Translate this page into:
Phenolic compounds from Actinidia deliciosa leaves: Caco-2 permeability, enzyme inhibitory activity and cell protein profile studies
⁎Corresponding author at: Faculdade de Ciências, Universidade de Lisboa, Edifício C8, Campo Grande, 1749-016 Lisboa, Portugal. mlserralheiro@fc.ul.pt (Maria Luísa Serralheiro)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Chemical compounds from leaves of fruit-producing trees, a waste from agricultural activity can be isolated and used as a source of natural bioactive chemicals. Boiling water was used as an extractant of bioactive compounds from Actinidia deliciosa leaves and co-extracted fibres were removed with ethanol precipitation. Rutin and quercitrin were the main flavonoids identified and quantified by RP-HPLC-DAD. No cytotoxicity was detected for any of the extracts towards Caco- 2 cell line. A permeation of approx. 14% of extract components through the cells monolayer was determined. The cell protein profile of Caco-2 cells was modified when in the presence of the fibre-free extract and transketolase was the protein over-expressed in the presence of polyphenols. Acetylcholinesterase inhibitory activity was also studied, IC50 of 0.56 mg/mL was obtained with the fibre-free extract. A. deliciosa leaves are a good source of phenolic compounds and, therefore, some advantage may be taken of this agricultural residue, due to their biological activity.
Keywords
Actinidia deliciosa leaves
Rutin
Quercitrin
Acetylcholinesterase
Transketolase
Polyphenols permeation
Caco-2 cells
1 Introduction
Leaves from plants used in agriculture in fruit production generate a huge quantity of industrial wastes that have been thought, nowadays, as a good source of bioactive compounds, mainly polyphenolics (Santana-Méridas et al., 2012; Manach et al., 2004). Polyphenolics are a diverse type of chemical compounds with several biological activities, among which antioxidant (Talukder et al., 2016), anti-inflammatory (Li et al., 2014), antiacetylcholinesterase (Falé et al., 2009; Neagu et al., 2016), antidiabetic (Asgar, 2013), antihypercholesteroliemia (Falé et al., 2014; Lee et al., 2013) can be referred. Actinidia deliciosa plantation has been increasing in Europe during the last decade (CONFAGRI, 2012; AREFLH, 2014). The generation of wastes from tree pruning is a general problem for the agriculture sector. Therefore, in those cases where the leaves are a source of bioactive compounds, instead of being discarded, they could be used with advantage. There are several processes to take advantage of compounds present in the plant leaves. Water, the solvent traditionally used to prepare medicinal decoctions and infusions, is able to extract several phenolic compounds (Falé et al., 2013a, 2013b; Falé et al., 2014; Henriques et al., 2017; Lizcano et al., 2010). Mucilage, a soluble fiber, polysaccharide-rich material, is co-extracted in these procedures (Prabakaran et al., 2011) and precipitation with ethanol has been used to separate this type of polymers from phenolic compounds (Ghanem et al., 2010). Taking advantage of phenolic compounds requires the evaluation of their toxicity and permeability through the intestinal cells. Caco-2 cells, a cell line derived from colorectal adenocarcinoma, are recommended by FDA to evaluate the permeation of drugs (Awortwe et al., 2014). These cells have the ability to grow in a monolayer, developing all the enzymatic machinery similar to the cells present in the small intestine (Engle et al., 1998). Several studies indicate that some polyphenols can permeate this barrier although in a low amount (Borrás-Linares et al., 2015; Falé et al., 2014). In the present work, the biological activity of A. deliciosa leaves will be studied, in vitro, using acetylcholinesterase (AChE, acetylcholine acetylhydrolase, E.C. 3.1.1.7) inhibition as model. This enzyme is located in the synapsis (Colovic et al., 2013) and neuromuscular junctions (Rotundo, 2003). AChE catalyses the hydrolysis of the neurotransmitter acetylcholine, ending the neuronal impulse. The inhibition of this activity is used in the symptomatic approach to the of Alzheimer's disease treatment (Colovic et al., 2013; Mehta et al., 2012) and to accelerate the gastrointestinal transit in severe constipation (Bharucha et al., 2013). To inhibit this enzyme but most of them have secondary effects (Colovic et al., 2013) so, the search for new and natural enzyme inhibitors is a matter of importance. It has been demonstrated that polyphenols are able to modify the cell protein expression (Pollio et al., 2016) what may explain some of the biological activities found for these compounds. The objective of the present work was to prepare an extract with bioactive compounds from an agricultural waste, with no cell toxicity and biological activity, which can be used in the development of new added-value products. The novelty of this work resides in the detection of several phenolic compounds in A. deliciosa leaves with biological activity and effect on cell proteome that have not been previously reported for these leaves.
2 Material and methods
2.1 General experimental procedure
All chemicals were analytical grade. MTT (thiazolyl tetrazolium bromide), bovine serum albumin, rutin, acetylcholinesterase (AChE), acetylthiocholine (AChI) and 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) were obtained from Sigma (Barcelona, Spain). Trifluoroacetic acid, quercitrin and methanol were obtained from Merck (VWR, International, Darmstadt, Germany). Ethanol was obtained from Carlo Erba (Peypin, France). Roswell Park Memorial Institute (RPMI) medium, Hank’s balanced salt solution (HBSS), glutamine, Pen-Strep (penicillin and streptomycin mixture), and fetal bovine serum (FBS) were bought from Lonza (Verviers, Belgium). For the one-dimensional electrophoresis gel GE Healthcare Amersham™ ECL™ was used in a 4–12% concentration. The marker protein sample buffer (5X SDS PAGE) and staining solution, BlueSafe, were purchased from NZYTech®.
2.2 Plant material
Fully expanded leaves from Actinidia deliciosa (A. Chev) C.F. Liang et A.R. Ferguson (Actinidiaceae) were collected in December 2016 from plants cultivated at the region of Beira Baixa, Portugal (lat. 39° 46′ 47,783″ N; long. 7° 48′ 20,171″ W, 244 m alt). Fresh leaves were washed and immediately used for extraction as decoction, 48 h after collection.
2.3 Preparation of plant extracts
Aqueous plant extracts were prepared as decoction by using 10 g of grounded leaves in 100 mL of distilled water, boiling for 10 min. The decoctions were filtered through number 1 Whatman grade paper and lyophilized. The yield of extraction was 114 mg of extract/g of plant. For the phenolic compounds isolation, the precipitation of fibers was developed as described in Ghanem et al. (2010), with small modifications (Henriques et al., 2017).
2.4 Reverse Phase High Performance Liquid Chromatography (RP-HPLC-DAD)
The chromatographic analysis was carried out using reverse phase liquid chromatography with a diode array detector (RP-HPLC-DAD) as described in Falé et al. (2013b). The quantification of rutin and quercitrin was carried out using the RP-HPLC-DAD by injecting standard solutions and preparing a calibration curve under the experimental conditions (Falé et al., 2013b). The results were analysed using Microsoft® Excel 2007.
2.5 Cytotoxicity studies in Caco-2 cells
This study was carried out as described in Falé et al. (2013a, 2013b) using the MTT test.
2.6 Permeation studies in Caco-2 cell line
Caco-2 cells (ATCC#HTB-37) a human colorectal adenocarcinoma epithelial cell line, were cultured as described in Falé et al. (2014). Non-purified extract and the fibres-free extract were introduced in the apical chamber and samples were withdrawn after 6 h from the apical and basolateral compartment. These samples were immediately analysed for the phenolic compounds by RP-HPLC-DAD as described in Falé et al. (2013b) and the cells were frozen at −80 °C for protein analysis. The permeation was measured as % of the amount introduced in the apical chamber and as apparent permeability coefficients (Papp). These were determined as indicated in Falé et al. (2014).
2.7 Polyacrylamide gel Electrophoresis (SDS–PAGE)
The cells (previously stored at −80 °C) that were under contact with the extracts and the control were disrupted by sonication during 10 min and then centrifuged at 4°C, 12,000g for 10 min. The supernatant was removed (150 µL) and proteins were quantified using the Bradford method (Bio-Rad®). A volume containing approximately 20 µg protein was used to obtain protein by precipitation with two volumes of acetone and left in the cold (4 °C) for 12 h. The samples were centrifuged at 4 °C, 12,000g for 10 min and then dried under nitrogen current. Electrophoresis was performed on a horizontal homogeneous precast gel 4–12% acrylamide (45 × 80 × 1.4 mm) using a GE Healthcare Life Sciences system and following the instructions provided. The precipitated protein from each sample, blank, cells under the influence of extract and mucilage-free extract, were dissolved with sample buffer and water in a ratio of 1/4. In each gel, well was added 20 µL of the sample (20 µg protein). Twenty microliters of molecular weight marker and sample buffer were also applied. Electrophoretic separation was carried out at 160 V for 80 min. The gel was stained with BlueSafe™ for one hour and photographed using ImageQuant LAS 500 system, GE Healthcare Life Sciences. The gel bands were treated and quantified using ImageJ software.
2.8 Protein identification by mass spectrometry
The protein identification was carried out as a service at Mass-Spectrometry Unit, ITQB-UNL, Oeiras, Portugal.
2.9 Acetylcholinesterase inhibition
Acetylcholinesterase inhibition was measured as described by Falé et al. (2013b) using the extract and the fibre-free extract.
2.10 Data analysis
The software used was Microsoft® Excel 2010, and the results were expressed as mean ± standard deviation. Additional analysis of variance (ANOVA) was performed with α = 0.05 using the software developed by Microsoft®.
3 Results and discussion
3.1 Polyphenols from A.deliciosa decoction
Previous work on the extraction of phenolic compounds from A.deliciosa leaves (Henriques et al., 2017) indicated that these agricultural waste was a good promising source of bioactive compounds. Leaves from this plant collected at the end of December, the pruning period in Portugal, were used for the extraction of phenolic compounds with boiling water (decoction), followed by purification with ethanol precipitation, as described previously (Henriques et al., 2017). Both complete extract and fibers-free extract were analyzed by RP-HPLC-DAD. The chromatographic profile was similar for both extracts, as shown in Fig. 1. The major compounds, peaks 1 and 2 were identified by comparing their retention times and UV–Vis spectra rutin and quercitrin standard compounds. Both compounds are quercetin glycoside derivatives, Fig. 2. These flavonoids were quantified using the external standard method, Table 1. The amount of quercitrin in the extract is twice the value of rutin (significant differences at α = 0.05). Discarding fibers from the extract caused an increase in approximately 50% in the rutin and quercitrin content, keeping the proportionally between the two flavonoids, Table 1. Several phenolic compounds were also determined in the leaves of other plants (Alabri et al., 2014; Iqbal et al., 2015).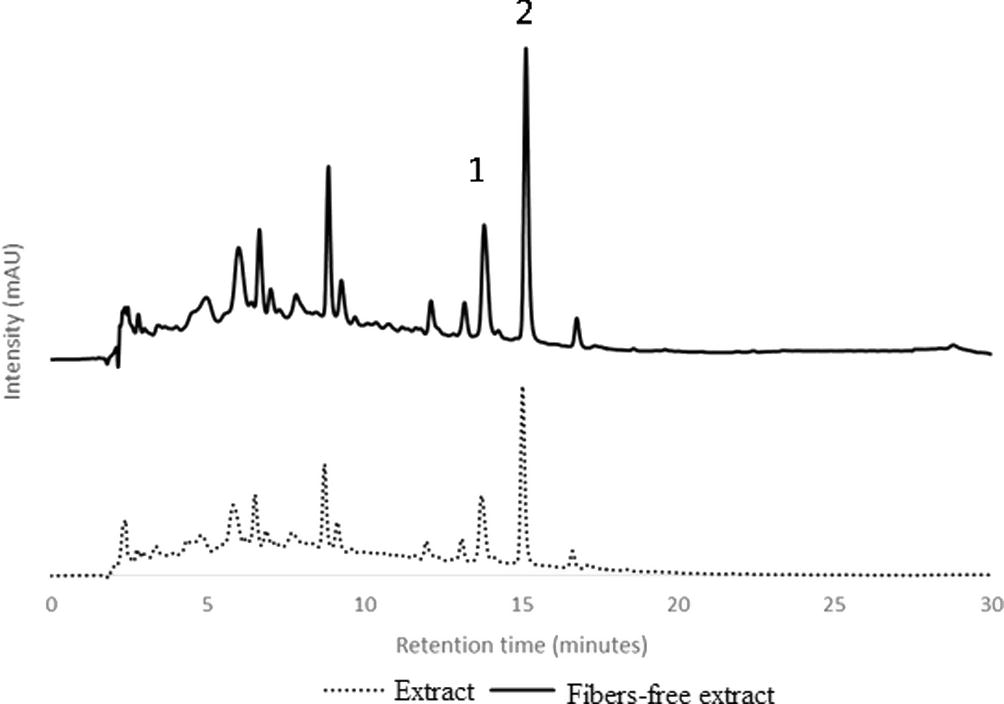
RP-HPLC-DAD of Actinidia deliciosa leaves’decoction. (……) extract; (–) fiber-free extract.
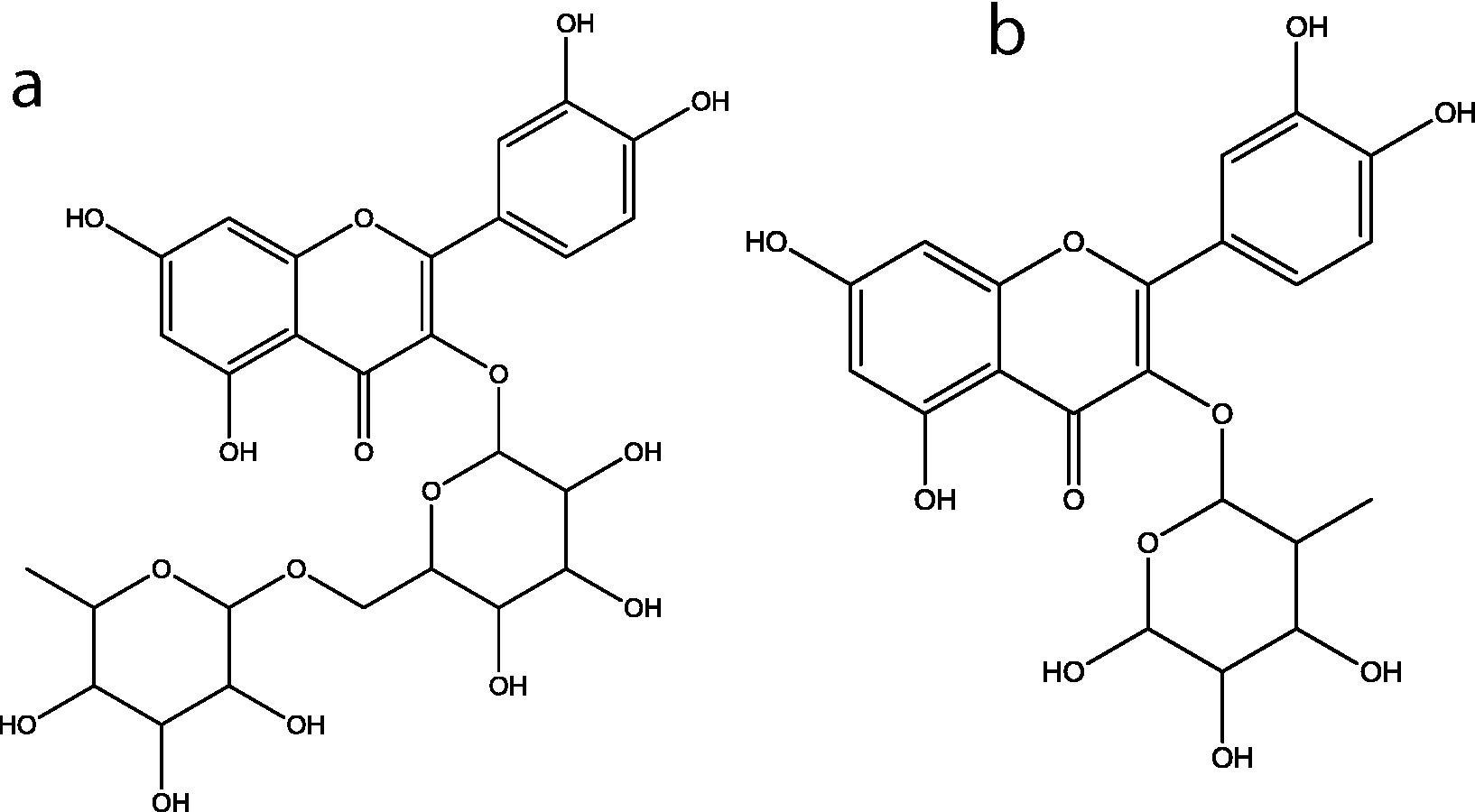
Chemical structure of: (a): Rutin; (b): Quercitrin.
Before ethanol precipitation (total extract)
After ethanol precipitation (fiber-free extract)
Rutin
7.7 ± 0.9
11.9 ± 0.5
Quercitrin
14.9 ± 0.7
22.4 ± 0.8
cytotoxicity for 0.1 mg.mL−1
2.3 ± 2
19 ± 4.1
AChE
0.79 ± 0.01
0.56 ± 0.03
3.2 Permeation of flavonoid derivatives through Caco-2 cells
To study the phenolic compound permeation the toxicity of the extract towards Caco-2 cells has to be evaluated. Neither the complete extract nor the fibers-free extract were toxic to these cells according to Okonogi et al. (2007) (Table 1). In order to evaluate the permeation of polyphenols through the intestinal barrier, and the effect of fibers on their permeation, Caco-2 cells were allowed to grow and differentiate as a monolayer on a Transwell™ system (Falé et al., 2013a, 2014). Extract and fiber-free extract were introduced in the apical chamber, with the same amount of polyphenols, incubated for six hours, after which samples were withdrawn from the apical and basolateral compartments. Only rutin and quercitrin were detected in the basolateral compartment by RP-HPLC-DAD, Fig. 3, and the quantification is shown in Table 2. Permeation of rutin and quercitrin through a monolayer of Caco-2 cells is similar for both flavonoid derivatives, around 7.6 and 8.1 × 10−6 cm/s, corresponding to approximately 10% of the initial amount detected in the basolateral chamber (not statistically different at α = 0.05). These values are in accordance with Papp values determined in other studies (D’Archivio et al., 2010). When using the fiber-free extract, the permeation values for both rutin and quercitrin, were also similar 3.6 and 3.4 × 10−6 cm/s, (α = 0.05), but the permeation without fiber represents a decrease of 41% and 56% comparatively to the complete extract (significantly different, α = 0.05). Our results showed the role of fibers on the permeation of flavonoids through the intestine, increasing the permeation of these compounds. Previous works have also shown that the bioavailability of phenolic compounds can be affected by the food matrix (D’Archivio et al., 2010; Pérez-Jiméne et al., 2009). Pectin, a glycoside-based polymer, also favored the permeation of phenolic compounds (Tamura et al., 2007). Permeation of rutin through Caco-2 cells monolayer can differ when the compound is within an extract containing several other phenolic compounds or when isolated, as a pure compound (Falé et al., 2013a, 2014).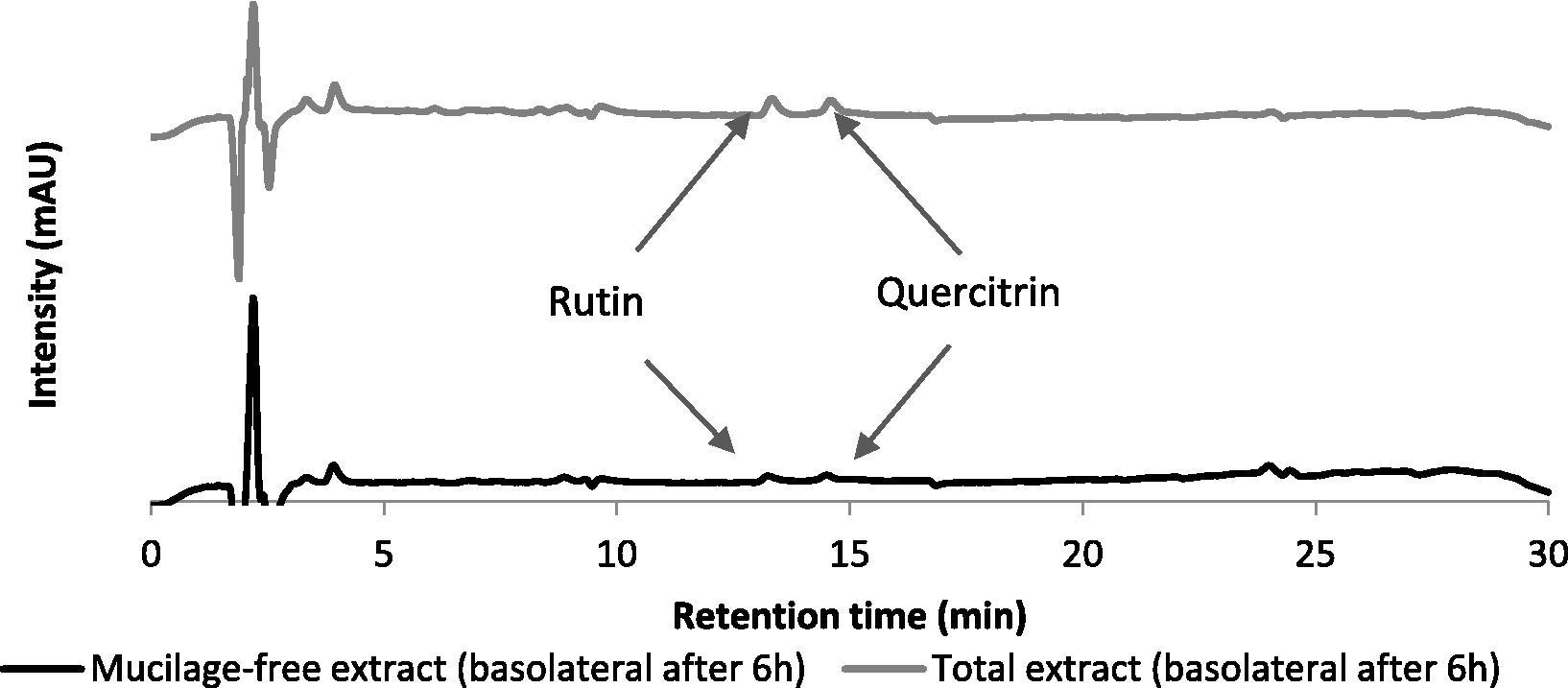
RP-HPLC-DAD of the total extract and mucilage-free extract in basolateral side after 6 h of permeation through Caco-2 monolayers.
Sample
Compound
Bioavailability
Basolateral (%)
Papp AP-BL (×10−6 cm/s)
Intracellular (%)
Total Extract
Rutin
10.4 ± 1.2
8.1 ± 0.9
0.09 ± 0.01
Quercitrin
10 ± 0.9
7.6 ± 0.7
0.14 ± 0.01
Fiber-free Extract
Rutin
5.7 ± 1.3
3.6 ± 1.6
0.36 ± 0.03
Quercitrin
4.4 ± 2.3
3.4 ± 1.8
0.47 ± 0.01
Cells were lysed after the permeation experiments to analyze the intracellular content in flavonoid derivatives. Only 0.09% and 0.14% of the initial amount of rutin and quercitrin were detected inside Caco-2 cells using the complete extract, Table 2. Previous work also detected low quantities of rutin inside cells (Falé et al., 2013a). Within fiber-free extract, rutin and quercitrin showed an intake of 0.36 and 0.47%, respectively, representing an increase of 300% and 235% comparatively to the complete extract. Although our results indicated an increase in flavonoid permeation in the presence of fibers, the effect of these polymers on the permeation of phenolic compounds is still a matter of discussion and research (Bohn, 2014). The increase in permeation and the smaller intracellular amount observed with fibers (polymers having glycosylic units) is in agreement with other works showing that mucin, proteins containing glycosylic groups may enlarge tight junctions between the cells, facilitating the paracellular transport of compounds (Lis et al., 2013). Several studies point out the impact of mucus-secretory cells on the permeation of nutritional food compounds, increasing it (Pelaseyed et al., 2014). As a whole, the compounds could permeate the apical membrane of Caco-2 cells, corresponding to the luminal surface of the intestinal barrier.
3.3 Effect of phenolic compounds on protein-profile Caco-2 cells
As flavonoids were detected inside the cells, the effect on the cell proteins was also investigated. The cells that had been disrupted for the flavonoid analysis were also analyzed in relation to the protein profile. A strong effect on the protein content can be noticed by SDS-PAGE analysis, indicating that flavonoids can modify the protein profile, Fig. 4. Protein with molecular weight around 52 kDa showed an increase in 8% intensity under the effect of extract and the same protein increased 164% intensity relatively to the blank when the fiber-free extract was used. Similar results were obtained using aqueous extract of leaves from a medicinal plant (Falé et al., 2012) and it is known (Salucci et al., 2002) that flavonoids interfere with Caco-2 cell cycle which means that they cause modifications in protein expression inside the cell.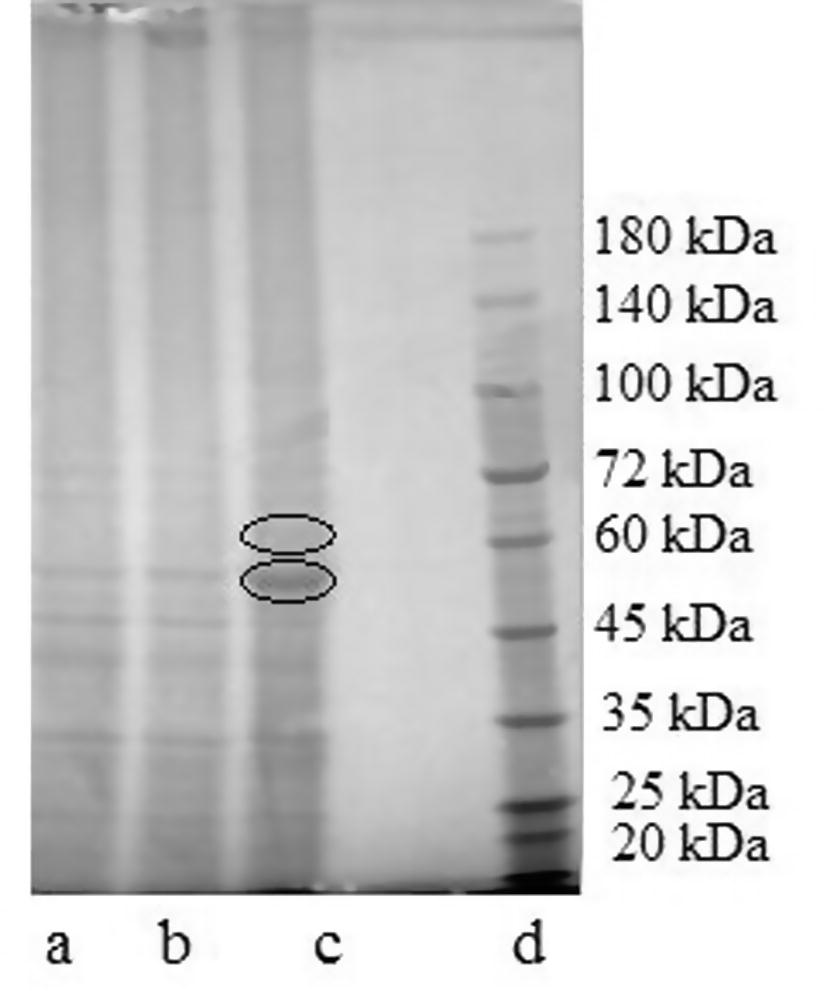
Gel obtained from SDS-PAGE of protein cells under the effect of: (a) control; (b) extract; (c) fiber-free extract; (d) proteins marker.
Proteins present in higher amount in the treated cells were identified by mass spectrometry. The protein with molecular weight 52 kDa was identified as albumin, and the one with molecular weight of approximately 61 kDa was identified as transketolase (E.C. 2.2.1.1). Albumin is coming from the culture medium, as intestinal cells do not produce this protein and it is the main protein in the culture medium. It is relevant that the cells in contact with the polyphenols have a much higher amount of this protein, although all the cells had been washed before disruption and extraction of the proteins. This fact may be explained by the binding of polyphenols to proteins (Rawel et al., 2005; Falé et al., 2012). Polyphenols can permeate the cell membrane and be found inside the cells, as was shown in Table 2 due to several membrane transporter proteins (Gonzales et al., 2015). It is possible that medium proteins bound to polyphenols may enter the cells, explaining the higher amount of albumin in treated cells.
Transketolase (TKT) is involved in the phosphate pentose pathway (PPP), and recent research work has disclosed its relationship with cancer (Patra and Hay, 2014; De Preter et al., 2015; Xu et al., 2016; Kowalik et al., 2017). Cancer cells have an overproduction of this enzyme, and we observed that the presence of polyphenols increased its biosynthesis. This metabolic pathway is involved in energy production, with concomitant production of NADPH and reducing power (Xu et al., 2016). This molecule can participate in the decrease of oxidative stress found in cells. In cancer cells, it has been found that the knocking-out this enzyme avoids cells division, decreasing proliferation of tumour cells (De Preter et al., 2015). Decreasing oxidative stress in normal cells will help cells to proceed their normal biochemical activity, preventing diseases like cancer, diabetes and atherosclerosis (Reuter et al., 2010). The over production of TKT will mean increased synthesis of antioxidant power (NADPH), fighting oxidative stress. This situation is advantageous in normal cells but not for cancer cells.
These results also help to interpret cell toxicity. It is reported in this study that the leaves’ extract were not toxic to the cells, this means also that the compounds present cannot kill cancer cells. In fact, recent research has shown that knock-out of TKT will decrease cancer cells (Xu et al., 2016). As these polyphenols did not decrease TKT, but increased its production, they are not toxic to cancer cells neither to normal cells, but may be beneficial when a decrease in oxidative stress is needed. Therefore our research shows that polyphenols may favour replication of cancer cells, but may prevent oxidative stress-related diseases in normal cells.
3.4 Acetylcholinesterase inhibitory activity
In order to evaluate whether the compounds could inhibit some of the enzymes involved in the treatment of several important diseases, AChE was chosen as a model enzyme. IC50 value for the extract and the fiber-free extract are indicated in Table 1. Mucilage-free extract originated a product with higher inhibitory activity. An increase in 30% of activity that can be attributed to a higher amount of rutin and quercitrin determined in this extract, Table 1. According to previous studies, plant extracts containing rutin and quercitrin also inhibit AChE activity (Hernandez et al., 2010; Neagu et al., 2016; Ressaissi et al., 2016), although with a lower inhibitory activity than the drug galantamine (Hernandez et al., 2010). A.delicosa leaves’ extract has AChE inhibitory capacity within the values found for plant extracts (Kamal et al., 2015). In the present work, these compounds justify approximately 25% of the inhibitory activity of the fiber-free extract, suggesting that the enzyme inhibitory activity is the added contribution of all the phenolic compounds present, several flavonoid derivatives and a triterpene acid-O-hexoside (Henriques et al., 2017).
4 Conclusion
Actinidia deliciosa leaf decoction containing rutin and quercitrin showed promising activities as modulator of the cell protein profile and enzyme activities, as no toxicity towards Caco-2 human cell line was detected. Rutin and quercitrin were able to permeate through Caco-2 monolayers, but fiber removal decreased their permeation. Protein profile showed some modification in the presence of the phenolic compounds, mainly overexpressing transketolase. Leaves from A. deliciosa may have added-value and be used with advantage for the extraction of bioactive compounds.
Notes
The authors declare no competing financial interest.
Acknowledgments
We acknowledge Fundação para a Ciência e Tecnologia (FCT) for financial support to Centro de Química e Bioquímica (PEst-OE/QUI/UI0612/2013; UID/MULTI/00612/2013). Authors are also grateful to Prof. Maria Helena Mendonça (DQB, FCUL) for having collected the A. deliciosa leaves for this study.
References
- Comparative study of phytochemical screening, antioxidant and antimicrobial capacities of fresh and dry leaves crude plant extracts of Datura metel L. J. King Saudi Univ. Sci.. 2014;26:237-243.
- [Google Scholar]
- AREFLH: Assemblée des régions européenes fruitières, légumières et horticoles, Données. Le Kiwi dans le Monde, La Production de Kiwi et les Exportations 2014. http://www.areflh.org/index.php?option=com_content&view=article&id=63%3Ale-kiwi-dans-le-monde&catid=16%3Aetudes&Itemid=116&lang=en accessed on 6th July 2016.
- Anti-diabetic potential of phenolic compounds: a review. Int. J. Food Prop.. 2013;16:91-103.
- [Google Scholar]
- Application of Caco-2 cell line in herb-drug interaction studies: current approaches and challenges. J. Pharm. Pharm. Sci.. 2014;17:1-19.
- [Google Scholar]
- A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation. Gut. 2013;62:708-715.
- [Google Scholar]
- Permeability study of polyphenols derived from a phenolic-enriched Hibiscus sabdariffa Extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int. J. Mol. Sci.. 2015;16:18396-18411.
- [Google Scholar]
- Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol.. 2013;11:315-335.
- [Google Scholar]
- CONFAGRI: Confederação Nacional de Cooperativas Agrícolas e do Credito Agrícola de Portugal. 2012. Kiwis: Produção nacional vai duplicar em dois anos. http://www.confagri.pt/Noticias/Pages/noticia44750. aspx accessed on 6th July 2016.
- Bioavailability of the polyphenols: status and controversies. Int. J. Mol. Sci.. 2010;11:1321-1342.
- [Google Scholar]
- Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation. Oncotarget. 2015;7:2910-2920.
- [Google Scholar]
- Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J. Cel. Physiol.. 1998;174:362-369.
- [Google Scholar]
- Rosmarinic acid, scutellarein 40-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (‘‘falso boldo”) Food Chem.. 2009;114:798-805.
- [Google Scholar]
- Acetylcholinesterase inhibition, antioxidant activity and toxicity of Peumus boldus water extracts on HeLa and Caco-2 cell lines. Food Chem. Toxicol.. 2012;50:2656-2662.
- [Google Scholar]
- Evaluation of cholesterol absorption and biosynthesis by decoctions of Annona cherimola leaves. J. Ethnopharmacol.. 2013;150:718-723.
- [Google Scholar]
- Antioxidant and anti-acetylcholinesterase activity of commercially available medicinal infusions after in vitro gastrointestinal digestion. J. Med. Plants Res.. 2013;7:1370-1378.
- [Google Scholar]
- Studies on the molecular mechanism of cholesterol reduction by Fraxinus angustifolia, Peumus boldus, Cynara cardunculus and Pterospartum tridentatum infusions. J. Med. Plants Res.. 2014;8:9-17.
- [Google Scholar]
- Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: localization and composition in relation to salt stress. J, Plant Physiol. 2010;167:382-392.
- [Google Scholar]
- Review on the use of cell cultures to study metabolism, transport, and accumulation of flavonoids: from mono-cultures to co-culture systems. Compr. Rev. Food Sci. Food Saf.. 2015;14:741-754.
- [Google Scholar]
- Valorization of kiwifruit production: leaves of the pruning branches of Actinidia deliciosa as a promising source of polyphenols. Eur. Food Res. Technol. 2017
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibition and antioxidant activity of the water extracts of several Hypericum species. Food Chem.. 2010;120:1076-1082.
- [CrossRef] [Google Scholar]
- Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci.. 2015;27:224-232.
- [Google Scholar]
- Anticholinesterse and antioxidant investigations of crude extracts, subsequent fractions, saponins and flavonoids of atriplex laciniata L.: potential effectiveness in Alzheimer’s and other neurological disorders. Biol. Res.. 2015;48:1-11.
- [Google Scholar]
- Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front. Oncol.. 2017;7 article 87
- [Google Scholar]
- Hypolipidemic and Antioxidant Properties of Phenolic Compound-Rich Extracts from White Ginseng (Panax ginseng) in Cholesterol-Fed Rabbits. Molecules. 2013;18:12548-12560.
- [Google Scholar]
- Anti-inflammatory and antioxidant activities of phenolic compounds from Desmodium caudatum leaves and stems. Arch. Pharm. Res.. 2014;37:721-727.
- [Google Scholar]
- Development of an improved three-dimensional in vitro intestinal mucosa model for drug absorption evaluation. Tissue Eng. Part C. 2013;19:1-12.
- [Google Scholar]
- Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food Chem.. 2010;119:1566-1570.
- [Google Scholar]
- Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr.. 2004;79:727-747.
- [Google Scholar]
- New Acetylcholinesterase Inhibitors for Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012
- [CrossRef] [Google Scholar]
- Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J. Biol. Sci. 2016
- [Google Scholar]
- Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem.. 2007;103:839-846.
- [Google Scholar]
- The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev.. 2014;260:8-20.
- [Google Scholar]
- Bioavailability of phenolic antioxidants associated with dietary fiber: plasma antioxidant capacity after acute and long-term intake in humans. Plant Foods Hum. Nutr.. 2009;64:102-107.
- [Google Scholar]
- Polyphenolic profile and targeted bioactivity of methanolic extracts from mediterranean ethnomedicinal plants on human cancer cell lines. Molecules. 2016;21:395-418.
- [Google Scholar]
- Extraction and characterization of Hybiscus Rosa-Sinensis leaves mucilage for pharmaceutical applications. J. Pharm. Sci.. 2011;1:232-238.
- [Google Scholar]
- Binding of selected phenolic compounds to proteins. J. Agric. Food Chem.. 2005;53:4228-4235.
- [Google Scholar]
- Aqueous extracts from nopal (opuntia ficus-indica): antiacetylcholinesterase and antioxidant activity from phenolic bioactive compounds. IJGHC. 2016;5:337-348.
- [Google Scholar]
- Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med.. 2010;49:1603-1616.
- [Google Scholar]
- Expression and localization of acetylcholinesterase at the neuromuscular junction. J. Neurocytol.. 2003;32:743-766.
- [Google Scholar]
- Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2) Br. J. Cancer. 2002;86:1645-1651.
- [Google Scholar]
- Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. J. Sci. Food Agr.. 2016;96:232-244.
- [Google Scholar]
- Effect of pectin enhancement on plasma quercetin and fecal flora in rutin-supplemented mice. J. Food Sci.. 2007;72:648-651.
- [Google Scholar]
- Transketolase counteracts oxidative stress to drive cancer development. PNAS 2016:E725-E734.
- [Google Scholar]







