Translate this page into:
Wheat blast: Aggressiveness of isolates of Pyricularia oryzae and effect on grain quality
⁎Corresponding author at: Centro de Investigaciones de Fitopatologia (CIDEFI), Universidad Nacional de la Plata, 60 y 119 s/n La Plata, CP 1900 Buenos Aires, Argentina. Ivan.martinez.19912@gmail.com (Sergio Iván Martínez)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Four wheat cultivars commonly used in Argentina displayed different pattern of reaction depending of the Pyricularia oryzae isolate inoculated, according to the observed lesions and type of reaction both on seedlings and spikes of the plants tested grown under greenhouse conditions. At seedling stage, Buck Meteoro showed a susceptible reaction with seven of the eight isolates tested (phenotypic reaction 3 and 4). On the contrary, Baguette 11 showed a resistant reaction with most of the isolates tested (phenotypic reaction 1 and 2). At heading stage, cultivars Klein Proteo and Baguette 11 showed the greatest susceptibility to P. oryzae (more than 95% severity with isolates ArW22 and ArR1). On the contrary, low disease severity was shown in ACA 303 particularly in combination with isolates ArR3, ArR4, BrW27 and BolW8 with mean values of 2.18%–11.50%. The results indicate variation in aggressiveness among P. oryzae isolates evaluated. A low negative correlation between seedling disease severity and spike disease severity was found. The 1000-grain weight was negatively correlated with spike blast severity. Reduction values induced by each isolates ranged from 18.53% to 74.94%. ACA 303 showed the highest reduction in 1000-grain weight. The lower weight registered, in the most affected genotype ACA 303 infected with ArR2, was of 9.32 g. Protein extractability was higher in Wheat blast (WB) infected grains compared to the control healthy wheat. Protein values increased with the increasing severity of WB infection demonstrating P. oryzae interfered with the grain protein profile, thereby altering the grain protein content and quality.
Keywords
Pyricularia oryzae
Magnaporthe sp-wheat
Pathogenicity
Wheat blast disease severity
1 Introduction
Wheat plays an important role in the world. It is grown in more than 70 countries on 5 continents (Dixon, 2007) and is the most widely grown crop in the world (Baenziger et al., 2009).
Wheat blast (WB), caused by Magnaporthe oryzae Triticum pathotype (MoT) (anamorph Pyricularia oryzae Triticum) is a serious constraint to wheat production in several South American countries (Kohli et al., 2011). The disease was reported for the first time in Brazil, from the northern part of the state of Paraná in 1985 (Igarashi et al., 1986), and then rapidly spread to other countries. Wheat blast was identified in 1996 in the Santa Cruz department of Bolivia (Barea and Toledo, 1996) and in 2002 in the Alto Paraná and Itapúa departments of Paraguay (Viedma, 2005). MoT was identified in a small area in northeast in Argentina (Alberione et al., 2007; Cabrera and Gutierrez, 2007) and recently in some wheat plants in Buenos Aires Province (Perelló et al., 2015) and barley plants in Corrientes Province (Cabrera and Gutierrez, 2007) in Argentina. Little information is available until now regarding WB in Argentina (Perelló, 2016; Perelló et al., 2017). In adjacent countries with agroecological regions with higher rainfall (Brasil, Paraguay, Bolivia) considerably more is known about the disease and cultivar resistance (Kohli et al., 2011; Urashima et al. 2004). Knowledge of the reaction to WB of Argentinian wheat cultivars infected with isolates from neighbour countries under greenhouse conditions will enable growers to make informed decisions regarding the behaviour of cultivars and the level of risk they could have during the occurrence of an eventual outbreak of the disease.
Wheat blast was previously detected in India in 1922 and in Pakistan in 1943 (Diekmann and Putter, 1995), nevertheless the first serious outbreak reported outside South America, was recently reported in Bangladesh (Aman, 2016; BARI, 2016) – the fungus was coming from South America (Islam et al., 2016) – then in northern India (Bhattacharya and Pal, 2017) (northern India and there is an increasing fear that it could become a threat to wheat production in other parts of Asia and the world (Callaway, 2016). Under favorable conditions, wheat blast can be devastating resulting in 100% yield loss (Cruz et al., 2012; Kohli et al., 2011). Higher yield losses occur when favorable climatic conditions occur at the critical growth stages for infection (Urashima et al., 2009). Infected spikes produce small and shriveled grains with low specific weight (Miranda et al., 2015), however, little is known about the effect on grain protein content (GPC). Some studies have suggested that hemibiotrophic fungi can either increase GPC or have no effect (Blandino and Reyneri, 2009; Clare et al., 1993; Gooding et al., 1994; Ruske et al., 2001). However, most of these reports have been done on Septoria leaf blotch cause by Zymoseptoria tritici (Desm.) Quaedvl. & Crous teleomorph Mycosphaerella graminicola (Fuckel) J. Schröt, in Cohn, and studies about the effect of WB on GPC are scarce (; Miranda et al., 2015; Urashima et al., 2009).
The best disease management strategy for WB should combine cultivar resistance with appropriate agronomic practices. The identification of resistance genes to wheat blast fungus could be helpful for developing a disease management strategy. Due WB is a new disease in Argentina there is lack of information on wheat cultivars resistance and its effect on grain quality.
The aims of this work were (1) To investigate the level of susceptibility or resistance among four wheat genotypes to Pyricularia oryzae selected isolates at seedling and heading stage (2) To analyse the effect on 1000-grain weight and grain protein content (GPC) after artificial inoculations with the fungus.
2 Material and methods
2.1 Isolation and preparation of Pyricularia oryzae inoculum
Cultures of P. oryzae assayed in this study were previously recovered from diseased rice plants (ArR1, ArR2, ArR3; ARr4) and diseased wheat plants from Argentina (ArW22). One isolate from infected wheat rachis from Bolivia (BolW8), one isolate from Brazil (BrW27) and one isolate from Paraguay (PgW1) were also included. The isolates, molecularly identified previously, belong to a culture collection of the fungus maintained under refrigerator (−20 °C) onto filter paper preservation technique (Jia, 2009) at the CIDEFI and the INBIOTEC. For artificial inoculation, the inoculum was multiplied on plates containing oat-meal agar and incubated at alternating temperatures of about 25 to 30 °C for eight days in alternating cycles of 12 h light and 12 h darkness to induce growth of P. oryzae and were kept upside down. Sporulation was obtained by flattening 12 days old colony with an L-shaped glass rod and incubating under fluorescent light for three days. Sporulating plates were flooded with distilled water and the spores were removed by a paint brush. Spore suspension was adjusted to 5 × 104 conidia/ml in two independent assays for evaluate the effect of the isolates on wheat plants at seedlings stage and head stage. Plants were later transferred to the glasshouse bench and maintained inside a growth chamber at 23–25 °C and 12 h/12 h light/darkness conditions.
2.2 Aggressiveness of Pyricularia isolates on seedlings and adults wheat plants
The isolates were evaluated for pathogenicity on four wheat cultivars (ACA 303, Baguette 11, Buck Meteoro and Klein Proteo) of common use in Argentina chosen for their diverse origins and agricultural characteristics. The plants grown in plastic flats (60 cm × 40 cm × 8 cm) under greenhouse conditions at 22 to 25 °C and 12 h/12 h light/darkness. Wheat plants were inoculated by a hand sprayer at tillering stage (Z2.1) (Zadoks, 1972) with 5 × 104 conidia/ml of each isolate until run off. Concentration was calculated by harvesting the conidia/mycelia by flooding the Petri dish with 5 ml of sterile distilled water and dislodging the conidia with a bent glass rod. The resulting suspension was filtered through cheesecloth and the number of conidia were determined with a hemocytometer.
Then, plants were covered with plastic bags during two days to maintain high humidity conditions and evaluated 7 and 14 days after inoculation for severity considering the percentage of infected area (%)/ total area of leaf, and lesion type according to.
At mid-anthesis (Z6.5) (34) heads were sprayed with 1,4 ml/head with 5 × 104 conidia/ml using a hand sprayer and individually covered during two days with a black bag moistened with water on the inside to maintain high humidity conditions. Heads were rated for disease symptoms 14 days post inoculation considering the number of diseased spikelets for each spike of 8 plants per pot and two pots as replication. Spike disease severity is the average of the percentage of diseased spikelets within each diseased spike. Spikes were harvested when grain moisture content dropped below 15%. A 5 grades scale was used considering the spike disease severity (%) according Chavez et al. (2016). 0 = without symptoms; 1 = 10% affected spike; 2 = 40% affected spike; 3 = 60% affected spike; 4 = 100% affected spike. Grades 0 and 1 are considered resistant (R), 2: moderately resistant (MR); 3 = moderately susceptible (MS) and 4 = susceptible (S).
2.3 Effect of wheat blast disease on 1000-grain weight (g) and wheat grain protein content (%)
Harvested wheat grains were analyzed for grain quality such as chalky kernel, 1,000-grain weight (g) and grain protein content (%) (GPC) at the Laboratory of Cereals, Faculty of Agronomical Sciences and Forestry, UNLP. The observations were recorded in both inoculated and uninoculated conditions of all combinations cultivars × isolates tested. Grain protein content (%) was calculated in grains from harvested spikes inoculated with P. oryzae as was indicated previously. The seeds were separated into two using levels of grain disease severity, LS1 and LS2. LS1 = Seeds free from visual symptom or scarcely affected; LS2 = LS2 seeds with variable size and shape usually deformed in the structure and texture with rough and wrinkled external surface termed as shriveled seeds. The LS2 seed are usually small, narrow and lighter in weight. For both LS1 and LS2 grains, the nitrogen (N) concentration was determined by Micro-Kjeldahl method (AOAC, 1970) and GPC was expressed as crude protein multiplying the value of N obtained by 5.7 (IRAM 15852, 2002). Value of each sample was obtained and compared with the uninoculated controls.
2.4 Data analysis
Each experiment was repeated twice with nearly identical results. Data were statistically analyzed by ANOVA and Tukey’s test (α = 0.05). Data shown are the average of two replicates.
3 Results
3.1 Pyricularia oryzae isolates on leaf seedlings
Eight days later, for every line-strain combination, we monitored the number of completely resistant plants, i.e. for which we observed no lesions, small pinpoint-sized brown specks or larger brown specks without a differentiated center with the potential to produce mycelium and spores to spread the disease. Non-pathogenic isolate causing no lesions, small pinpoint-sized brown specks or larger brown specks without a sporulating differentiated center were not found. First symptoms appeared 4 days post inoculation in cultivars Klein Proteo and Buck Meteoro with all the isolates tested. Cultivars Baguette 11 and ACA 303 did not shown any symptoms at that time. Seven days after inoculation most combinations cultivar x isolate showed any of the typical phenotypic reactions already described according to.
The seddling disease severity measured 7 days after inoculation highly varied among the type of isolates studied. Its values after 7 days from inoculation ranged between 0 to 32.67% for ACA 303; 0 to 8.83% for Baguette 11; 5.17 to 17.08% for Buck Meteoro and 1.71 to 10.42% for Klein Proteo (Fig. 1a, b, c, d). Baguette 11 infected with isolate ArR2 and ACA 303 with isolates ArR1, ArR2 and ArR4, did not show any symptoms of the disease. However, in the second evaluation date (14 days), all the combinations variety × isolate shown any type of symptom like minute pinhead-sized spots, small brown to dark lesions with or without grey centers or the typical blast lesions elliptical with gray centers (Table 1). The highest seedling disease severity were reached 14 days after inoculation (60.91%, 37.5% and 26.91%) by the isolate ArR3 in combination with cultivars ACA 303, Buck Meteoro and Klein Proteo respectively. Regarding the phenotypic reactions, ACA 303 and Buck Meteoro with isolate ArR3, Baguette 11 with isolate ArW22 and Buck Meteoro with isolate PgW1 reached lesions type 4 (Table 1). These lesions showed mycelia of the fungus under 24 h of humidity chamber at Lab conditions. This is an important information from an epidemiological point of view and indicative that even with low severity values, the infected leaves can the potential to produce mycelium and spores to spread the disease under favorable field conditions. According to the observed lesions and type of reaction, the cultivars tested displayed a different pattern of reaction across leaves depending of the inoculated strain. Particularly, Buck Meteoro showed a susceptible reaction with 7 of the 8 isolates tested (phenotypic reaction 3 and 4). The opposite was noticed for Baguette 11 that showed a resistant reaction with most of the isolates tested (phenotypic reaction 1 and 2). The results indicate variation in aggressiveness among P. oryzae isolates evaluated. 0: no visible reaction. 1: minute, pinhead-sized spot. 2: small Brown to dark. 3: small with grey centers. 4: Typical blast lesions elliptical with grey centers.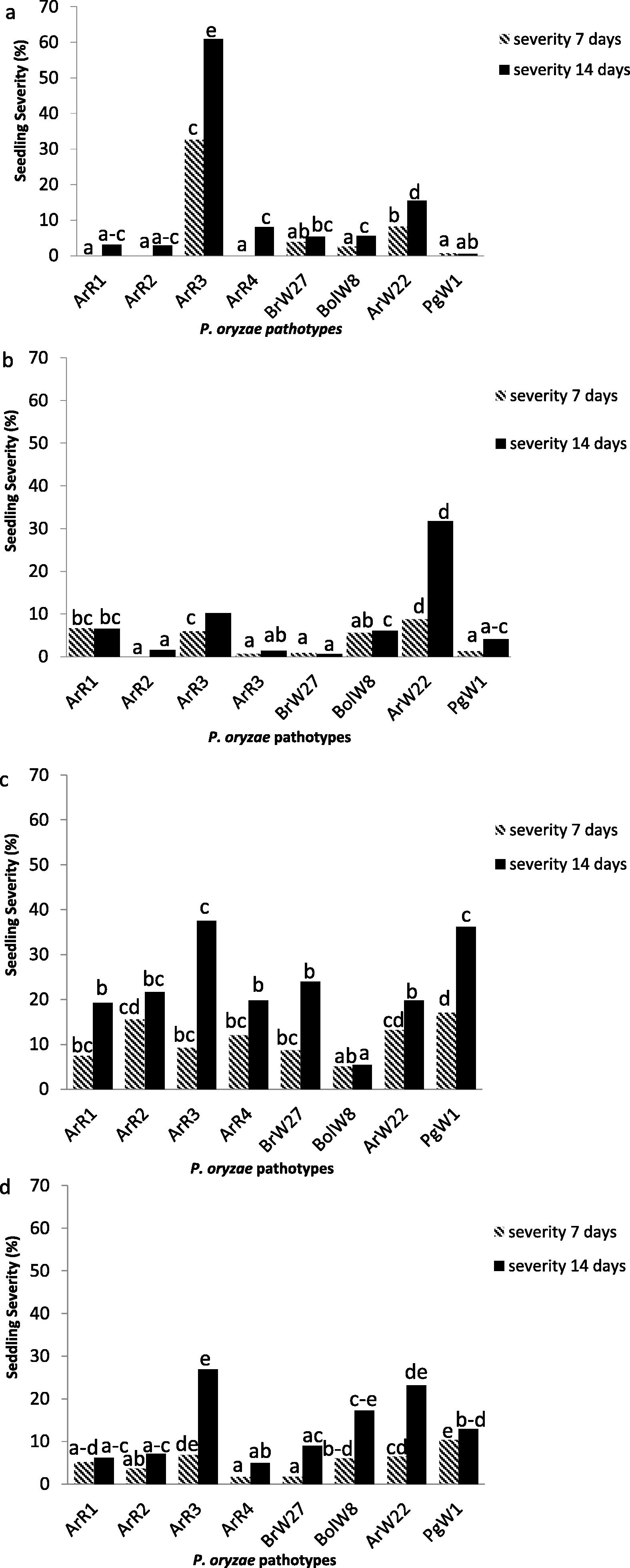
Seedling severity on 4 wheat cultivars to eight isolates of Pyricularia oryzae different pathotype. ACA 303 (a), Baguette 11 (b), Buck Meteoro (c) and Klein Proteo (d).
Variety/Isolate
ArR1
ArR2
ArR3
ArR4
BrW27
BolW8
ArW22
PgW1
Baguette 11
2
1
2
1
1
2
4
1
Klein Proteo
2
2
2
2
2
2
3
3
Buck Meteoro
3
3
4
3
3
2
3
4
ACA 303
1
1
4
2
2
2
3
1
3.2 Pyricularia oryzae on adult wheat plants
The statistical analysis showed highly significant differences in susceptibility levels of different wheat cultivars used in the experiment (Fig. 2a, b, c, d). First signs of the disease were developed after a week of inoculation on Buck Meteoro and Baguette 11 cultivars as small necrotic spots in the basal part of the spikes. The progress of the disease showed variation in susceptibility according the wheat cultivar and isolate inoculated. Low spike disease severity was particularly shown in ACA 303 in combination with isolates ArR3, ArR4, BrW27 and BolW8 with mean values of 2.18% to 11.50%. Similarly, Buck Meteoro with ArR2, ArR4 and BolW8 reached mean values of spikes disease severity among 9.09% and 14.43%. By other hand, Klein Proteo with isolates ArW22 and ArR1 was highly susceptible (97.38% and 99.20% of severity respectively). Similar behavior was shown by Baguette 11 inoculated with isolates ArR1 (95.20%), ArR3 (97.57%) and ArW22 (97.77%).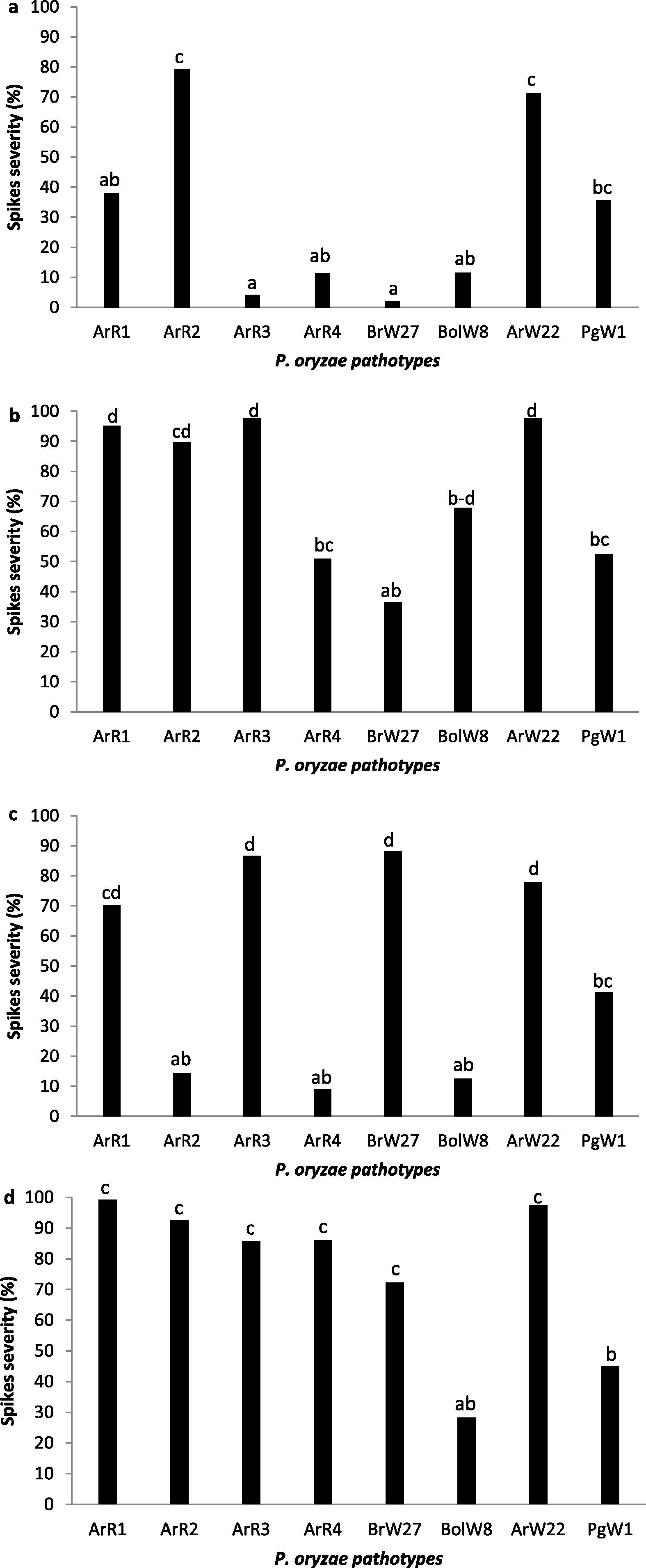
Spikes severity on 4 wheat cultivars to eight isolates of Pyricularia oryzae different pathotype. ACA 303 (a), Baguette 11 (b), Buck Meteoro (c) and Klein Proteo (d).
Analyzing isolates, a similar spike disease severity pattern was shown by the isolates ArW22 and PgW1 on the four cultivars tested. Interestingly, isolate ArR2 behaved as aggressive on ACA 303, Baguette 11 and Klein Proteo but shown low severity on Buck Meteoro. Similarly, isolate ArR3 behaved highly aggressive on Buck Meteoro, Klein Proteo and Baguette 11 but showed low aggressiveness on ACA 303.
Analyzing the values of R (Fig. 3a, b, c, d) in the nonlinear regression of seedling disease severity vs spike disease severity, it is observed that the value of the slope in the line is negative which is indicating a low negative correlation between these two parameters.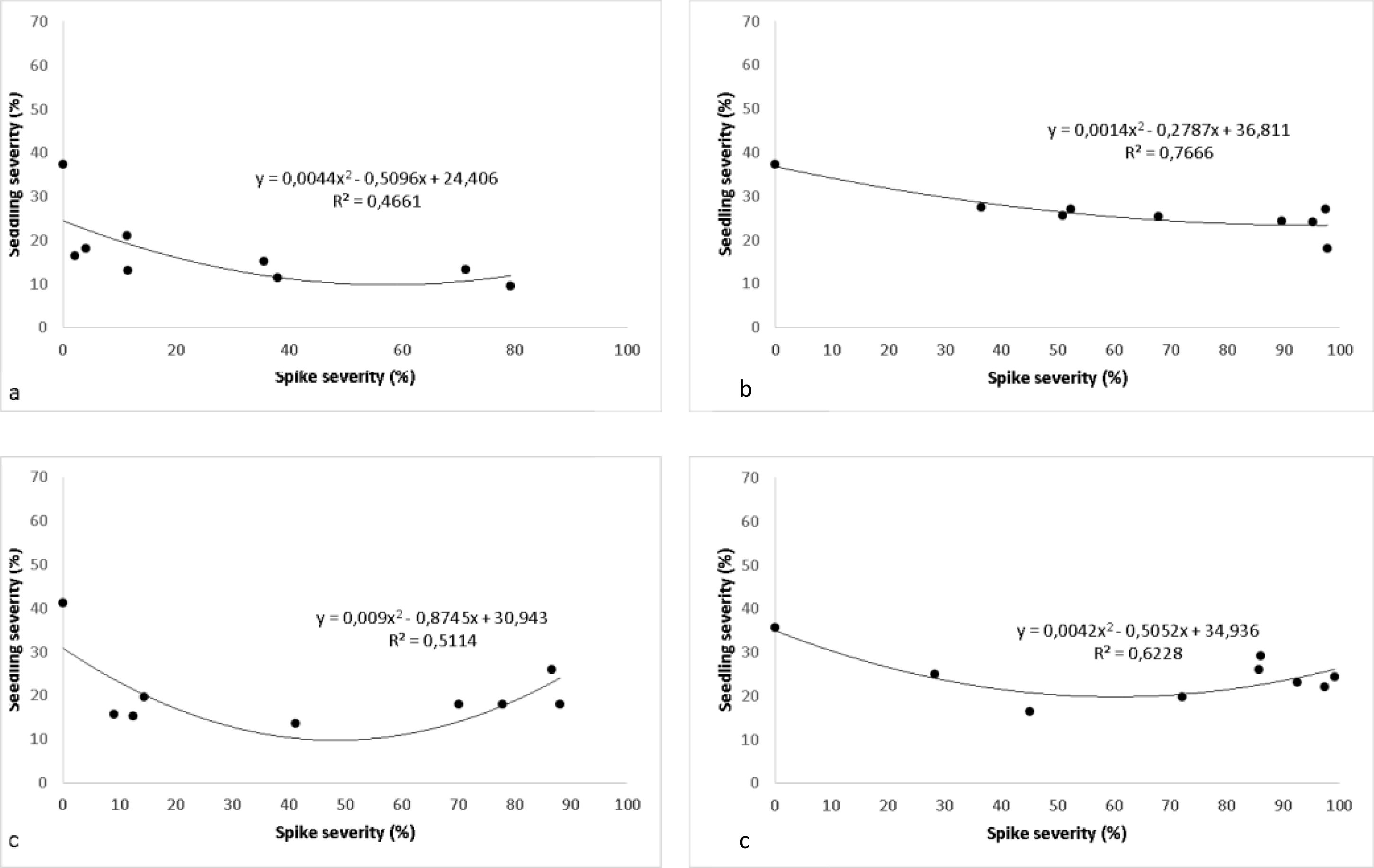
No Linear regression between Spikes severity (%) vs Seedling severity (%) on cvs ACA 303 (a), Baguette 11 (b), Buck Meteoro (c) and Klein Proteo (d).
3.3 Effect of wheat blast disease on 1000-grain weight (g) and wheat grain protein content (%)
The relative 1000-grain weight of the four cultivars used in the experiment was significantly affected by the infection of the eight isolates of P. oryzae tested when compared with the controls. The lower weight registered, in the most affected genotype ACA 303 infected with ArR2, was of 9.32 g (Fig. 4). Reduction values induced by each isolates ranged from 18.53% to 74.94% (Table 2). ACA 303 showed the highest reduction in 1000 grain weight in combination with six of the eight isolates tested (ArR1, ArR2, ArR3, BrW27, BolW 8 and ArW22). MS: moderately susceptible; S: susceptible Different letters shown significant differences according Tukey’s Test (α: 0,05).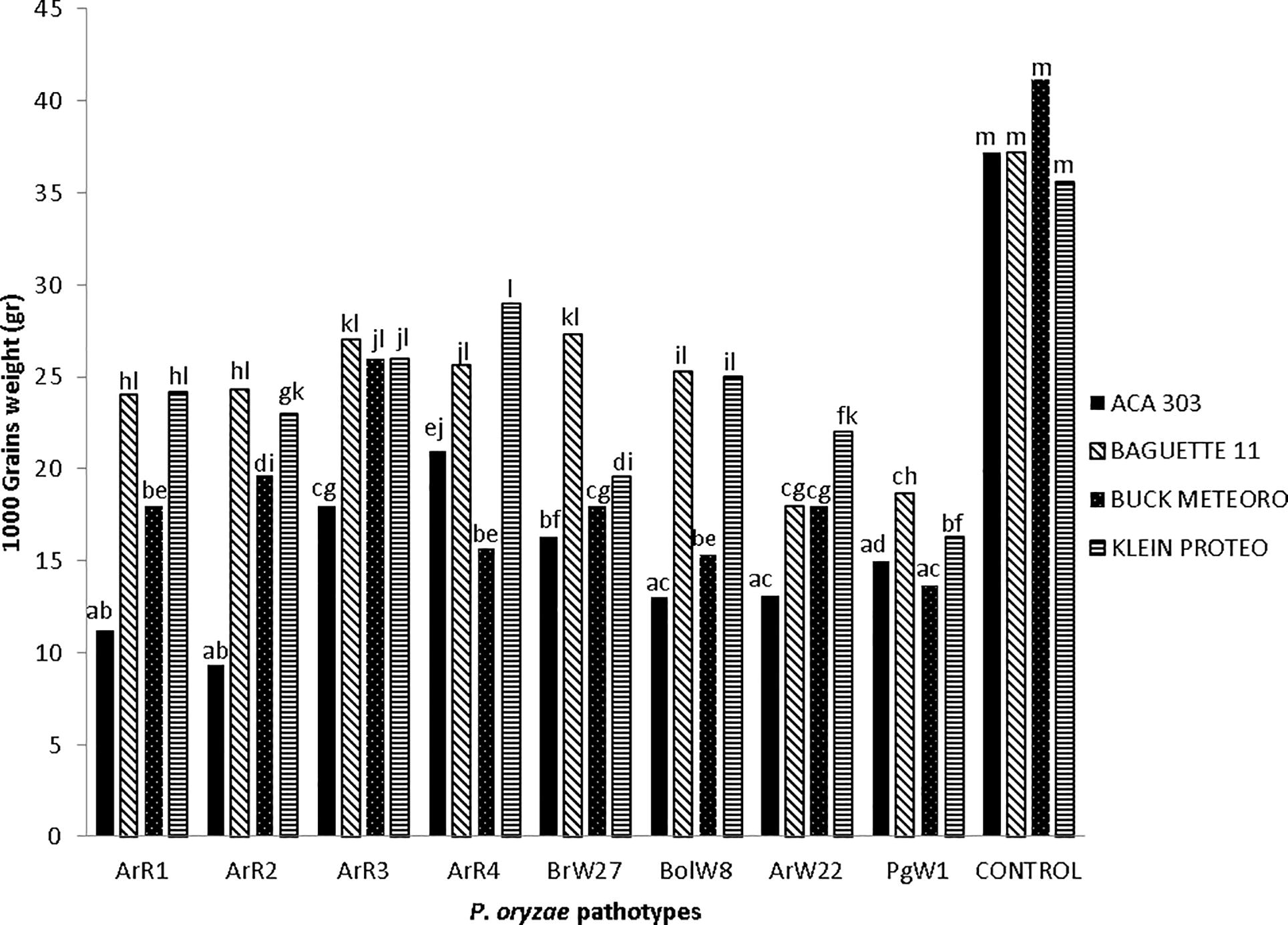
1000-Grains weight of four wheat cvs inoculated with eight isolates of Pyricularia oryzae different pathotype.
Wheat Varieties
Disease Severity (%)
Host response to infection
1000-grain weight
Control (grams)
Disease (grams)
Kernel weight loss (%)
ACA 303
37,30 a
MS
37,2
14,62 a
60,69 b
Baguette 11
74,80 c
S
37,2
23,77 c
36,28 a
Buck Meteoro
53,59 b
MS
41,2
18,03 b
57,00 b
Klein Proteo
78,26 c
S
35,6
23,14 c
34,78 a
The correlation coefficient between spike disease severity and 1000 grain weight (R2:0.67) was high in Baguette 11 and low (R2: 0.05; R2:0.15) in Buck Meteoro and Klein Proteo respectively (Fig. 5a, b, c, d). This results indicates that there is a change to identify large numbers of tolerant genotypes.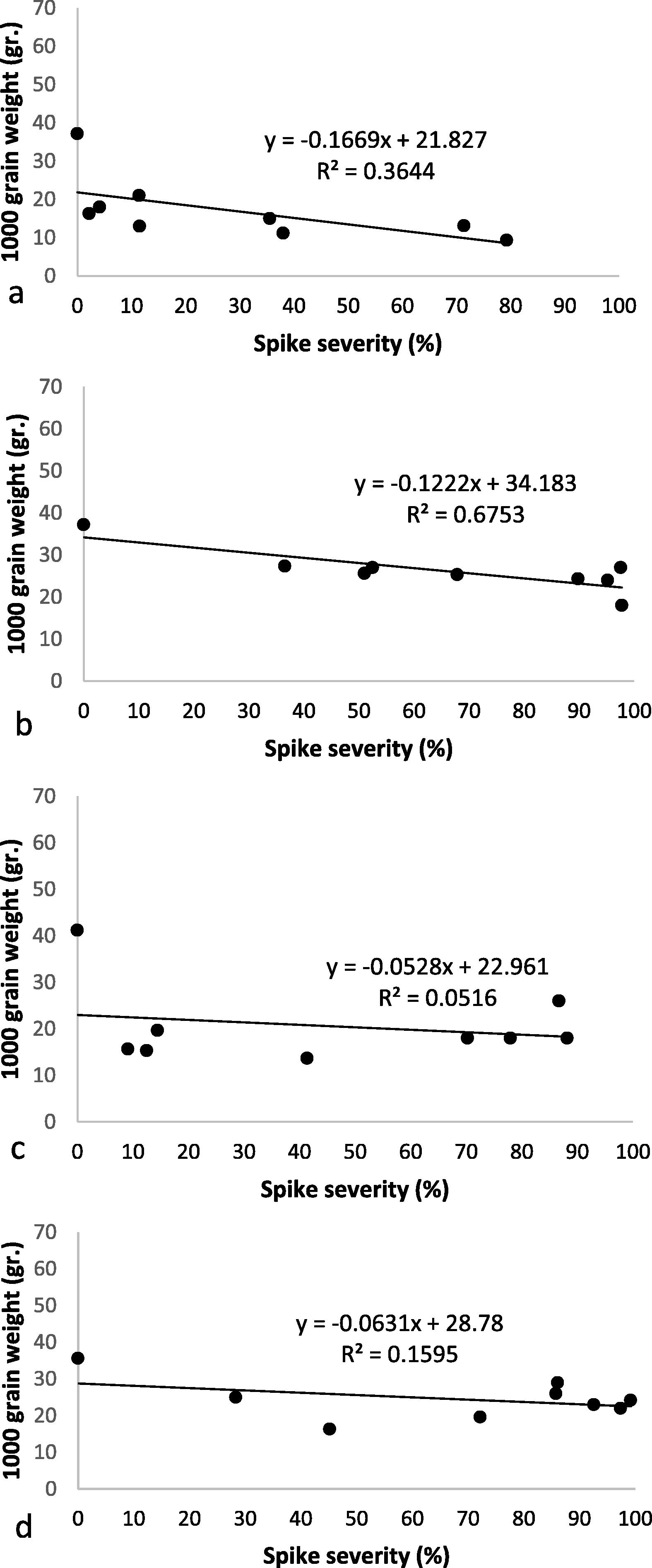
Correlation between spikes severity (%) vs 1000-grain weight (gr) on cvs ACA 303 (a), Baguette 11 (b), Buck Meteoro (c) and Klein Proteo (d).
By other hand, Wheat blast infection of grains showed marked influence on GPC. Significant variations in these characters were found between two levels of grain disease severity (LS1 and LS2). The GPC was the lowest in apparently healthy grains (LS1), which was statistically similar to that recorded under the affected grains of LS2. A negative correlation between GPCand spikes severity was shown for the four cultivars tested (Table 3). The GPC values (average) increased between 35.76 and 50% in diseased grains compared to healthy grains of uninoculated spikes. Significant differences between GPCof test and infected grains for the four tested cultivars and both severity levels were shown. In general, the higher GPCvalues, the highest visual harmfulness of grains. However, LS1 also correlated with significant increase of GPC. This demonstrates that both, either infected grains without or with visible symptoms, might affect the industrialization and grain quality of wheat. According the present results the higher GPC were shown in the combination Klein Proteo/ ArR3, Klein Proteo/PgW1, Buck Meteoro/ArR1, Buck Meteoro/ArR4 y Baguette 11/ArR1 and Baguette 11/ArR2, with an increasing of more than 50% of GPCin ACA 303/ArR1 and ACA 303/PgW1. Different letters shown significant differences according Tukey’s Test (α: 0, 05). LS1: Seeds free from visual symptoms or scarcely affected. LS2: Shriveled seeds.
ACA 303
ArR1
ArR2
ArR3
ArR4
BrW27
BolW8
ArW22
PgW1
CONTROL
LS1
16,98 c–e
15,93 b–d
16,08 b–d
15,42 bc
16,39 b–d
16,54 b–d
15,32 bc
16,92 c–e
9,66 a
LS2
19,10 f
17,67 d–f
16,04 b–d
14,69 b
17,82 d–f
17,57 d–f
17,96 d–f
18,65 ef
9,66 a
Baguette 11
ArR1
ArR2
ArR3
ArR4
BrW27
BolW8
ArW22
PgW1
CONTROL
LS1
13,99 bd
12,79 bc
15,04 cd
14,49 bd
14,30 bd
14,42 bd
12,78 bc
12,39b
9,02 a
LS2
16,42 d
16,40 d
15,70 d
14,71 bd
16,18 d
15,37 d
15,54 d
14,84 cd
9,02 a
Buck M.
ArR1
ArR2
ArR3
ArR4
BrW27
BolW8
ArW22
PgW1
CONTROL
LS1
19,45 f–h
14,74 b
17,98 c–g
18,45 d–g
15,27 b
19,24 f–h
15,62 bc
15,91 b–d
11,82 a
LS2
20,53 gh
17,15 b–f
18,84 e–h
21,17 h
16,30 b–e
18,90 e–h
16,10 bd
18,21 c–g
11,82 a
Klein Proteo
ArR1
ArR2
ArR3
ArR4
BrW27
BolW8
ArW22
PgW1
CONTROL
LS1
16,03 bc
15,97 bc
18,12 d–g
17,16 c-e
15,49 b
18,91 fg
16,80 b–d
17,96 d–g
12,45 a
LS2
17,68 d–f
17,52 c–f
19,32 g
17,18 c–e
15,98 bc
18,67 e–g
17,08 cd
19,43 g
12,45 a
4 Discussion
Few studies have been done in Argentina to evaluate wheat cultivars for resistance to WB. This study provides information on the reaction to WB of wheat cultivars. Cultivars differed in their reaction to WB both in seedling and adult plants trials. The cultivar Buck Meteoro was consistently the most susceptible to WB (seedling disease severity) at seedling stage whereas the cultivar Baguette 11 was among those with low WB seedling disease severity according the evaluation of infection after 14 days of inoculation. At heading stage, ACA 303 behaved with low severity following by Buck Meteoro; Baguette 11 and Klein Proteo showed high severity level. Based on the results of this study, cultivars tested were classified as moderately susceptible and susceptible respectively.
Nevertheless, more research is needed to demonstrate that our greenhouse results are congruent with the field evaluation of wheat blast resistance of these cultivars. Therefore, it would be of great interest to achieve projects in common with other countries from which non-Argentine strains come.
Furthermore, in our conditions, rice strains infected wheat, but this compatibility could be not consensual for others authors considering the wheat and rice derived populations of Pyricularia oryzae were described as genetically distinct and host specific based on distinct DNA-fingerprinting profiles, the absence of cross pathogenicity between wheat and rice- derived strains, and sexual incompatibility between the two host specialized populations (Maciel et al., 2014). However, in agreement with our results, exists others reports were Magnaporte oryzae oryzae patotype were highly virulent on the original rice host (Oryza sativa) and also on wheat (Castroagudin et al., 2016)
In the present study, and in line with other findings (Islam et al., 2016), 1,000-grain weight was significantly reduced by WB. Previous works indicates that the 1,000-grain weight is the direct component most important in the selection of yield of wheat in the Pampa’s region of Buenos Aires Province, Argentina (Miranda et al., 1994). In Agreement García del Moral et al. (2003) concluded that wheat yield in the north of Spain is also determined by grain weight as principal component. Thus, we could conclude that probably this parameter has a highly significant direct influence on grain yield of wheat infected by P. oryzae under field conditions.
However, GPC was statistically higher in diseased grains independently of isolate type or cultivar, mainly explained by the grain size reductions caused by the disease which produced smaller and shrivelled grains. Shriveled grains are undesirable due to its association with poor milling quality because they may contribute to impurities and had reduced flour extraction rates with lower contents of metabolizable energy (Gooding and Davies, 1997). Our findings are in line with those reported by Urashima et al. (2009) who found that grains from blast diseased spikes had higher GPC. Recent studies (Miranda et al., 2015) have also reported changes in physicochemical and rheological parameters and bread making test caused by the diseased, however, the authors do not specify whether GPC was increased. Most of the studies available in literature regarding the effect of an hemibiotrophic fungus on wheat GPC have been done on M. graminicola (Dimmock and Gooding, 2002). Although the two diseases are caused by an hemibiotrophic pathogen, the level of known about the effect of WB on GPC, in relation to Septoria leaf blotch, is a lot lower, in partly due to the relatively recent emergence of the first one (Nunes Maciel et al., 2014). Hence, more research is needed that focuses on the potentially negative consequences of WB on grain and bread-making quality of wheat.
Evaluation of a wider range of winter wheat cultivars grown in the region for resistance to WB will provide more choices and increased benefits to producers and the food processing industries in case the disease spread in Argentina.
Acknowledgments
The authors thank the financial support provided by CONICET (Project PIP 819/14) and FCAyF-UNLP (Project 11A 296).
References
- Alberione, E., Bainotti, C., Cettour, I., Salines, J., 2008: Evaluación de enfermedades en trigos en siembra de verano en el NEA argentino-Campaña 2007/2008. In: 7 Congreso Nacional de Trigo. Santa Rosa, La Pampa, Argentina.
- Aman, A., 2016.‘Wheat blast’ threatens yield – farmers in 6 districts complain of infection. (http://www.thedailystar.net/backpage/wheat-blast-threatens-yield-784372). Accessed 29 November 2016.
- AOAC, 1970. Official methods of analysis. Association of Official Analytical Chemist. 11th Ed. Washington DC, USA, pp. 1015.
- Baenziger, P., Graybosch, R., Van Sanford, D., Berzonsky, W., 2009. Winter and specialty wheat, in: Cadena, M. J., ed; Cereals. Springer, pp. 251–265.
- Bangladesh Agricultural Research Institute (BARI), 2016. Anual report. http://bari.portal.gov.bd/sites/default/files/files/bari.portal.gov.bd/annual_reports/0f72f23b_1489_4db9_893e_0e8548d36ae9/BARI%20Annual%20Report%202015-16%20(1).pdf.
- Identificación y zonificación de Pyricularia o brusone (Pyricularia oryzae) en el cultivo de trigo en el departamento de Santa Cruz. Bolivia: Santa Cruz de la Sierra; 1996. p. :76-86.
- Bhattacharya, R. and Pal, S., 2017. Deadly wheat blast symptoms enters India through the Bangladesh border, Bengal govt burning crops on war footing. Hindustan Times. http://www.hindustantimes.com/kolkata/deadly-wheat-blast-symptoms-enters-india-through-the-bangladesh-border-bengal-govt-burning-crops-on-war-footing/story-3zoWQ0H7sdMU4HxQyzWUsN.html.
- Effect of fungicide and foliar fertilizer application to winter wheat at anthesis on flag leaf senescence, grain yield, flour bread-making quality and DON contamination. Eur. J. Agron.. 2009;30:275-282.
- [CrossRef] [Google Scholar]
- Primer registro de Pyricularia oryzae en cultivos de trigo del NE de Argentina. In: Astiz Gasso M., Molina M., eds. Jornada de Actualización en Enfermedades de Trigo. Instituto Fitotécnico de Santa Catalina: Lavallol, Buenos Aires; 2007. p. :18-180.
- [Google Scholar]
- Devastating wheat fungus appears in Asia for first time. News in Focus. Nature. 2016;532:421-422.
- [Google Scholar]
- Castroagudin, V.L., Moreira, S.I., Pereira, D.A.S., Moreira, S.S., Brunner, P.C., Maciel, J.L.N., Crous, P.W., McDonald Alves, B.E., Ceresini, P.C., 2016. Wheat blast disease caused by Pyricularia graminis-tritici sp. Nov. Persoonia doi: 10.3767/003158516X692149.
- Factors affecting the quality of milling wheats produced in a high yield situation. Aspects of Applied Biology 36. Cereal Quality. 1993;III:241-250.
- [Google Scholar]
- Preliminary assessment of resistance among U.S. wheat cultivars to the Triticum pathotype of Magnaporthe oryzae. Plant Dis.. 2012;96:1501-1505.
- [Google Scholar]
- Diferencia en la reacción a Pyricularia oryzae de materiales de trigo en los estadios vegetativo y reproductivo. (Difference in the reaction to Pyricularia oryzae of wheat materials in the vegetative and reproductive stages) Inv. Agr.. 2016;19(1):56-63.
- [Google Scholar]
- Diekmann, M., Putter, C.A.J. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
- The influence of foliar diseases, and their control by fungicides, on the protein concentration in wheat grain: a review. J. Agric. Sci.. 2002;138:349-366.
- [Google Scholar]
- The economics of wheat. Research challenges to field to fork. In: Buck H.T., Nisi J.E., Salomon N., eds. Wheat Production in Stressed Environments. Springer, Dordrecht: The Netherlands; 2007. p. :9-22.
- [Google Scholar]
- Evaluation of grain yield and its components in durum wheat under Mediterranean conditions: an ontogenic approach. Agron. J.. 2003;95:266-274.
- [Google Scholar]
- Effects of late season applications of propiconazole and tridemorph on disease, senescence, grain development and the breadmaking quality of winter wheat. Crop Prot.. 1994;13:362-370.
- [Google Scholar]
- Wheat Production and Utilization: Systems, Quality and the Enviroment. Wallingford, U.K.: CAB International; 1997. p. :355.
- Igarashi, S., Utiamada, C.M., Igarashi, L.C., Kazuma, A.H., Lopes, R.S., 1986. Pyricularia in wheat. 1. Occurrence of Pyricularia sp. In Parana State. (In Portuguese) Fitopatol. Bras. 11;351–352.
- IRAM 15852., 2002. Cereales. Determinación de proteínas totales. Método Kjeldahl modificado [Cereals. Determination of total proteins. Modified Kjeldahl method.]
- Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol.. 2016;14:84.
- [Google Scholar]
- Jia, Y., 2009. A user-friendly method to isolate and single spore the fungi Magnaporthe oryzae and Magnaporthe grisea obtained from diseased field samples. Online. Plant Health Progress doi:10.1094/PHP-2009-1215-01-BR.
- Pyricularia blast – A threat to wheat cultivation. Czech J. Genet. Plant Breed.. 2011;47:S130-S134.
- [Google Scholar]
- Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathology. 2014;104:95-107. 10.1094
- [Google Scholar]
- Miranda, R., Morant, A.E., Junquera, A.A., Borsetti, S.C., 1994. Ideotipos de trigo. Criterios de selección 1: componentes de rendimiento en ambiente semiárido. 111 Congreso Nacional de trigo. Actas pp.126- 127. Bahía Blanca. 26–28 Octubre.
- Miranda, M., Torres, G., Santana, F., Sussel, A., Goulart, A., Coelho, M., 2015. Effect of blast disease incidence on wheat technological quality. En: Actas del 9th Inernational Wheat Conference, 20th to the 25th of September, Sydney, Australia. pp. 185.
- Population Structure and Pathotype Diversity of the Wheat Blast Pathogen Magnaporthe oryzae 25 Years after Its Emergence in Brazil. Phytopathology. 2014;104:95-107.
- [Google Scholar]
- First report of virulence and effects of Magnaporthe oryzae isolates causing wheat blast in Argentina. Plant Dis.. 2015;99:1177.
- [Google Scholar]
- Perelló, A., 2016. Update on recent wheat blast research progress in Argentina. 5th International Symposium on Fusarium Head Blight and the 2nd International Workshop on Wheat Blast, held on April 6 – 9, 2016 in Florianópolis, Universidad de Passo Fundo, Brazil. pp. 146.
- Pathogenicity of isolates of Magnaporthe oryzae from wheat and grasses infecting seedlings and mature wheat plants in Argentina. Plant. Pathol. 2017
- [CrossRef] [Google Scholar]
- Nitrogen accumulation in grains of winter wheat in response to strobilurin fungicides. Aspects Appl. Biol. Wheat Qual.. 2001;64:227-234.
- [Google Scholar]
- Resistance spectra of wheat cultivars and virulence diversity of Magnaporthe grisea isolates in Brazil. Fitopatol. Bras.. 2004;29:511-518.
- [Google Scholar]
- Effect of Magnaporthe grisea on seed germination, yield and quality of wheat. In: Xiaofan W., Valent B., eds. Advances in Genetics, Genomics and Control of Rice Blast Disease. New York, USA: Springer; 2009. p. :267-277.
- [Google Scholar]







