolymers for photonic applications. J. Colloid Interf. Sci.. 2010;348:424-430.
- [Google Scholar]
- Soluble polymers of monosubstituted acetylenes with quaternary ammonium pendant groups: structure and morphology. Polym. Int.. 2011;60:8.
- [Google Scholar]
- Electrodeposited ZnO with squaraine sentisizers as photoactive anode of DSCs. Mater. Res. Express. 2014;1:015040.
- [Google Scholar]
- Bioconjugation of gold-polymer core-shell nanoparticles with bovine serum amine oxidase for biomedical applications. Colloids Surf. B: Biointerfaces. 2015;134:314-321.
- [Google Scholar]
- The effects of polymeric nanostructure shape on drug delivery. Adv. Drug Delivery Rev.. 2011;63:1228-1246.
- [Google Scholar]
- Cyclosporine a loaded PLGA nanoparticles for dry eye disease: in vitro characterization studies. J. Nanotechnology. 2014;2014 Article ID 683153
- [Google Scholar]
- Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res.. 2003;63:5838-5843.
- [Google Scholar]
- Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Delivery Rev.. 2008;60:1037-1055.
- [Google Scholar]
- Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. U.S.A.. 2011;108:2426-2431.
- [Google Scholar]
- Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Delivery Rev.. 2012;64:686-700.
- [Google Scholar]
- What are determining factors for stable drug incorporation into polymeric micelle carriers? Consideration on physical and chemical characters of the micelle inner core. J. Controlled Release. 2007;123:11-18.
- [Google Scholar]
- Pursuing “zero” protein adsorption of poly(carboxybetaine) from undiluted blood serum and plasma. Langmuir. 2009;25:11911-11916.
- [Google Scholar]
- Nanoparticulate Drug Delivery Systems; Strategies, Technologies and Applications. Wiley; 2013.
- Engineered nanoparticles as precise drug delivery systems. J. Cellular Biochemistry. 2006;97:1184-1190.
- [Google Scholar]
- pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int. J. Pharm.. 2011;403:262-267.
- [Google Scholar]
- Onion-like multilayered polymer capsules synthesized by a bioinspired inside-out technique. Nat. Commun.. 2017;8:193.
- [Google Scholar]
- Zwitterionic hydrogels: an in vivo implantation study. J. Biomater. Sci. Polymer Ed.. 2009;20:1845-1859.
- [Google Scholar]
- Nanomaterials for energy conversion and storage. Chem. Soc. Rev.. 2013;42:3127-3171.
- [Google Scholar]
- Construction and optoelectronic properties of organic one-dimensional nanostructures. Acc. Chem. Res.. 2010;43:409-418.
- [Google Scholar]
- Architecture of stable and water-soluble CdSe/ZnS core-shell dendron nanocrystals via ligand exchange. J. Colloid Interface Sci.. 2009;339:336-343.
- [Google Scholar]







