Translate this page into:
Essential oil of Xylopia aethiopica from Cameroon: Chemical composition, antiradical and in vitro antifungal activity against some mycotoxigenic fungi
⁎Corresponding author. alphonsesoker@gmail.com (Alphonse Sokamte Tegang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aim of this work was to evaluate the chemical composition, antiradical activity and antifungal activity of Xylopia aethiopica essential oil from Cameroon against five mycotoxigenic fungal strains responsible for the biodegradation of foodstuffs. The Clevenger apparatus was used for the extraction of essential oil from dried fruits, which was subsequently analyzed by CG and CG/ MS for determination of its chemical profile. The incorporation method and the method using the DPPH radical were used respectively for the in vitro evaluation of the antifungal and antioxidant activity of the essential oil of X. aethiopica. The yield of essential oil was 4.2% (v/w). The main components of Xylopia aethiopica essential oil were β-pinene (32.16 ± 3.69%), β-phellandrene (10.71 ± 3.05%), Z-γ-bisabolene (10.07 ± 2.61%) and α-pinene (7.39 ± 1.69%). The essential oil of X. aethiopica showed a low antiradical activity (SC50 = 594.58 ± 57.37 μg/mL) as compared to that of BHT (SC50 = 65.03 ± 0.99 μg/mL). The essential oil has a good antifungal activity against Aspergillus niger and Fusarium oxysporium with a minimum inhibitory concentration of 3000 ppm for the two fungal strains and a minimum fungicidal concentration of 3000 and 4000 ppm respectively. The essential oil of X. aethiopica did not exert a fungicidal effect against A. flavus, A. fumigatus and A. versicolor, for which the MIC was 4000 ppm. The most resistant fungal strain was Aspergillus fumigatus. This study shows that the essential oil of X. aethiopica has a real potential as an antifungal agent for controlling fungal growth on foodstuffs.
Keywords
Essential oil
Xylopia aethiopica
Chemical composition
Antioxidant activity
Antifungal activity
1 Introduction
For many developing countries, cereals are the staple food for populations because of their high food value and ease of cultivation (McKevith, 2004). To cover food needs throughout the year, part of the harvest is stored. However, the lack of system controls of preservation by producers and the bad handling of crop products expose cereals to the problems of mold contaminations. This could lead to bio-deterioration resulting to reduce food values (nutrients loss) and market quality of cereals (color, appearance, texture and taste). The post-harvest losses vary from one region to another, but generally they range between 5% and 40% of total cereals production, which is a very significant shortfall for a grower usually with limited income (Hodges et al., 2013). The northern region of Cameroon, due to its temperate climate, is very favorable to the development of a large number of mold species which are able to synthesize and excrete toxic secondary metabolites (mycotoxins), which could seriously affected the health of consumers upstream of the food chain (humans and animals). The main species producing mycotoxins belong mainly to the genera Aspergillus, Penicillium, and Fusarium (AFSSA, 2006).
Free radicals produced naturally by the oxidation of food substances during technological treatments and sometimes throughout the preservation of manufactured products constitute a non-negligible concern for agro-food industries. These free radicals can initiate in the organism of the consumers, chain reactions, leading to the oxidative stress, which can damage besides DNA, proteins and lipids (Ayepola et al., 2014).
To control the damage caused by mold and the oxidation of foodstuffs, producers, and the food industries use synthetic chemicals (BHT, BHA, benzoic acid, etc.) whose use is more and more limited due to their side effects on health (liver cancer, etc…) and food quality (Witschi, 1986). Consumers have become suspicious of products so preserved, and claim of products that are not risky for health. To satisfy their expectations, the search for alternative methods of preservation by natural substances has become a great necessity.
The renewed interest and valorization of essential oils are due to the fact that they have excellent antimicrobial and antioxidant properties and are potential natural preservatives (Celikel and Kavas, 2008). In Cameroon, Xylopia aethiopica (Annonaceae) commonly known as Ethiopian pepper is widely exploited as a spice. Its seeds have an aromatic pungent taste and dried fruits are important as flavourings. Medicinally, the fruit is used for the treatment of a cough, stomachache, dizziness, amenorrhea, bronchitis, dysentery, headache, neuralgia, carminative, female sterility, purgative, biliousness and skin infections (Okwu, 2001). Several studies have shown that X. aethiopica extracts possess antibacterial and antifungal activities. Asekun and Adeniyi (2004) reported that X. aethiopica essential oil from Nigeria inhibited the growth of five fungi strains (Stellocapella madis, Candida albicans, Aspergillus flavus, Aspergillus ocheraccus and Fusarium oxysporum). Thus, many researches has been conducted to evaluate the antibacterial (Tatsadjieu et al., 2003) and anti-insecticidal (Kouninki et al., 2007) activities of X. aethiopica from Cameroon whereas very few were interested in the evaluation of its antifungal potential.
The aim of this study was to evaluate the antioxidant potential of the essential oil of X. aethiopica as well as its antifungal effectiveness to control the growth of A. niger, A. fumigatus, A. versicolor, F. oxysporium, and A. flavus.
2 Materials and methods
2.1 Plant material
Dried fruits of X. aethiopica were collected in Bafoussam (West Cameroon) in July 2015. They were identified by Mr Nikio Kaji, a botanist of the National Herbarium of Yaoundé, Cameroon (located in West Africa between latitude 6°00′N and longitude 12°00′E with the maximum altitude of 4095 m above sea level). The voucher specimen of this plant were deposited (53821/HNC).
The samples were dried at 40 °C for 48 h in a ventilated dryer and then crushed using a mixer to have small particle size used for extraction.
2.2 Fungal strains
The fungal strains used include Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Aspergillus versicolor and Fusarium oxysporium. They were chosen because of their high frequency of contamination of food products and their involvement in human and animal pathology. All strains were provided by the Microbiology Laboratory of the National High School of Agro-Industrial Sciences of the University of Ngaoundéré, Cameroon.
Throughout the study, the fungal strains were maintained at 4 °C in tubes containing inclined Sabouraud Dextrose Agar medium (SDA) supplemented with chloramphenicol. For their reactivation, the fungal strains were transferred to the surface of a fresh SDA medium, contained in Petri dishes. The Petri dishes were incubated for 7 days at 25 ± 2 °C. After incubation, fungal spores were scraped from the surface of each dish with a sterile material, and introduced into a sterile tween solution (1%), further adjusted to give a final concentration of 106 spores/ml before being seeded uniformly to the surface of SDA medium. The Petri dishes were incubated for an additional 48 h at 25 ± 2 °C, and a mycelial disk was removed for the antifungal test (Yaouba et al., 2011).
2.3 Extraction of essential oil
100 g of dried and crushed plant material was introduced into a flask and 500 ml of distilled water was added. The Clevenger was adapted to the upper part of the flask and the mixture was boiled at 95 °C for 4 h (Oliveira et al., 2017). After condensation of the heterogeneous vapours of water, the essential oil harvested was subsequently dried with an anhydrous sodium sulfate column and stored at 4 °C in a hermetically sealed brown glass vial until used. The extraction yield was calculated and estimated as a percentage (v/w) based on the weight of the dried fruit used for extraction.
2.4 Chemical analysis of essential oil
Analysis of essential oil was performed by gas chromatography (GC) and gas chromatography coupled with mass spectrometry (GC/MS).
2.4.1 Gas chromatography analysis (GC)
The chemical composition of the essential oil was analyzed using Varian CP-3380 GC (Varian, Inc. USA) coupled to an injector. It was equipped with a DB-5 capillary column (30 mm 0.25 mm d.i; film thickness: 0.25 μm), a FID detector set at 200 °C and a split-splitless injector set at 250 °C. The split (leakage ratio: 1/50, flow rate: 66 ml min−1) injection mode was used. Nitrogen was used as carrier gas with a flow rate of 1.7 ml/min. The increasing temperature in the oven during the analysis was set up from 50 to 200 °C with a speed of 5 °C/min. The essential oil was dissolved in hexane (1/10 v/v) and 1ul of this solution was injected into the chromatograph.
2.4.2 Gas Chromatography–Mass Spectrometry (GC/MS)
The GC/MS analyses were carried out using a Hewlett-Packard chromatograph equipped with an automatic injector and an HP1 column (30 mm 0.25 mm d.i, film thickness 0.25 μm), coupled to a mass detector (GC-quadrupole MS system, model 5970). The programming of temperature involves raising from 70 to 200 °C at a speed of 10 °C/min. Helium was used as carrier gas with a flow rate of 0.6 ml/min. The injection is carried out by the split mode with an injection temperature set at 200 °C. The molecules are bombarded by an electron beam of 70 eV and the detection is performed by an analyzer quadrupole filter. The mass spectra obtained by electron impact were acquired over the mass range of 35–350 m/z. The ion source temperature is 200 °C.
The identification of essential oil constituents was performed by comparing their retention index (IK) with those of the reference data obtained in the literature review (Adams, 2007). The IK are determined by injecting a mixture of the C6 to C24 n-alkanes under the same operating conditions.
2.5 Antifungal activity
The antifungal assays were carried out using the incorporation method as described by De Billerbeck et al. (2001). Due to the non-miscibility of the essential oil with water and thus with the culture medium, an emulsification of the essential oil was previously carried out using an aqueous solution of dimethylsulphoxide (DMSO). The essential oil is then incorporated at a different volume into the Sabouraud Dextrose Agar (SDA) medium to have desired concentrations (1000 ppm, 2000 ppm, 3000 ppm, 4000 ppm, 5000 ppm, 6000 ppm and 7000 ppm). The medium thus supplemented is poured into Petri dishes of 90 mm in a proportion of 20 ml and left for about 15–30 min for solidification. For each fungal strain, a mycelial disc of 6 mm in diameter is taken from a preculture of 48 h and seeded directly in the center of each dish. The positive control of fungal growth is carried out according to the same procedure for dishes containing the culture medium not supplemented with essential oil. The dishes are incubated in an inverted position at 25 ± 2 °C and the diameter is measured along two perpendicular lines passing through the center of the plate, at regular time intervals (48 h) until the stop of control radial growth. The minimum inhibitory concentration (MIC) was determined and is equivalent to the lowest concentration of essential oil in which no growth was observed. The following formula was used to calculate the antifungal index (AI):
Dt = diameter of growth zone in the test plate
Dc = diameter of growth zone in the control plate.
The discs exhibiting no fungal growth were seeded again in Petri dishes containing the SDA medium not supplemented with essential oil. Seven days after incubation at 25 ± 2 °C, the Petri dishes in which there was no resumption of growth are noted, which makes it possible to determine the minimum fungicidal concentration (MFC).
2.6 Evaluation of antiradical activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay was performed to assess the antiradical activity of essential oil (Brand-Williams et al., 1995). Different volumes of essential oil were introduced into tubes, and the final volume was made up to 2 ml by addition of the methanolic solution of 100 μM DPPH so as to have essential oil concentrations range from 9.77 to 80,000 µg/mL. The BHT used as a reference antioxidant was evaluated according to the same procedures. The reaction mixture is subsequently incubated in the dark and at room temperature for 60 min. Absorbance is measured at 517 nm using a spectrophotometer (RAYLEIGH VIS-723N). The percentage of free radical trapping (AA) is calculated relative to the control containing only 100 μM DPPH solution and methanol, using the following formula:
ODc = Absorbance of control
ODt = Absorbance of test
AA (%) = Antioxidant Activity
The SC50 representing the concentration of antiradical substance allowing for trapping 50% of the free radical and obtained graphically from the plot of the trapping curves of the free DPPH radical was used as an element of comparison of the antiradical activity.
2.7 Statistical analysis
Each assay was done in triplicate to minimize the experimental error, and the average was calculated. Data were analyzed by Statistica .06, Statistical package (Statsoft, 1995). Differences between means were tested using Duncan Multiple Range Test with p ≤ 0.5.
3 Results and discussion
3.1 Chemical analysis
The extraction yield of the essential oil of X. aethiopica was 4.2%. These results are comparable to those previously obtained by Bakarnga-Via et al. (2014) who found extraction yields of 3.57% and 4.68%, for dry fruits of X. aethiopica from Chad and Cameroon, respectively. But, the extraction yields of the essential oil of dry fruits of X. aethiopica obtained from local market in Keffi, Nasarawa State, Nigeria, was too lower; 1.2% (Olonisakin et al., 2007).
Besides, the analysis of the chemical composition of the essential oil of X. aethiopica allowed to identified 70 compounds listed in their order of elution from DB-5 column, the results are presented in Table 1. All compounds identified are divided into four major chemical classes: monoterpenes hydrocarbons (69.41%), oxygenated monoterpenes (8.42%), sesquiterpenes hydrocarbons (17.58%) and oxygenated sesquiterpenes (1.73%). The major compounds identified are β-pinene (32.16%), β-phellandrene (10.71%), α-pinene (7.39%), α-phellandrene (6.8%) for the hydrocarbon monoterpene class, and finally, the Z-γ-bisabolene (10.07%) belonging to the oxygenated monoterpenes. The chemical composition analysis of X. aethiopica essential oil from Cameroon shows that it is represented only by a single chemotype since β-pinene always appears as the major compound. However, in Sudan, two other chemotypes have been postponed. Indeed, EL-Kamali and Adam (2009), Elhassan et al. (2010) have examined the chemical composition of essential oil of the dry fruits of X. aethiopica obtained from Sudan and found that the major component was the 4-isopropylbenzyl alcohol (16.67%) and 4-terpineol (11.30%), respectively.
Number
Compounds
IK
Percentage (%)
1
α-thujene
924
1.92 ± 0.08
2
α-pinene
931
7.39 ± 1.69
3
α-fenchene
946
0.12 ± 0.04
4
ß-pinene
977
32.16 ± 3.69
5
α-phellandrene
994
6.8 ± 1.62
6
α-terpinene
1006
0.61 ± 0.28
7
p-cymene
1013
0.44 ± 0.16
8
β-phellandrene+1,8-cineole
1019
0.03 ± 0.01
9
Z-β-ocimene
1027
1.12 ± 0.13
10
limonene
1034
0.68 ± 0.11
11
β-Phellandrene
1038
10.71 ± 3.05
12
cis-β-ocimene
1046
2.37 ± 0.47
13
γ-terpinene
1055
2.09 ± 0.35
14
Camphenilone
1060
0.18 ± 0.05
15
p-cymenene
1075
1.94 ± 0.23
16
terpinolene
1079
0.39 ± 0.13
17
ß-thujone
1100
0.46 ± 0.11
Total Monoterpenes Hydrocarbons
69.41 ± 2.68
18
cis-p-menth-2-en-1-ol/
1110
0.22 ± 0.09
19
Nopinone
1112
0.19 ± 0.08
20
myroxyde E
1130
0.24 ± 0.06
21
isopulegol
1141
0.18 ± 0.02
22
β-pinene oxide
1145
0.02 ± 0.01
23
p-mentha-1,5-dien-8-ol
1151
0.44 ± 0.08
24
terpinene-4-ol
1160
0.11 ± 0.05
25
cryptone
1166
0.03 ± 0.01
26
α-terpineol
1171
0.05 ± 0.03
27
myrtenal
1175
0.13 ± 0.04
28
methyl chavicol
1198
0.04 ± 0.02
29
verbenone
1182
4.67 ± 1.52
30
trans carveol
1186
0.06 ± 0.02
31
(R)-(+)-beta citronellol
1203
0.74 ± 0.21
32
(E)-citral
1207
0.21 ± 0.07
33
cuminal
1216
0.2 ± 0.08
34
Carvone
1223
0.12 ± 0.03
35
Peryllaldehyde
1248
0.33 ± 0.11
36
bornyl acetate
1270
0.04 ± 0.01
37
thymol
1277
0.35 ± 0.12
38
2E,4Z-decadienal
1301
0.05 ± 0.01
Total Oxygenated Monoterpenes
8.42 ± 1.39
39
longycyclene
1358
1.37 ± 0.15
40
α-copaene
1363
0.2 ± 0.08
41
β-bourbonene
1372
0.63 ± 0.2
42
α-cubebene
1377
0.04 ± 0.02
43
cyperene
1386
0.13 ± 0.05
44
ß-elemene
1395
0.49 ± 0.15
45
Z-caryophyllene
1405
0.47 ± 0.13
46
cis-prenyl limonene
1430
0.6 ± 0.14
47
β-copaene
1444
0.08 ± 0.02
48
aromadendrene
1447
0.21 ± 0.08
49
α-humulene
1457
0.95 ± 0.23
50
trans-prenyl limonene
1459
0.09 ± 0.02
51
aromadendr-9-ene
1467
0,2 ± 0.06
52
germacrene D
1485
0.19 ± 0.05
53
Z-γ-bisabolene
1501
10.07 ± 2.61
54
δ-cadinene
1509
0.13 ± 0.05
55
E-γ-bisabolene
1518
0.87 ± 0.1
56
α-cadinene
1523
0.12 ± 0.04
57
α-calacorene
1528
0.14 ± 0.04
58
selina-3,7(11)-diene
1535
0.56 ± 0.12
59
Germacrene-B
1550
0.04 ± 0.02
Total Sesquiterpenes Hydrocarbons
17.58 ± 2.03
60
elemol
1544
0.04 ± 0.01
61
caryophyllene oxyde
1568
0.1 ± 0.03
62
thujopsan-2-α-ol
1574
0.56 ± 0.13
63
neryl isovalerate
1596
0.19 ± 0.05
64
epi-globulol
1606
0.16 ± 0.04
65
epoxy-allo alloaromadendrene
1613
0.06 ± 0.02
66
isospathulénol
1632
0.04 ± 0.01
67
α-eudesmol
1642
0.25 ± 0.05
68
vélérianol
1656
0.11 ± 0.05
69
α-cadinol
1676
0.14 ± 0.04
70
E-apritone
1704
0.08 ± 0.02
Total Oxygennated Sesquiterpenes
1.73 ± 0.22
Total
97.14 ± 1.95
Several factors can influence the yield of the essential oil and its chemical composition, including plant age, place and time of harvest, and presence of parasites (Zantar et al., 2015).
3.2 Antifungal activities
3.2.1 Effect of X. aethiopica essential oil on all fungal strains
The effects of X. aethiopica essential oil on mycelial growth of Aspergillus niger, Aspergillus fumigatus, Aspergillus versicolor, Aspergillus flavus and Fusarium oxysporium are expressed in antifungal index and are illustrated in Fig. 1. These figures show that for all fungal strain, the antifungal index increases with the increase in the concentration of essential oil, indicating its antifungal activity. Furthermore, throughout the incubation period and for a defined concentration of essential oil of X. aethiopica, significant differences (p > 0.05) was observed in the antifungal index against A. flavus, F. oxysporium, A. versicolor, A. fumigatus and A. niger. This shows the variability of the resistance level exerted by each strain under the effect of the supplementation of the culture medium with the essential oil.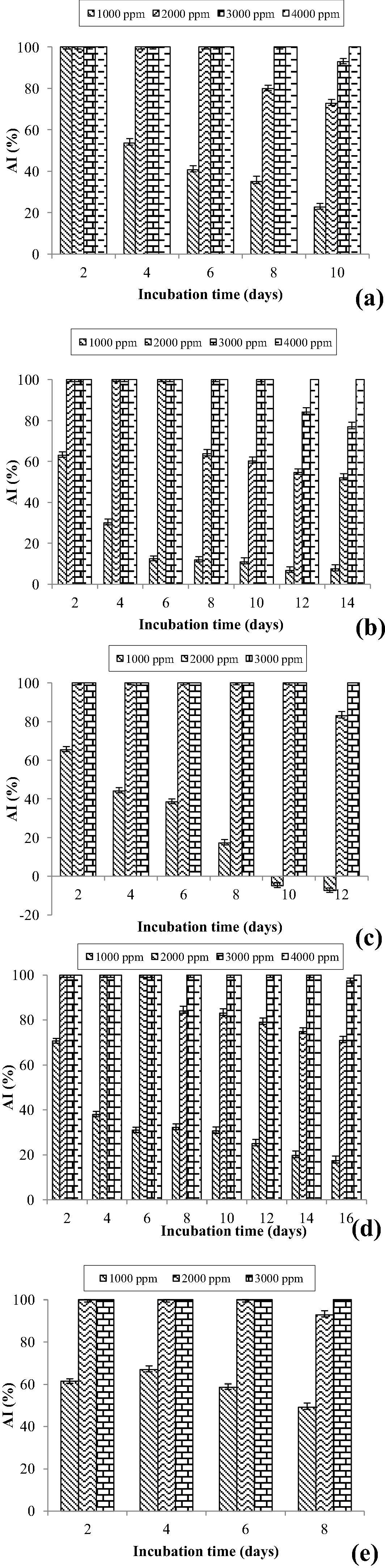
Antifungal Index of X. aethiopica essential oil against: A. flavus (a); A. fumigatus (b); A. niger (c); A. versicolor (d) and F. oxysporium (e).
3.2.2 Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
Last day of the incubation time, which was variable from one strain to another (8–16 days) and who was defined by the stopping of the radial growth in the control Petri dish, the minimum inhibitory concentration was determined for all fungal strains, and it is presented in Table 2.
Fungal species
MIC (ppm)
MFC (ppm)
Aspergillus flavus
4000
>6000
Aspergillus fumigatus
4000
>6000
Aspergillus versicolor
4000
>6000
Aspergillus niger
3000
3000
Fusarium oxysporium
3000
4000
X. aethiopica essential oil exhibited the lowest MIC values with 3000 ppm against A. niger, F. oxysporium and 4000 ppm against A. flavus, A. versicolor and A. fumigatus. This table also presents the minimum fungicidal concentrations of the essential oil of X. aethiopica. It can be observed that this essential oil exhibits fungicidal activity on some fungal strains. Indeed, the essential oil of X. aethiopica showed a fungicidal effect on A. niger and F. oxysporium from 3000 and 4000 ppm respectively. However, A. flavus, A. versicolor and A. fumigatus exhibited resistance up to 6000 ppm of essential oil concentration.
The antimicrobial activity of X. aethiopica essential oil can be attributed to its high content of bioactive compounds such as β-pinene (32.16%) and α-pinene (7.39%) which have been isolated, purified and extensively studied for their antimicrobial activity (Lis-Balchin et al., 1998; Magiatis et al., 1999; Karioti et al., 2004). This essential oil, due to its chemical complexity, contains minor compounds such as monoterpenes (p-cymene, γ-terpinene, terpinolene, and α-terpinene) and sesquiterpenes (β-elemene, α-humulene), whose antifungal activity has been proven and who can interact synergistically to potentiate the biological effectiveness of essential oil against certain fungal strains as reported by Rattanachaikunsopon and Phumkhachorn (2010). The antimicrobial mechanism of these monoterpenes is to cause significant damage to the membrane of the microbial cell (Cristani et al., 2007).
Soro et al. (2010) showed that essential oil of dry fruits of X. aethiopica from Ivory Coast; totally inhibit the growth of Fusarium oxysporum as from 4000 ppm. This could be attributed to the high inhibitory capacity of its chemical compounds and their ability to be metabolized into culture medium in products strongly inhibiting activity of hydrolytic enzymes. Indeed, β-pinene and α pinene are oxidable in quinone, inhibiting the hydrolytic enzymes of moulds (Kouninki et al., 2007; Da Silva et al., 2012). For López-Malo et al. (2005), the phenolic terpenes act by binding to the amines and hydroxyl amines groups of the microbial membrane proteins causing alteration of the membrane permeability and leakage of intramembranous constituents.
Fig. 1c also shows that when the medium is supplemented with a low concentration (1000 ppm) of the essential oil of X. aethiopica, A. niger can, after a longer latency period (8th day), have a higher growth compared to the control. This results in a negative antifungal index observed after the 8th day of incubation. Indeed, A. niger is known for its ability to produce certain lipases that could cut fatty acids into smaller molecules and use them as a nutrient for their growth.
3.3 Antiradical activities of essential oil
The DPPH assay was used to evaluated the scavenging activity of the essential oil of X. aethiopica and that of BHT and the results are reported in Table 3. It is apparent from this table that the increase in the concentration of essential oil leads to an increase in the percentage of trapping of the free DPPH radical. This shows that it possesses, like the reference antioxidant, an anti-radical activity. To compare their antioxidant potential, the SC50, which refers to the minimum concentration of anti-radical substance capable of trapping 50% of free radicals was used. The lower its value, the more the substance tested has a good antiradical efficacy. The graphical plot was used to determine the SC50 values of the essential oil and the BHT: 594.58 ± 57.37 μg/mL and 65.03 ± 0.99 μg/mL, respectively. This shows that the anti-radical activity of the essential oil is very low compared to that of the reference antioxidant. As has been shown in several studies, the biological activity of essential oils is closely related to their chemical profile. Thus, the low antioxidant potential of the essential oil of X. aethiopica could be due to its low content of γ-terpinene, p-cymene, thymol known for their considerable antioxidant activity and its high content of monoterpenes hydrocarbons (69.41%) and sesquiterpenes hydrocarbons (17.58%). The terpene compounds are endowed with a low antioxidant activity. They are incapable of giving their hydrogen atom necessary for the reduction of the free DPPH radical, and they have a low solubility in alcoholic medium for DPPH test (Mata et al., 2007).
Concentration (µg/mL)
Essential oil
BHT
Inhibition percentage (%)X
Inhibition percentage (%)X
0.0
0.0
0.0
9.77
–
11.91 ± 0.61
19.53
–
15.85 ± 0.49
39.06
–
37.53 ± 0.72
78.13
–
54.07 ± 0.23
156.25
17.63 ± 1.05
60.41 ± 0.44
312.50
34.59 ± 1.82
76.01 ± 0.34
625
51.86 ± 1.98
78.32 ± 0.86
1250
61.45 ± 1.35
79.24 ± 0.88
2500
65.36 ± 0.57
82.81 ± 0.29
5000
66.75 ± 0.74
83.26 ± 0.26
10,000
67.58 ± 0.52
84.00 ± 0.52
20,000
79.52 ± 1.47
–
40,000
72.51 ± 1.71
–
80,000
72.60 ± 0.5
–
SC50 (µg/mL)Y
594.58±a
65.03±b
Konan et al. (2009) showed that the antiradical potential of dry fruits of X. aethiopica essential oil (SC50 = 4100 µg/mL) which originated from Langossou, in Ivory Coast was close to that of the reference antioxidant solution which was the α-tocopherol (SC50 = 4300 µg/mL).
4 Conclusion
The results of this study showed that the essential oil of X. aethiopica consists mainly of monoterpenes hydrocarbons and sesquiterpenes hydrocarbons. It has a good antifungal activity and could be used effectively to control the fungal growth of A. niger and F. oxysporium for which it has exerted a fungicidal activity at 3000 and 4000 ppm, respectively. The other fungal species (A. flavus, A. versicolor and A. fumigatus), despite their complete inhibition from 4000 ppm, showed a high resistance to the essential oil of X. aethiopica, particularly A. fumigatus, which proved to be the most resistant fungal strain. Concerning the antioxidant activity of the essential oil X. aethiopica, it was found to be very low compared to the reference antioxidant. To be used as a preservative, additional work should be done to improve its stability in food matrices and to evaluate its synergistic potential with other antifungal molecules against fungal strains such as A. fumigatus.
Acknowledgement
Authors are grateful to University of Ngaoundere (Cameroon) for support in the form of infrastructural facilities made available for undertaking the present study.
Conflict of interest
The authors declare no financial or other conflicts of interest.
References
- Identification of essential oil components by gas chromatography/mass spectroscopy (fourth ed.). USA: Allured Publishing Corporation; 2007. p. :803.
- Agence Française de Sécurité Sanitaire des Aliments., 2006. Évaluation des risques liés à la présence de mycotoxines dans les chaînes alimentaires humaine et animale (Rapport synthétique). France.
- Antimicrobial and cytotoxic activities of the fruit essential oil of Xylopia aethiopica from Nigeria. Fitoterapia. 2004;75(3/4):368-370.
- [Google Scholar]
- Ayepola, O.R., Brooks, N.L., Oguntibeju, O.O., 2014. Oxidative Stress and Diabetic Complications: the role of Antioxidant and Flavonoids. In Antioxidante-Antidiabetic Agents and Human Health. InTech. Rijeka. 25–58.
- Composition and cytotoxic activity of essential oils from Xylopia aethiopica (Dunal) A. Rich, Xylopia parviflora (A. Rich) Benth.) and Monodora myristica (Gaertn) growing in Chad and Cameroon. BMC Complement. Altern. Med.. 2014;14(1):1-8.
- [Google Scholar]
- Use of free radical method to evaluate antioxidant activity. LWT – Food Sci. Technol.. 1995;28(1):25-30.
- [Google Scholar]
- Antimicrobial properties of some essentials oils towards some pathogenic microorganisms. Czech J. Food Sci.. 2008;26(3):174-181.
- [Google Scholar]
- Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J. Agric. Food Chem.. 2007;55(15):6300-6308.
- [Google Scholar]
- Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules. 2012;17(12):6305-6316.
- [Google Scholar]
- Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J. Microbiol.. 2001;47(1):9-17.
- [Google Scholar]
- Chemical composition of essential oil in dried fruits of Xylopia aethiopica from Sudan. J. Med. Aromat. Plants.. 2010;1(1):24-28.
- [Google Scholar]
- Aromatic Plants from the Sudan: Part II. Chemical composition of the essential oil of Xylopia aethiopica (Dunal) A. Rich. – Existence of chemotype species. Adv. Nat. Appl. Sci.. 2009;3(2):166-169.
- [Google Scholar]
- Lutte contre les pertes de céréales post-récolte en Afrique subsaharienne. Int. J. Rural Develop. 2013
- [Google Scholar]
- Composition and antioxidant activity of the essential oils of Xylopia aethiopica (Dun) a. rich. (Annonaceae) leaves, Stem Bark, root Bark, and fresh and Dried fruits, Growing in Ghana. J. Agric. Food Chem.. 2004;52(26):8094-8098.
- [Google Scholar]
- Chemical Composition and Antioxidant Activities of Essential Oils of Xylopia Aethiopica (Dunal) a. Rich. Eur. J. Sci. Res.. 2009;37(2):311-318.
- [Google Scholar]
- Toxicity of some terpenoids of essential oils of Xylopia aethiopica from Cameroon against Sitophilus zeamais Motschulsky. J. Appl. Entomol.. 2007;131(2):269-274.
- [Google Scholar]
- Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr. J.. 1998;13(2):98-104.
- [Google Scholar]
- Aspergillus flavus growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds. Int. J. Food Microbiol.. 2005;99(2):119-128.
- [Google Scholar]
- Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med.. 1999;65(8):749-752.
- [Google Scholar]
- Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem.. 2007;103:778-786.
- [Google Scholar]
- Evaluation of the chemical composition of indigenous spices and flavouring agents. Glob. J. Pure Appl. Sci.. 2001;7(3):455-459.
- [Google Scholar]
- Chemical composition of essential oil extracted from leaves of Campomanesia adamantium subjected to different hydrodistillation times. Ciênc. Rural.. 2017;47(1):1-7.
- [Google Scholar]
- Composition and Antibacterial Activity of Steam Distilled Oils of Xylopia aethiopica and Syzigium aromaticum. J. Eng. Appl. Sci.. 2007;2:236-240.
- [Google Scholar]
- Synergistic antimicrobial effect of Nisin and para-Cymene on Salmonella enterica Serovar Typhi in vitro and on ready to-eat food. Biosci. Biotechnol. Biochem.. 2010;74(3):520-524.
- [Google Scholar]
- Effet Inhibiteur in Vitro et in Vivo de l'extrait de Poudre et de l'huile Essentielle de Xylopia Aethiopica (Dunal) A. Rich. (Annonaceae) sur Fusarium oxysporum f. sp Radicis-lycopersici (Forl), Champignon Parasite des Cultures de Tomate. Eur. J. Sci. Res.. 2010;39(2):279-288.
- [Google Scholar]
- Statistica for Windows (second edition). Tulsa, Okla: Statsoft, Inc.; 1995.
- Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloïdes and Zanthoxylum leprieurii from Cameroon. Fitoterapia. 2003;74(5):469-472.
- [Google Scholar]
- Enhanced tumor development by butylated hydroxytoluene (BHT) in the liver, lung and gastro-intestinal tract. Food Chem. Toxicol.. 1986;24(10–11):1127-1130.
- [Google Scholar]
- Mycelia growth inhibition of some Aspergillus and Fusarium species by essential oils and their potential use as antiradical agent. Agric. Biol. J. N. Am.. 2011;2(11):1362-1367.
- [Google Scholar]
- Effect of harvest time on yield, chemical composition, antimicrobial and antioxidant activities of Thymus vulgaris and Mentha pulegium essential oils. Eur. J. Med. Plants.. 2015;8(2):69-77.
- [Google Scholar]







