New group of azastilbene analogs of resveratrol: Synthesis, anticandidal activity and toxicity evaluation
⁎Corresponding author. nadiacritt@gmail.com (Nádia Rezende Barbosa Raposo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Infections caused by microorganisms of the Candida genus represent a major cause of morbidity and mortality among the population. Therefore, this study aimed at evaluating the antifungal activity and toxicity of resveratrol-analog Schiff bases. Faced with Candida albicans ATCC 10,231, the broth microdilution method was used, along with amphotericin B as the reference drug. The toxicity was evaluated in human keratinocyte cells and against Artemia salina. All analogs were active against the microorganism and caused structural changes in the yeast. The minimum inhibitory concentration was lower for analog A (156.3 µg mL−1), followed by B (312.5 µg mL−1), C (312.5 µg mL−1) and D (625 µg mL−1); while the minimum fungicidal concentration was lower for A (1,250 µg mL−1), B (1,250 µg mL−1) and D (1,250 µg mL−1), followed by C (2,500 µg mL−1). In keratinocytes, the compounds presented cell viability between 50.9 and 93%. Against A. salina, the compounds presented moderate cytotoxic activity. The compound with the nitro group, an electron withdrawing group, in the para position presented better antifungal activity. Resveratrol analogs can be promising in the development of new antifungal agents.
Keywords
Resveratrol analogs
Schiff bases
Candida albicans
Antifungal activity
Toxicity
1 Introduction
Yeasts of the Candida genus are considered opportunistic fungi, responsible for the infection known as candidiasis (Deorukhkar and Saini, 2013; Lim et al., 2012). Within this genus, the Candida albicans species is considered the most prevalent fungus in human infections (Guirao-Abad et al., 2013).
About 75% of women may be affected by vulvovaginal candidiasis throughout their lives and 5–10% have chronic recurrent episodes which affect their quality of life (Kim and Sudbery, 2011; Sudbery, 2011). Furthermore, urinary tract infections by microorganisms belonging to this genus can be serious and may evolve to pyelonephritis, candidemia and even death (Wernli et al., 2013).
Invasive infections by these fungi are considered serious (Sherry et al., 2014) and represent the fourth leading cause of bloodstream infections in the hospital environment, with a mortality rate of approximately 50% (Andes et al., 2012). Often, they can also form biofilms in medical and hospital devices and implants (Lee and Lee, 2015), which can worsen the patient's condition.
There are few classes of antifungal drugs available for medical treatment. Moreover, both the human host and fungi are eukaryotic organisms, limiting the cellular targets for these drugs. These factors, associated with the agents’ toxicity, resistance of microorganisms to such agents (Singh et al., 2014; Torabzadeh and Panahi, 2013) and the emergence of new virulence factors (Guirao-Abad et al., 2013), lead to the search for compounds with novel mechanisms of action or actions which are synergistic with the mechanisms already assessed (Favre-Godal et al., 2014).
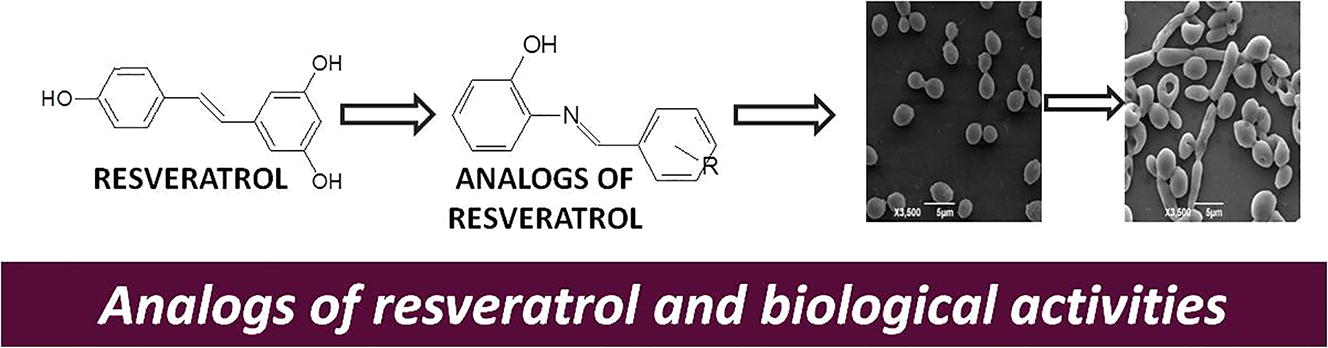
Resveratrol is a stilbene derived from the secondary metabolism of certain spermatophytes and, among their biological properties already described, the antimicrobial activity and the activity against the phytopathogenic fungi Penicillium expansum and Aspergillus niger stand out (Collado-González et al., 2012). In addition, resveratrol analogs have shown biological activity, including antifungal, antibacterial, antimalarial, photoprotective, antioxidant (Polonini et al., 2013; Santos et al., 2013), antioxidant activity on bovine embryo development (Patrocínio et al., 2016), antiproliferative, antiaging antiviral, anti-inflammatory and antipyretic properties. The imine group seems to be related to such properties (Chedea et al., 2017; Silva et al., 2011). It is possible to mention activity in tumor suppression, increase in insulin sensitivity and effects on obesity and adipogenesis (Azhar et al., 2016). It has the capacity to cross the blood–brain barrier and may exert a protective effect on the brain tissue, being considered promising about cognitive function and memory (Farzaei et al., 2017). The activities of this compound can be attributed not only to its antioxidant action but also to the ability to trigger cellular signaling pathways and gene expression related to cellular defense (Chedea et al., 2017).
Through the use of classical bioisosterism in the stilbenoid nucleus, it is possible to obtain active resveratrol analogs, with significant antitubercular and leishmanicidal activities (Polonini et al., 2013; Coimbra et al., 2016). Therefore, the purpose of this study was to synthesize new resveratrol-analog Schiff bases, evaluate their antifungal potential against Candida albicans ATCC 10,231 and determine their toxicity against human keratinocytes (HaCaT) and A. salina.
2 Material and methods
2.1 Synthesis of Schiff bases
The four resveratrol analogs, designated A, B, C and D, were synthesized and characterized in the chemical laboratory of the Federal University of Juiz de Fora, as described by and in accordance with other data in the literature (Lima et al., 2013).
Resveratrol analog D was synthesized by means of condensation between 2-hydroxyaniline with a variety of aromatic aldehydes in ethanol. This compound was characterized by 1H and 13C nuclear magnetic resonance (NMR), infrared (I.R.), and melting point (M.P).
2.2 Antifungal activity
The Candida albicans American Type Culture Collection (ATCC) 10,231 species was used in this study, obtained from the André Tosello Foundation (Campinas, São Paulo, Brazil). The procedures were performed according to the M27-A2 protocol from the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2002). The fungal suspension was prepared in sterile saline (0.85% NaCl w/v) and then it was diluted in RPMI 1640 culture medium, buffered with 3-(N-morpholino)-propanesulphonic acid (MOPS) and the pH was adjusted to 7.0 ± 0.1, so as to obtain from 5 × 102 to 2.5 × 103 colony forming units (CFU) per mL. The analogs were diluted in RPMI 1640 medium buffered with MOPS and 20 µL mL−1 Tween-80/Dimethyl Sulfoxide (DMSO) (1:1, v/v) in a concentration range of 10–5000 µg mL−1. The assay was performed in 96-well sterile microplates (Sarstedt, Germany), to which 100 µL of analogs dilutions and 100 µL RPMI 1640 were added, buffered with MOPS and inoculated with a suitable number of the microorganism’s colony forming units. The growth control consisted of 100 µL of the same inoculated culture medium and 20 µL mL−1 Tween 80/DMSO (1:1, v/v) and a sufficient quantity of the uninoculated medium to add up to 200 µL. The negative control was prepared by adding 200 µL of uninoculated medium. The drug Amphotericin B (Cristália, Brazil) was used as positive control at concentrations from 0.0313 to 16.0 µg mL−1. The microplates were incubated at 35 °C/48 h. The minimum inhibitory concentration (MIC) was established as the lowest concentration at which no turbidity was observed in the culture medium. After determining the MIC, an aliquot of 20 µL was retained from those wells which showed no visible growth and re-incubated with 4 mL of Tryptic Soy Broth (TSB) without the addition of an antifungal agent, for another 48 h at 35 °C. The lowest concentration at which no turbidity was noticed after this period was considered to be the Minimum Fungicidal Concentration (MFC). Results were expressed in µg mL−1.
2.3 Scanning electron microscopy
The material used for observation by scanning electron microscopy was formed by colonies of fungal species that were not subjected to treatment (negative control), and colonies exposed to the tested compounds and Amphotericin B (reference drug).
The sample preparation was performed according to the method described by Gao et al. (2011) with some modifications. The same antifungal activity standardization procedure was used in the preparation of the fungal suspension, in order to obtain a suspension between 5 × 102 and 2.5 × 103 CFU mL−1.
The suspensions were transferred to a microplate with 100 μL inoculated medium, and, after incubation in an oven at 35 °C for 48 h, both the compounds and the reference drug were transferred to their respective wells. After this procedure, the microplate was re-incubated. In this study, the concentrations used for the compounds and reference drug were the MFC values found. After that, the samples were then centrifuged at 5000 rpm for 10 min (Eppendorf, 5417R, Germany) and the pellets formed were transferred to glass slides and fixed with 2.5% glutaraldehyde solution for 12 h. After this period, coverslips were washed with 0.1 M phosphate buffer (pH = 7.4) and dehydrated with increasing concentrations of ethanol (50% to 100%), with an interval of 20 min between each exchange. Then the coverslips were dried at room temperature and fixed in stubs with double-sided carbon tape. Subsequently, the coverslips were placed in the metallizer (Balzers Union FL – 9496, Balzers, Germany) for 5 min, resulting in a layer of gold–palladium thereon. Samples were observed by scanning electron microscope (JSM 5310, Jeol, Japan), at 25 kV power and 17 mm work distance.
2.4 Brine shrimp lethality test
The assay was carried out according to Meyer et al. (1982) Artemia salina Leach eggs were cultured in artificial sea water: NaCl 24.0 g L−1, CaCl2·2H2O 1.5 g L−1, KBr 0.1 g L−1, KCl 0.7 g L−1, Na2SO4 4.0 g L−1, NaHCO3 0.3 g L−1, MgCl2.6H2O 11.0 g L−1. The resveratrol and analogs were dissolved in Tween 80 and dimethyl sulfoxide (DMSO) (1:2, v/v), and then in artificial sea water. Ten brine shrimps (A. salina) were transferred to test tubes containing the following concentrations of the compounds: 10, 50, 100, 250, 500 and 1000 μg mL−1 (n = 5). The tubes were kept in constant illumination and thymol was used as standard control. Living nauplii were counted after 24 h of exposition to the compounds tested. The final volume used of the solution of Tween 80 and DMSO (1:2, v/v) was also tested.
For the LC50 calculations, the probit analysis method was used, which is defined by the necessary concentration to cause death of 50% of the A. salina larvae in a 24 h period. The calculations were performed in the statistical computer program “MICRO PROBIT”.
The compounds were considered highly toxic when the LC50 was lower than 80 µg mL−1, moderately toxic with LC50 between 80 µg mL−1 and 250 µg mL−1 and with low toxicity or not toxic with LC50 higher than 250 µg mL−1 (Pompilho et al., 2014).
2.5 Evaluation of cellular viability using MTT assays
Cell viability of human keratinocytes (HaCaT) was evaluated after culture in Dulbecco's Modified Eagle Medium (DMEM) (Nutricell, Brazil) supplemented with 10 % fetal bovine serum (SFB) (Invitrogen, USA), 100 U mL−1 penicillin, 100 U mL−1 streptomycin, and 10 mM 4-(2-HidroxiEthil)-1-PiperazinEthanolSulfonic buffer (HEPES) (Mosmann, 1983). Cells were cultured in sterile 96-well plates with level depths (Sarstedt, Germany) at a density of 5 × 103 cells per well and were then incubated in an oven at 37 ± 2 °C in an atmosphere containing 5 % CO2 for 48 h. Subsequently, the culture media were replaced with sample solutions (resveratrol and analogs) at concentrations from 10 to 100 µM. Plates were, then incubated at 37 ± 2 °C in an atmosphere containing 5% CO2 for 48 h. Control was performed under the same conditions, but without adding the tested substance. After 48 h, culture media were removed and 100 µL aliquots of 10 % [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT; 5 mg/mL) in DMEM were added to all wells and the plates were immediately incubated at 37 ± 2 °C in an atmosphere containing 5 % CO2 for 3 h. Finally, the resulting formazan crystals were dissolved in DMSO and the absorbance was evaluated using a spectrophotometer (SpectraMax 190, Molecular Devices, Sunnyvale, CA, USA) at 570 nm (Twentyman and Luscombe, 1987).
2.6 Statistical analysis
Data were expressed as means ± standard errors of the mean (SEM) and are representative of 5 replicates. Differences were identified using analyses of variance (ANOVA) followed by Bonferroni’s test with the help of the Statistical Package for Social Sciences software, version 21.0 (IBM SPSS Statistics 21) and were considered significant when p < 0.05.
3 Results and discussion
3.1 Antifungal activity
The results obtained can be observed in Table 1. The capacity to inhibit fungal growth was decreasing in the following order: A, B/C and D (p < 0.05). The assessed fungicide potential was the same for A, B and D (p > 0.05), followed by C (p < 0.05) (Table 1). Resveratrol was not active in the experimental conditions. The microorganism tested was considered sensitive to amphotericin B, since the MIC value was lower than 1.0 µg mL−1 (CLSI, 2002).
| Compound | Chemical structure | MIC (µg mL−1) | MFC (µg mL−1) |
|---|---|---|---|
| Analog A |
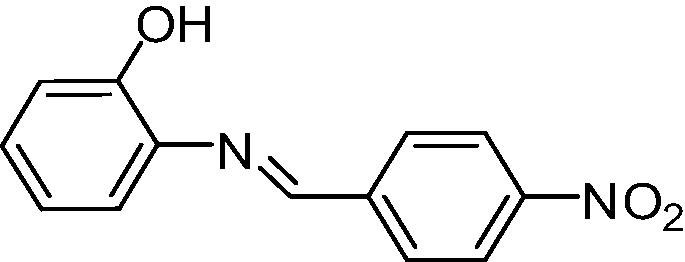
|
156.3 | 1250 |
| Analog B |
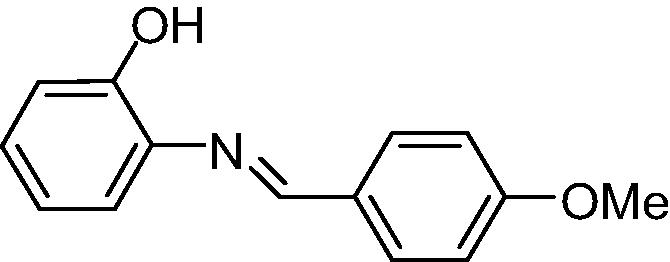
|
312.5 | 1250 |
| Analog C |
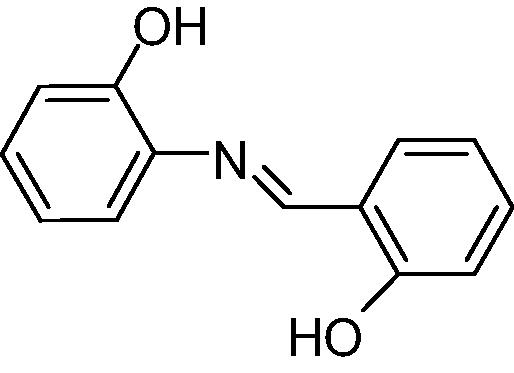
|
312.5 | 2500 |
| Analog D |
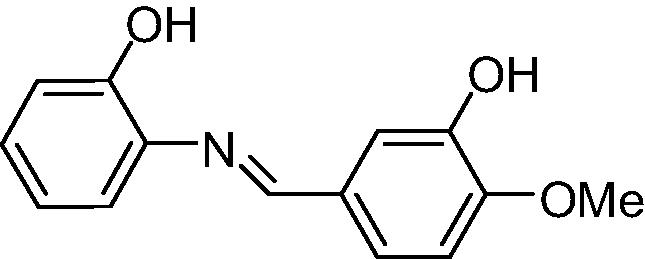
|
625.0 | 1250 |
| Resveratrol |
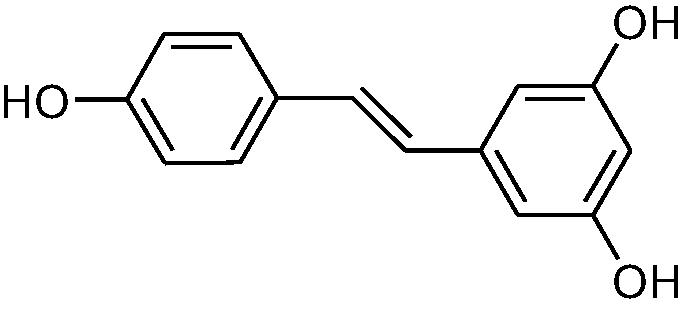
|
>5000 | >5000 |
| Amphotericin B |
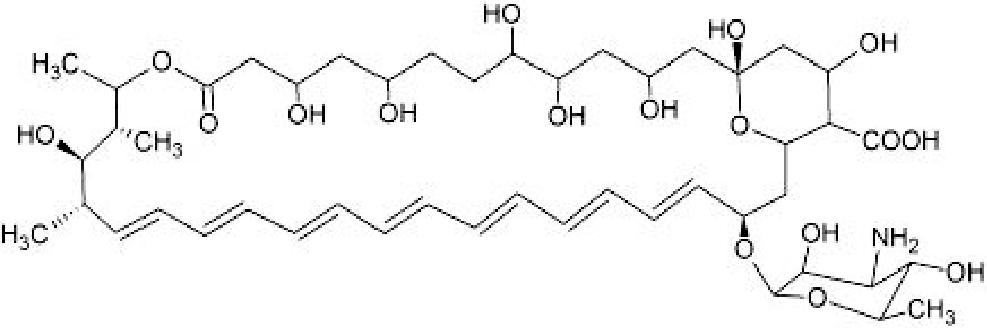
|
0.13 | 0.50 |
All analogs were active under the experimental conditions. It is noteworthy that the use of Amphotericin B is restricted to invasive and disseminated fungal infections and severe adverse reactions, such as nephrotoxicity, limit its application (Karimzadeh et al., 2015; Rocha et al. 2015). Furthermore, cases of cardiotoxicity, hepatotoxicity, neurotoxicity, phlebitis, anemia and others have also been reported due to its use (Karimzadeh et al., 2015).
The A, B, C and D analogs differ from each other due to the group linked to the second benzene ring. According to Anthony et al. (2014), the groups attached to the aromatic ring influence the antifungal activity.
The analysis of the structure–activity relationship of resveratrol analogs showed that the presence of nitro and metoxi group in the para position, favored antifungal activity. In particular, the analog with the nitro group (A) in this position was the most promising antifungal agent.
Anitha et al. (2012) pointed out that the presence of nitrogen in the molecule is important for antifungal activity, as hydrogen bonds may form between cell components and this atom, consequently, interfering in the cell metabolism. The fact that resveratrol does not have such an atom can justify its inactivity in this experiment. Kingsbury et al. (2012) found no resveratrol activity against C. albicans SC5314 in their experiments. On the other hand, Lee and Lee (2015) have observed that at low concentrations, resveratrol was able to permeate the cell membrane without causing damage, and induce apoptosis via metacaspase activation and release of cytochrome c.
Lakum et al. (2014) synthesized Schiff bases and chalcone derivatives and found MICs ranging between 0.78 and 200 μg mL−1 against C. albicans MTCC 183. These studies showed that the compounds presenting halogenated groups were more potent against the specific fungal strain and promoted a selective action. For C. albicans MTCC 183 (MIC = 0.78 µg mL−1), the 2,5-dichlorophenyl group was responsible for the highest activity observed when connected to the triazinyl chalcone derivative.
On the other hand, Patil et al. (2012) found that the Schiff bases presented higher fungistatic activity than the parent compounds against Aspergillus niger MTCC 1881, A. flavus MTCC 1883 and Cladosporium MTCC 1777 when complexed with Co (II), Ni (II) or Cu (II). In this case, the authors attributed the increased activity to the presence of metals, with possible interference in the microorganisms’ membrane permeability and alteration of parameters such as solubility, conductivity and dipole moment. Futhermore, the Cu (II) complexes were more active, followed by Ni (II) and Co (II).
These results are promising, since the analogs in the present study can be complexed with metals, which can lead to increased antifungal activity. That occurs because coordination and chelating change the structure of the molecule, producing more potent compounds. Additionally, there may be a reduction in the polarity of the metal compound, favoring penetration in the fungal cell and the action of the compound (Anitha et al., 2012; Dhanaraj and Johnson, 2014).
3.2 Scanning electron microscopy
The electron micrographs of the active analogs and amphotericin B against C. albicans ATCC 10,231, as well as the one for microorganism that was not subjected to the pharmacological treatment, can be observed in Fig. 1. In all of the pharmacological tests performed, invaginations were observed on the plasma membrane (Fig. 1 - II, III, IV, V and VI).
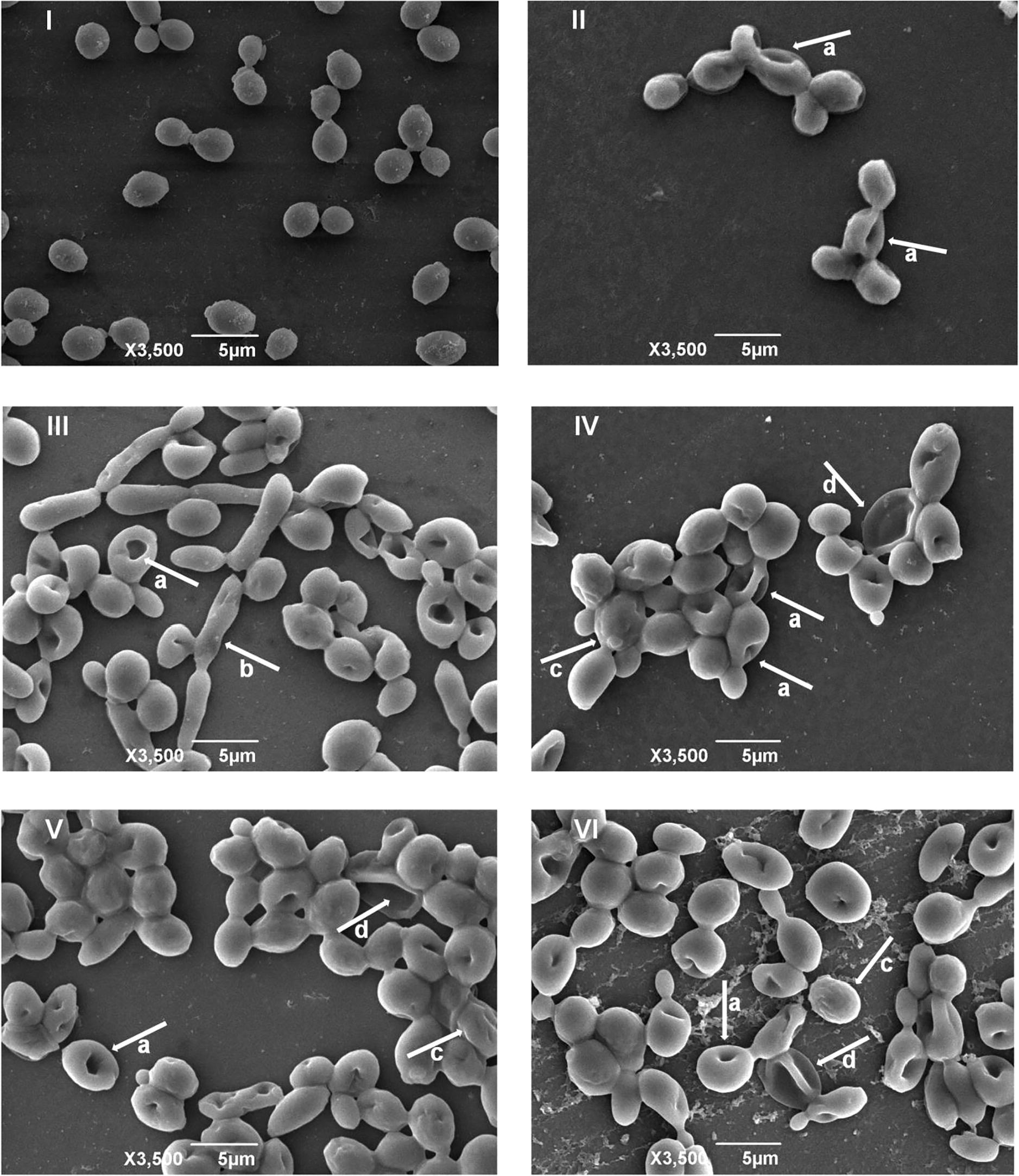
- Electron micrographs of Candida albicans ATCC 10,231 not subjected to pharmacological treatment and submitted to treatment with the resveratrol analogs and the reference drug. I: C. albicans not subjected to pharmacological treatment. II: C. albicans subjected to treatment with amphotericin B. III: C. albicans subjected to treatment with analog A. IV: C. albicans subjected to treatment with analog B. V: C. albicans subjected to treatment with analog C. VI: C. albicans subjected to treatment with analog D. a: invaginations in the plasma membrane. b: pseudo-hyphae. c: irregular plasma membrane. d: leakage of intracellular material.
In Fig. 1 - I, it was possible to notice that the fungal structure presented a well-defined, smooth and regular surface. Treatment with analogs B, C and D (Fig. 1 - IV, V e VI) led to the appearance of roughness on the membrane and cells with leakage of intracellular material. In the treatment with analog A (Fig. 1 - III) pseudohyphae were verified, which may be related to the virulence of the fungus’ mechanisms (Grow et al., 2012).
3.3 Brine shrimp lethality test
For all the tested compounds, a moderate toxicity was observed against the microcrustacean A. salina (Table 2). The analog A, considered in this study as the most promising compound regarding antifungal activity (MIC = 156 µg mL−1), presented the most significant lethality values (LC50 = 112.07 µg mL−1), followed by resveratrol (LC50 = 145.11 µg mL−1). In regard to the reference drug (LC50 = 14.22 µg mL−1), the analog A was 8 times less toxic.
| Compound | LC50 (µg mL−1) after 24 h |
|---|---|
| Analog A | 112.07 ± 3.48* |
| Analog B | 170.39 ± 1.26* |
| Analog C | 200.22 ± 2.69* |
| Analog D | 185.07 ± 3.01* |
| Resveratrol | 145.11 ± 4.30* |
| Thymol | 14.22 ± 2.23 |
Data are expressed as means ± standard errors of the mean (SEM) and are representative of 5 replicates. Data marked with an asterisk are significantly different (p < 0.05) with respect to the positive control (Thymol). Differences were identified using analyses of variance (ANOVA) (IBMS SPSS Statistical 21).
Among the bioassays that aim to guide the drug discovery process, the A. salina assay is considered efficient, quick, cheap and require only from 2 to 20 mg of the compounds (Pimenta et al., 2003; Ajaiyeoba et al., 2006; Dutra et al., 2012). This method has a high correlation with the acute toxicity recorded in rodents and can be predictive to the cytotoxicity in human cell cultures (Hartl and Humpf, 2000). Besides that, it correlates with numerous biological activities (Pompilho et al., 2014; Ngoumfo et al., 2010; El- Menshawi and Linder, 2010) such as antitumoral activity (McLaughlin et al., 1993), activity against Trypanosoma cruzi (Zani et al. 1995), antibacterial activity (Brasileiro et al., 2006) and antifungal activity (Niño et al., 2006). It is commonly considered that compounds which present toxicity to A. salina (LC50 < 250 μg mL−1) also present high potential of having the biological activities cited (Costa et al., 2009). Thus, it is suggested that the compounds hereby studied present this potential.
3.4 Evaluation of cellular viability using MTT assays
Resveratrol and its analogs were evaluated between 10 µM and 100 µM and the results can be observed below (Fig. 2).
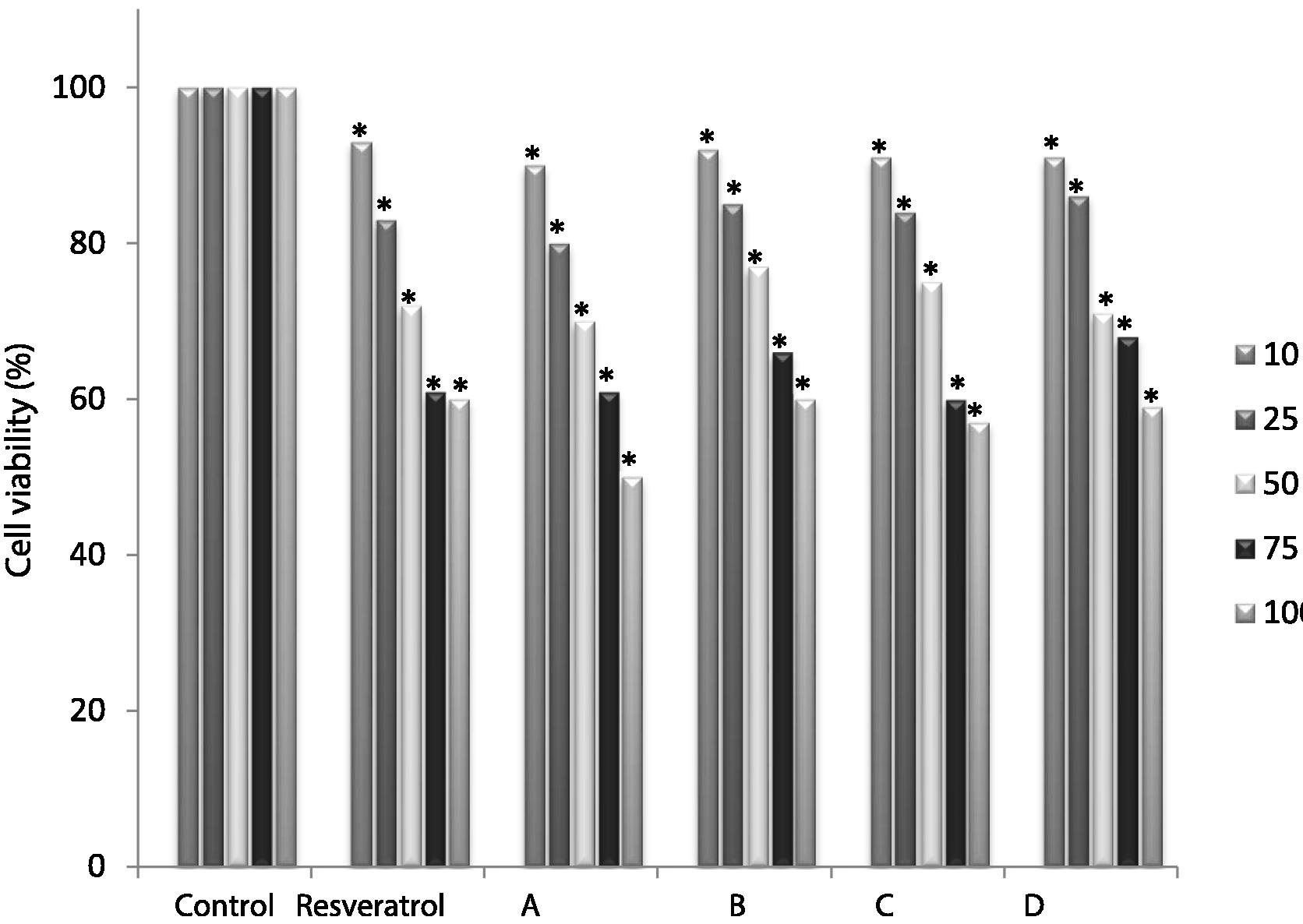
- Human keratinocytes viability after treatment with resveratrol or analogs for 48 h. Non-treated group viability (control) was set as 100%. Values represent mean ± SEM (n = 6). Data marked with an asterisk are significantly different with respect to the non-treated group (p < 0.05). Data were statistically analyzed by variance analysis followed by Bonferroni post hoc test (IBMS SPSS Statistical 21).
Resveratrol is a polyphenol that possesses great biological properties (Baur and Sinclair, 2006). Concerning the keratinocyte cells (HaCaT) exposed in this study to resveratrol and analogs for 48 h with increasing concentrations (10 to 100 µM), the results demonstrate a dose-depending increase in cell mortality (Fig. 2). Comparing the groups treated with resveratrol and the analogs A, B, C and D, statistical differences were verified in all concentrations used in the test (p < 0.05). The cell viability of resveratrol was 60.7% and 68.1% at 100 and 75 µM concentrations, respectively. At 50 µM, the analog B presented a better result than resveratrol (77.4% cell viability). In comparison to the untreated group of cells, there was a 7% drop in the viability with the resveratrol treatment at 10 µM. Analog A presented cell viability in a range from 50.6 (100 μM = 2.28 µg m −1) to 90% (10 μM = 228 µg mL−1). The results obtained in the MTT cytotoxicity assay suggest that all compounds tested had low cytotoxic activity in keratinocyte cell (HaCaT) cultures (Fig. 2). These results are important since both resveratrol and its derivatives can be used in dermal preparations, being effective in wound healing (Chedea et al., 2017; Tsai et al., 2013). According to Almeida et al. (2014), the compounds can be classified as not presenting cytotoxic activity (NA), as presenting low activity (LA), when the cell growth inhibition ranges from 0 to 50%, as presenting moderate activity (MA), when the inhibition ranges from 50 to 75% and as presenting high activity (HA), when the cytotoxicity is higher than 75%.
An in vitro study conducted by Clement et al. (1998) showed resveratrol as a potent natural chemopreventive agent in the HL60 cell line. This happened due to a better expression of the CD95L in HL60 cells treated with resveratrol. On the other hand, resveratrol presented low cytotoxicity to normal human lymphocyte cell cultures (PBLs) in concentrations up to 32 µmol L−1, suggesting CD95L specificity in resveratrol antitumoral activity, as well as its therapeutic efficacy.
Thus, these results are promising for the development of new drugs since other modifications in the structures of the analogs can be performed in order to enhance their fungicidal activity. More studies are needed to establish the mechanism of action of the new compounds.
4 Conclusions
The lowest drug concentration to inhibit fungal growth was observed for analog A. With regard to the fungicidal concentration, A, B and D showed concentrations statistically similar and lower than the other analogs. All analogs led to structural changes in the Candida albicans ATCC 10,231 membrane.
Resveratrol and its analogs did not interfered in cell viability of human keratinocyte cells (HaCaT) even in high concentrations. They also presented moderate cytotoxic activity against A. salina, which suggested good biological activity.
Finally, it was observed that the presence of nitro and methoxy groups in the para position of the second benzene ring favored antifungal activity and that this first group was responsible for the smaller doses which inhibited or killed the microorganism.
Based on the presented results, the resveratrol analogs can be promising in the development of new antifungal agents.
Acknowledgment
The authors gratefully acknowledge FAPEMIG, CNPq, CAPES and PROPESQ/UFJF for financial support.
References
- In vitro cytotoxicity studies of 20 plants used in Nigerian antimalarial ethnomedicine. Phytomedicine. 2006;13:295-298.
- [Google Scholar]
- Atividade antioxidante, citotóxica e antimicrobiana de Annona vepretorum Mart. (ANNONACEAE) Rev. Bras. Frutic.. 2014;36:258-264.
- [Google Scholar]
- Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis.. 2012;54:1110-1122.
- [Google Scholar]
- Spectroscopic studies and biological evaluation of some transition metal complexes of azo Schiff-base ligand derived from (1-phenyl-2,3-dimethyl-4-aminopyrazol-5-one) and 5-((4-chlorophenyl)diazeny)-2-hydroxy benzaldehyde. Spectrochim. Acta A.. 2012;96:493-500.
- [Google Scholar]
- Regioselective synthesis of 1,4-disubstituted 1,2,3-bistriazoles and their antifungal and a anti-oxidant evaluation. AJBPS.. 2014;4:9-13.
- [Google Scholar]
- Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr. Metab.. 2016;13(89):1-11.
- [Google Scholar]
- Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug discov.. 2006;5:493-506.
- [Google Scholar]
- Antimicrobial and cytotoxic activities screening of some Brazilian medicinal plants used in Governador Valadares district. Rev. Bras. Cienc. Farm.. 2006;42:195-202.
- [Google Scholar]
- Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996-1002.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), M27-A2, 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, 2nd (ed.) vol. 22, pp. 15. CLSI, USA.
- Synthesis, antitubercular and leishmanicidal evaluation of resveratrol analogues. J. Bras. Chem Soc.. 2016;27:2161-2169.
- [Google Scholar]
- Resveratrol lacks antifungal activity against Candida albicans. World J. Microbiol. Biotechnol.. 2012;28:2441-2446.
- [Google Scholar]
- Estudos farmacognósticos, fitoquímicos, atividade antiplasmódica e toxicidade em Artemia salina de extrato etanólico de folhas de Montrichardia linifera (Arruda) Schott Araceae. Braz. J. Pharmacogn.. 2009;19:834-838.
- [Google Scholar]
- Vulvovaginal candidiasis due to non albicans Candida: its species distribution and antifungal susceptibility profile. Int. J. Curr. Microbiol. Appl. Sci.. 2013;2:323-328.
- [Google Scholar]
- Synthesis, characterization, electrochemical and biological studies on some metal (II) Schiff base complexes containing quinoxaline moiety. Spectrochim. Acta Mol. Biomol. Spectros.. 2014;118:624-631.
- [Google Scholar]
- Chemical composition and cytotoxicity activity of the essential oil of Pterodon emarginatus. Rev. Bras. Farmacogn.. 2012;22:971-978.
- [Google Scholar]
- Screening of natural products for therapeutic activity against solid tumors. Indian J. Exp. Biol.. 2010;48:258-264.
- [Google Scholar]
- M.H. Farzaei R. Rahimi S. Nikfar M. Abdollahi 2017. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients Pharmacol. Res. Available from <https://ac.els-cdn.com/S1043661817308125/1-s2.0-S10436 61817308125-main.pdf?_tid=2c7fdbda-f63b-11e7-bcb7-00000aab0f02&acdnat=1515612 147_7f48a4fe065c6e1a16a5d83347c5bb39> Access (01 10 2018.).
- Comprehensive apporach for the detection of antifungal compounds using a susceptible strain of Candida albicans and confirmation of in vivo activity with the Galleria mellonella model. Phytochem. Phytochem.. 2014;105:68-78.
- [Google Scholar]
- Essential oil composition and antimicrobial activity of Sphallerocarpus gracilis seeds against selected food-related bacteria. Food Control.. 2011;22:517-522.
- [Google Scholar]
- Candida albicans morphogenesis and host defense: discriminating invasion from colonization. Nat. Rev. Microbiol.. 2012;10:112-122.
- [Google Scholar]
- Analysis of validamycin as a potential antifungal compound against Candida albicans. Int. Microbiol.. 2013;16:217-225.
- [Google Scholar]
- Toxicity assessment of fumonisins using the brine shrimp (artemia salina) bioassay. Food Chem. Toxicol.. 2000;38:1097-1102.
- [Google Scholar]
- Preventing or attenuating amphotericin B nephrotoxicity with dopamine receptor agonists: a literature review. TIPS. 2015;1:129-138.
- [Google Scholar]
- Calcofluor white combination antifungal treatments for Trichophyton rubrum and Candida albicans. Plos One. 2012;7:e39405.
- [CrossRef] [Google Scholar]
- Novel s-triaziny Schiff base/chalcone congeners: ration, synthesis, antimicrobial and anti-TB evaluation. Int. Lett. Chem. Phys. Astron.. 2014;19:56-73.
- [Google Scholar]
- Novel antifungal mechanism of resveratrol: apoptosis inducer in Candida albicans. Curr. Microbiol.. 2015;70:383-389.
- [Google Scholar]
- Candida and invasive candidiasis: back to basics. Eur. J. Clin. Microbiol. Infect. Dis.. 2012;31:21-31.
- [Google Scholar]
- Azastilbene analogs as tyrosinase inhibitors: new molecules with depigmenting potential. Sci. World J. 2013:1-7.
- [Google Scholar]
- Simple bench-top bioassays (BS & PD) for discovery of plant antitumor compounds-review of recent progress in human medicinal agents from plants. Nova York: Kinghorn & Balandrini; 1993. p. :112-137.
- Brine Shrimp: a convenient general bioassay for active plant constituents. Planta Med.. 1982;45:31-34.
- [Google Scholar]
- Rapide Colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunolog. Meth.. 1983;65:55-63.
- [Google Scholar]
- In vitro cytotoxic activity of isolated acridones alkaloids from Zanthoxylum leprieurii Guill. et Perr. Bioorg. Med. Chem.. 2010;15:3601-3605.
- [Google Scholar]
- Antibacterial, antifungal and cytotoxic activities of eight Asteraceae and two Rubiaceae plants from colombian biodiversity. Braz. J. Microbiol.. 2006;37:566-570.
- [Google Scholar]
- In vitro antibacterial, antifungal, and DNA cleavage studies of coumarin Schiff bases and their metal complexes: synthesis and spectral characterization. Med. Chem. Res.. 2012;21:4017-4027.
- [Google Scholar]
- Reprod. Fert. Dev.. 2016;29:183.
- Biological screening of Annonaceous brazilian medicinal plants using Artemia salina (Brine Shrimp Test) Phytomedicine. 2003;2003(10):209-212.
- [Google Scholar]
- Photoprotective activity of resveratrol analogues. Bioorg. Med. Chem.. 2013;15:964-968.
- [Google Scholar]
- Bioatividade de três espécies vegetais nativas da Floresta Atlântica brasileira frente ao microcrustáceo Artemia salina. Rev. Bras. Pl. Med.. 2014;16:473-480.
- [Google Scholar]
- Incidence, predictors, and impact on hospital mortality of amphotericin B nephrotoxicity defined using newer acute kidney injury diagnostic criteria. AAC. 2015;59:4759-4769.
- [Google Scholar]
- Resveratrol and analogues: a review of antioxidant activity and applications to human health. Rec. Pat. Food Nutrit. Agri.. 2013;5:144-153.
- [Google Scholar]
- Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol.. 2014;14:182-195.
- [Google Scholar]
- Molecular caracterization of azole resistanc mechanisms in Candida albicans clinical isolates from HIV-infectecd patients in India. IJPSR. 2014;5:3924-3931.
- [Google Scholar]
- Evaluation of antifungal activity of Physalis alkekengi L. extracts on Microsporum canis, Candida albicans, Trichophyton mentagrophytes and Nocardia asteroids. Middle East. J. Sci. Res.. 2013;13:926-929.
- [Google Scholar]
- 3,5,4′-trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial–mesenchymal transition. Toxicol. Appl. Pharmacol.. 2013;272:746-756.
- [Google Scholar]
- A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer. 1987;56:279-285.
- [Google Scholar]
- Use of isothermal microcalorimetry to quantify the influence of glucose and antifungals on the growth of Candida albicans in urine. J. Appl. Microbiol.. 2013;115:1186-1193.
- [Google Scholar]
- Brine shrimp lethality assay as a pre-screening system for anti-Trypanosoma cruzi. Phytomedicine. 1995;2:47-50.
- [Google Scholar]







