Translate this page into:
Characterization of flavonoids from fern Cheilanthes tenuifolia and evaluation of antioxidant, antimicrobial and anticancer activities
⁎Corresponding author. lucki.chem09@gmail.com (L. Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
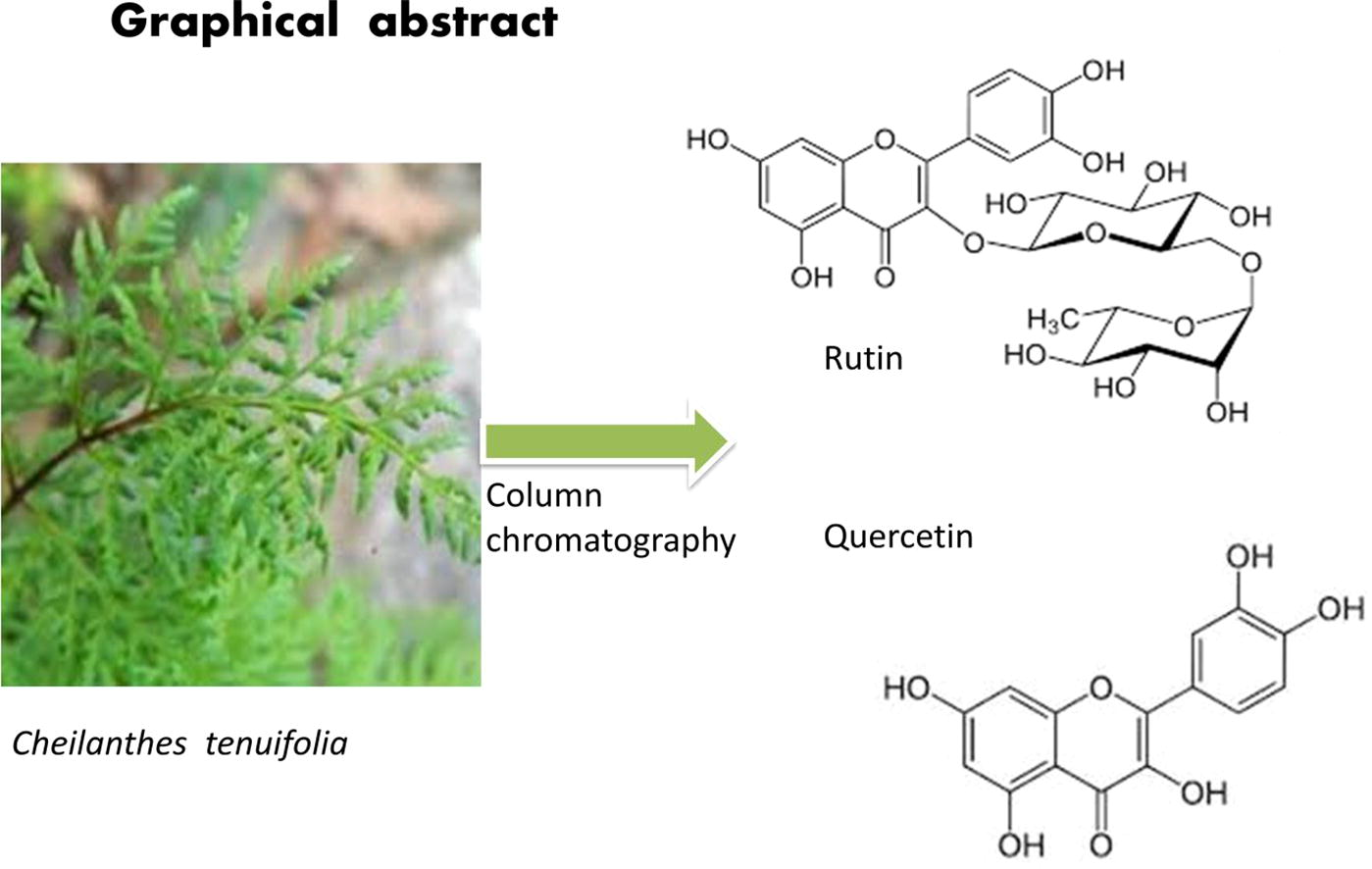
Abstract
The present study is designed to identify various bioactive flavonoid compounds from the methanolic fern extract (MFE) of Cheilanthes tenuifolia. Flavonoids derived from C. tenuifolia (fern) possess potent anti-cancerous, anti-bacterial, anti-oxidant activities that are responsible for their chemo-preventive potential against selected bacterial panel. A preparative column chromatography with a column Sephadex LH-20 was used to isolate and purify flavonoids from C. tenuifolia. Their structure and chemical bonds were identified by using Nuclear Magnetic Resonance (NMR) spectroscopy and Fourier Transform-Infra Red Spectroscopy (FTIR). Two flavonoids were identified as rutin (2.8 mg) and quercetin (3.34 mg) from 100 g of C. tenuifolia. The minimum inhibitory concentration (MIC) values of (2.25 and 0.45 µg/ml) were obtained in purified flavonoids against Staphylococcus aureus and Enterobacter sp., respectively. The two flavonoids (Rutin and quercetin) showed significant in vitro anti-oxidant activity; in this regard, the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging potential of quercetin (86.1%) was higher than that of rutin (73.2%). However, for human hepatoma HepG2 and human carcinoma HeLa cells, quercetin exhibited high anti-cancer activity than rutin. The purified compounds (rutin and quercetin) with having great potential of free radical inactivator in HepG2 cells. The results suggest that MFE of C. tenuifolia could potentially be employed in traditional medicine as they are rich in compounds with anti-oxidant, anti-microbial and anti-cancer properties.
Keywords
Cheilanthes tenuifolia
Column chromatography
Rutin
Quercetin
Fourier Transform-Infra Red Spectroscopy
Nuclear Magnetic Resonance
1 Introduction
Cheilanthes tenuifolia, a member of the Pteridaceae family, is an evergreen and small fern that can grow up to 70 cm in height. This fern grows suitably in warm, rocky regions and is often found in small crevices high up on cliffs. It is naturally abundant phytochemicals and many types of valuable flavonoids have been extracted from various species of ferns. Phytochemical investigations revealed that some ferns contain unusual flavan-4-ol glycosides and flavones modified in the benzene-ring (Kumar and Pandey, 2013). The flavonoids extracted from ferns have shown promising potential in view of their anti-cancer (Nithya et al., 2016; Bahadori et al., 2015; Hossaina and Rahman, 2015; Delmas and Xiao, 2012), anti-microbial (Nithya et al., 2016; Banerjee and Sen, 1980) anti-oxidant (Wang et al., 2016; Maruzzella, 2005) and anti-inflammatory activities (Babii et al., 2016; Wang et al., 2016; Singh et al., 2008) of the potential use in treating diabetes (Xiao, 2015). In ancient time, the rhizome juice of ferns was used to cure stomach disorders as well as peptic ulcer. In addition, the rhizome paste of ferns has generally been used to treat cuts and wounds (Nath et al., 2016). Ferns like Mycodium excertum and Tectaria zeylanica have been investigated to treat various kinds of disorders such as coronary heart disease, menopause syndrome, hypertension, rheumatism, osteoporosis arthritis, breast lumps, neurasthenia and bronchitis (Maridass and Ravichandran, 2009). Moreover, there have been reports in the literature (Rai et al., 2016) documenting that some species of the genus Adiantum exhibited higher antimicrobial activity than the commercial anti-microbial agents, gentamicin and ketoconazole.
It is evident that ferns contain various phytochemicals (flavonols, phenols, alkaloids) that exhibit clinical and pharmaceuticals activities.
Flavonoids belong to the phenolic class of phytochemicals and are regarded as fountains of health owing to their vast biological potential. Plants are the potential source for the isolation of these bioactive flavonoids. Azolla microphylla contains two flavonoids, namely rutin and quercetin that exhibited free radical scavenging activity against DPPH (Selvaraj and Bhattacharjee, 2013). Moreover, in Delphinium trichophorum, five hetisane-type C20-diterpenoid alkaloids with potential anti-cancer properties have been isolated (Lin et al., 2014). Cheilanthes albomarginata has been shown to contain different flavonoids, namely, kumatakenin 5-O-b-glucoside, kaempferol-7-methyl ether, rhamnocitrin 3-O-b-glucoside, and kaempferol 3-O-b-glucoside (Lamichhane et al., 2015; Jarial et al., 2018).
The main bio active constituents in C. tenuifolia fern are flavonoids and phenols yet, limited literature is available on the bioactive compounds of ferns including C. tenuifolia (Iwashina et al., 2002) .The study focuses on the isolation and free radical scavenger activities of flavonoids from MFE of C. tenuifolia. Two flavonoids namely, rutin and quercetin have been isolated from MFE of C. tenuifolia. Their structures have been elucidated through FTIR and NMR. To the best of our knowledge, the in vitro anti-oxidant, anti-bacterial and anti-cancer activities of purified flavonoids from MFE of C. tenuifolia were reported for the first time. Purified flavonoids from MFE of C. tenuifolia is not only a potent source of natural anti-oxidants and anti-bacterial activities but also possesses efficient anti- cancer activity.
2 Materials and methods
2.1 Material and General methods
The organic solvents used for the preparation of whole (leaves, stem and roots) plant crude extract and column chromatography were of analytical grade. D-101 macroporous resin obtained from Ftek lab of University Malaysia Pahang was used for sample preparation. Deuterated dimethyl sulfoxide (DMSO-d6) was used as the solvent for NMR determination. C. tenuifolia was collected from the out skirts of Kuantan, Pahang, for which identification was performed on the basis of ethno-medicinal properties in the literature (Rai et al., 2016). The fern specimens were identified and certified in H.P.U botany department Shimla. All chemicals were of the highest purity (≥99.0%). The reagents (NaCl, HCl, FeCl2, ethanol, methanol, acetone, H2SO4, ferric chloride, KCl, Na2HPO4, chloroform, conc. H2SO4, acetic anhydride, KH2PO4, NaNO2, AlCl3, NaOH, H2O2, Na2CO3, ferrozine, FeSO4 and glacial acetic acid) were purchased from Sigma Aldrich, Malaysia. Recorded NMR spectra on a JNM-A500 (Alpha series) 500 MHz NMR spectrometer (JEOL Ltd., UMP, Malaysia). Electrospray ionization mass spectrometry (ESI-MS) records were obtained on a MDS SCIEX API 2000 LC/MS apparatus (MDS Sciex, Malaysia). Column chromatography was accomplished over silica gel (100–200 or 200–300 mesh, Sigma Aldrich, Chemical Co., Ltd., Malaysia) and Sephadex LH-20 (University Malaysia Pahang) respectively. Thin layer chromatography was performed on pre-coated silica gel HSGF254 plates. Bacterial strains were bought from University Malaya (UM), Malaysia and were cultivated in nutrient broth. The human carcinoma HeLa cells had been provided by the UM research laboratory and were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma Chemical Company, MO, Saint Louis, USA) medium plus 10% heat-inactivated fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C.
2.2 Preparation of crude extract
The Plant material of C. tenuifolia was washed under running tap water, air-dried and then homogenized to fine powder. The powered preparations were stored in air-tight vials. For preparing tissue extracts, 100 g of homogenized powder was put in 1000 ml of methanol in a conical flask, plugged with cotton cap and kept on a rotary shaker (200 rpm for 48 h). Whatmann filter paper No. 1 was used for the filtration of extract and the filtrate was centrifuged at 10,000 rpm at 4 °C for 15 min. The supernatant was collected and completely evaporated at room temperature. The remaining MFE residue (50 mg) was dissolved in 5 ml PBS (0.05 M phosphate buffer saline, pH 7.2) to reach a final concentration (100 μg /ml) and was rendered sterilized by filtration (0.22 µm Millipore filter). The filter-sterilized preparation MFE obtained was stored in a freezer at −4 °C in air-tight vials for further studies.
2.3 Flavonoid assay
The assay was performed according to the reported procedure (Belmekki and Bendimerad, 2012). The determination of flavonoids was performed according to the colorimetric assay. 10% of Diluted Ammonia (5 ml) solution was mixed with the portion of filtrate plant extract followed by slowly addition of concentrated H2SO4. A yellow coloration, which disappeared on standing, observed in extract indicated the presence of flavonoids.
2.4 Quantitative assay for flavonoids
It was done according to the reported procedure .The aluminum chloride (AlCl3) method was used to determine the total flavonoids content in MFE (Marinova et al., 2005). Briefly, 0.5 ml of plant extract (2000 μg/ml) was individually added with 2.8 ml of diluted water, 0.1 ml of 10% aluminum chloride and 0.1 ml of 1 M potassium acetate. The content was mixed by using a vortex and incubated at room temperature for 30 min. At 415 nm we measured the absorbance of the content. The amount of aluminum chloride (10%) was substituted by the same amount of distilled water in blank.
2.5 Extraction of flavonoids
2.5.1 Column chromatography
Column chromatography of the MFE was performed with modifications according to the method described by (Yang et al., 2008). 2 ml prepared stored extract was loaded on glass column (22 mm i. d × 470 mm) packed with 25 g of silica gel column using CHCl3–MeOH solvent to obtain fractions E1-E20. Fractions E6 to E8 (6.318 mg) was again purified by silica gel to gain fractions E6–1 to E6–12, and fraction E6-5 (2.13 mg) was lastly purified by Sephadex LH-20 column; the resin was soaked in double distillation water (ddH2O) full-night and equilibrated (ddH2O) before use. Regenerated the column by washing with 10 column volumes of ddH2O. Fractions were again concentrated using a vacuum rotary evaporator at 35 °C, and the slurry was cleaned with ddH2O to a final volume of 3 ml, then transferred into a glass tube (10 ml) and evaporated using nitrogen flow. Lastly, the slurry was washed with 80% methanol, Teflon membrane used for filtration, and examined with FTIR and NMR. Flavonoids have been purified and claimed as the anti-oxidative principles of C. tenuifolia.
2.5.2 FTIR analysis and NMR measurements
To detect the UV–VIS spectrum profile of the fractions of extract C. tenuifolia (Adiantaceae) fern, the purified fractions were scanned under the wavelength, 200 nm to 800 nm by using UV spectrophotometer and the predictable, peaks were analyzed. FTIR detection was accumulated on the Perkin Elmer spectrophotometer system which was used to analyze the predictable peaks and their functional groups. The peaks results of the UV–VIS and FTIR were noted.
In NMR sample tubes (5-mm) content were loaded with CD3OD for rutin and with DMSO-d6 for quercetin. NMR spectra were used to identify constituents.
2.6 Antioxidant activity
2.6.1 DPPH free radicals scavenging assay
The 2, 2-diphenylpicryl-1-picryl-hydrazyl (DPPH) stable free radical assay is an easy, rapid and sensitive way to analyze the antioxidant activity of a specific compound or plant extract. The method described by (Zhu et al., 2005) was used to test for free radical scavenging activity. Specifically 100 mM of stock solution of DPPH (4 mg/10 ml) was diluted to 1: 10. Then 0.5 ml of this solution was added at different concentrations of purified flavonoids. An equal amount of distilled water was also added to make the final volume 1.0 ml. After 30 min. of incubation, the absorbance was measured at 517 nm against a blank on the UV–visible spectrophotometer. The radical scavenging activity was measured as a decrease in the absorbance of DPPH. The calibration curve was prepared by using ascorbic acid as standard. The DPPH radical scavenging capacity was estimated from the difference in absorbance for the sample and blank and expressed as percentage of DPPH scavenging. The degree of discoloration of violet color of DPPH radical, as it gets reduced, indicates the radical scavenging potential of the antioxidant.
DPPH radical-scavenging activities of the fractions were determined in vitro. Given that light-sensitive nature of DPPH, the solution was prepared in a vial wrapped with an aluminum foil. The mixture was shaken vigorously on a vortex mixer followed by incubation for 1 h in a water bath set at 37 °C, after which the A517 values of the reaction mixture was recorded. A reference profile was calibrated using appropriate concentrations of ascorbic acid.
The absorbance decreases as a result of a color change from purple to yellow as the radical is scavenged by anti-oxidants through the donation of hydrogen to form the stable DPPH molecule.
2.6.2 Cytotoxicity assay
The uniform volume of HepG2 and HeLa cell suspension (200 µl/well; ∼2 × 104 cell/ml) were poured in the selected wells of a 96-well sterile tissue culture plate. The filter-sterilized MFE (200 µg/ml stock) was dispensed in the wells of microtiter plate to achieve final concentrations of 0–600 µg/ml in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma Chemical Company, MO, Saint Louis, USA). The cells treated with purified flavonoids were incubated in a CO2 incubator with 95% humidity at 37 °C for 16–18 h. The DMEM (100 µl) was removed from each of the wells and 20 µl of freshly prepared MTT (5 mg/ml in distilled water) was added to these wells, followed by incubation for 1 h at 37 °C in a CO2 incubator. Thereafter, the DMEM containing MTT was completely removed from the wells using a multi-channel auto-pipette and the purple colored product produced by the cells in each of the wells was extracted in DMSO (100 µl/well) and A570 values were recorded in an ELISA reader. Each of the MFE a concentration was tested in quadruplicate and the mean values ± standard deviation (SD) was calculated after the MTT assay. The appropriate controls (containing appropriate volume of methanol alone) was also considered as a negative control to determine the effect of methanol on the viability of the proliferating cells cultured in vitro.
2.6.3 MIC values of Cheilanthes sp against selected bacterial strains
Micro-broth dilution method performed in 96-well plates to determine the MIC50 of test fractions. (Zavala et al., 1997). Test fractions were prepared at a concentration of 5.0 mg/mL, whilst rutin and quercetin was prepared at a concentration of 2 µg/mL and 200 µg/mL respectively. 12 wells in each of the rows of micro-titer plate were used, out of which the last two wells were taken as control [no flavonoids (rutin and quercetin) was added]. For nutrient broth twofold dilutions of every fraction were prepared in test wells. 10 wells received 100 µl of the MH broth; excluded the 1st well that received 200 µl of broth containing 500 µg/ml of the purified flavonoids (rutin and quercetin). From the 1st well, 100 µl of the MH-broth containing was withdrawn with a new sterile tip, and the same was added in the 2nd well containing 100 µl of the broth, contents were mixed 4 to 8 times, then 100 µl of MH-broth was withdrawn from 2nd well and was mixed to the 3rd well. A range of 2-fold serial dilutions was thus applied. 2 µl of the pure bacterial culture was inoculated in the MH broth of each well and the content was mixed by 10 clockwise and 10 anti-clockwise rotations on a flat surface, positive-control antibiotic was amoxicillin. The micro-titer plate was incubated at 37 °C for 24 h. Thereafter, the results for bacteria growth were visually made and MIC of purified flavonoids for each of the test bacteria were recorded and expressed as µg/ml of MFE.
2.7 Statistical analysis
To assure the reliability of data, not only the best value but also the mean are calculated. The statistical significance between the compound-treated and control groups were assessed by the Student’s t test. A p < 0.05 was considered to be statistically significant.
3 Results
3.1 Flavonoid compositions
The amount of phytochemicals in the Cheilanthes sp extract was quantitatively determined using standard procedure. C. tenuifolia extract contains 7.5 mg of flavonoids (data not shown).
3.2 Purification and identification of flavonoids from C. Tenuifolia extract
Two flavonoids were isolated from the MFE with silica gel column and Sephadex LH-20 column, was eluted with the solvents outlined in Table 1. Their chemical structures were identified on the basis of NMR and ESI-MS evidence and comparison with the literature. They were quercetin (Mahesh and Vidya, 2008) and rutin (Lallemand and Duteil, 1977) respectively, of which the chemical structures are shown in Fig 1.
Fraction
H2O conc. (%)
Acetone conc. (%)
MeOH conc. (%)
F1
100
0
0
F2
70
30
0
F3
50
50
0
F4
50
40
10
F5
50
30
20
F6
50
20
30
F7
50
10
40
F8
40
0
60
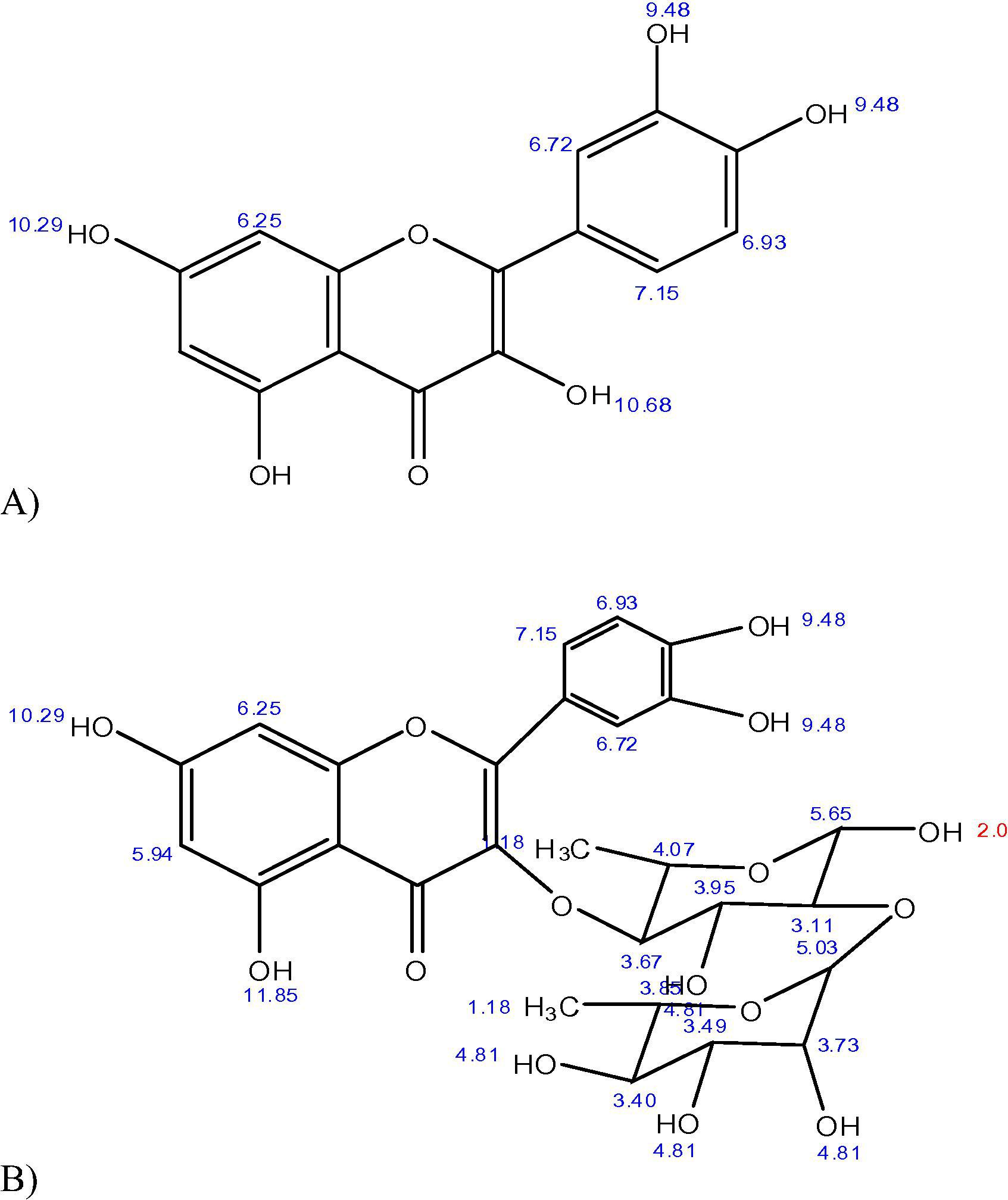
Chemical structures of components A and B (Quercetin and Rutin). The aforementioned 1H NMR spectra matches well with the reported values given in paper published by Qiu et al. (2000). The protons of quercetin are appearing well in the region of 6–13 ppm, whereas the peaks between 2 and 3.5 corresponds to the peaks of deuterated DMSO.
The record of the 1H NMR spectra were observed on a JNM-A600 in CDCl3 solution with internal standard TMS (0 ppm), and chemical shifts were reported in parts per million (δ/ppm). Peaks were observed at: 6.27 and 6.95 ppm given presence of vinylic hydrogen on the phenolic rings, at 7.25 ppm is due to the benzene ring and at 9.48 ppm indicated the presence of hydroxyl group, as shown in Fig 2(A, B).![(A): FTIR spectrum of Quercetin which is chemically called 2-(3,4-Dihydroxyphenyl)-5,7- dihydroxy-3-[[(2 S,3R,4R,5R,6 S)-3,4,5-trihydroxy-6-methyl-2-tetrahydropyranyl]oxy]-4-chromenone clearly showed a peak at value 3435.6 cm−1, 3368.9 cm−1, 3233 cm−1, were due to number of hydroxyl groups presented. (B) 1H NMR spectrum showed peaks with (δ/ppm): 6.27, 6.95 ppm was due to vinylic hydrogen present on the phenolic rings and a signal at value 7.25 ppm is due to benzene ring. The 1H NMR spectrum of the compound in DMSO-D6 exhibited two sets of ortho- and meta-coupling aromatic protons at δH, 7.67 (1H, d, J = 2 Hz, H-2′), 7.64 (1H, dd, J = 8.0, 2.0 Hz,), 6.92 (1H, d, J = 8.0 Hz) as well as 6.23 (1H, d,) and 6.42 (1H, d, J = 2.0 Hz, H-8). The 1H NMR spectrum also supported the presence of two sugar moieties with the anomeric proton signals at δH 5.12 (1H, d, J = 7.5 Hz, Glc-H-1″) and δH 4.59 (1H, s, Ram-H-1‴) related to glucose and rhamnose respectively. The spectra also showed corresponding peaks with the well reported spectras.](/content/185/2018/30/4/img/10.1016_j.jksus.2017.04.007-fig3.png)
(A): FTIR spectrum of Quercetin which is chemically called 2-(3,4-Dihydroxyphenyl)-5,7- dihydroxy-3-[[(2 S,3R,4R,5R,6 S)-3,4,5-trihydroxy-6-methyl-2-tetrahydropyranyl]oxy]-4-chromenone clearly showed a peak at value 3435.6 cm−1, 3368.9 cm−1, 3233 cm−1, were due to number of hydroxyl groups presented. (B) 1H NMR spectrum showed peaks with (δ/ppm): 6.27, 6.95 ppm was due to vinylic hydrogen present on the phenolic rings and a signal at value 7.25 ppm is due to benzene ring. The 1H NMR spectrum of the compound in DMSO-D6 exhibited two sets of ortho- and meta-coupling aromatic protons at δH, 7.67 (1H, d, J = 2 Hz, H-2′), 7.64 (1H, dd, J = 8.0, 2.0 Hz,), 6.92 (1H, d, J = 8.0 Hz) as well as 6.23 (1H, d,) and 6.42 (1H, d, J = 2.0 Hz, H-8). The 1H NMR spectrum also supported the presence of two sugar moieties with the anomeric proton signals at δH 5.12 (1H, d, J = 7.5 Hz, Glc-H-1″) and δH 4.59 (1H, s, Ram-H-1‴) related to glucose and rhamnose respectively. The spectra also showed corresponding peaks with the well reported spectras.
The 1H NMR spectrum of rutin revealed the following peaks: one at 7.82 ppm reflecting the benzene ring; a cluster of signals between 4.2 and 4.9 ppm, given presence of hydroxyl group in the moiety: those between 6.45 and 6.52 ppm reflecting the vinylic groups of benzene ring and the signal value between 2 and 3.5 ppm is due to presence of pyrrole ring, as shown in Fig 3(A, B). Collectively, these findings suggest the isolation of flavonoids from the plant extract.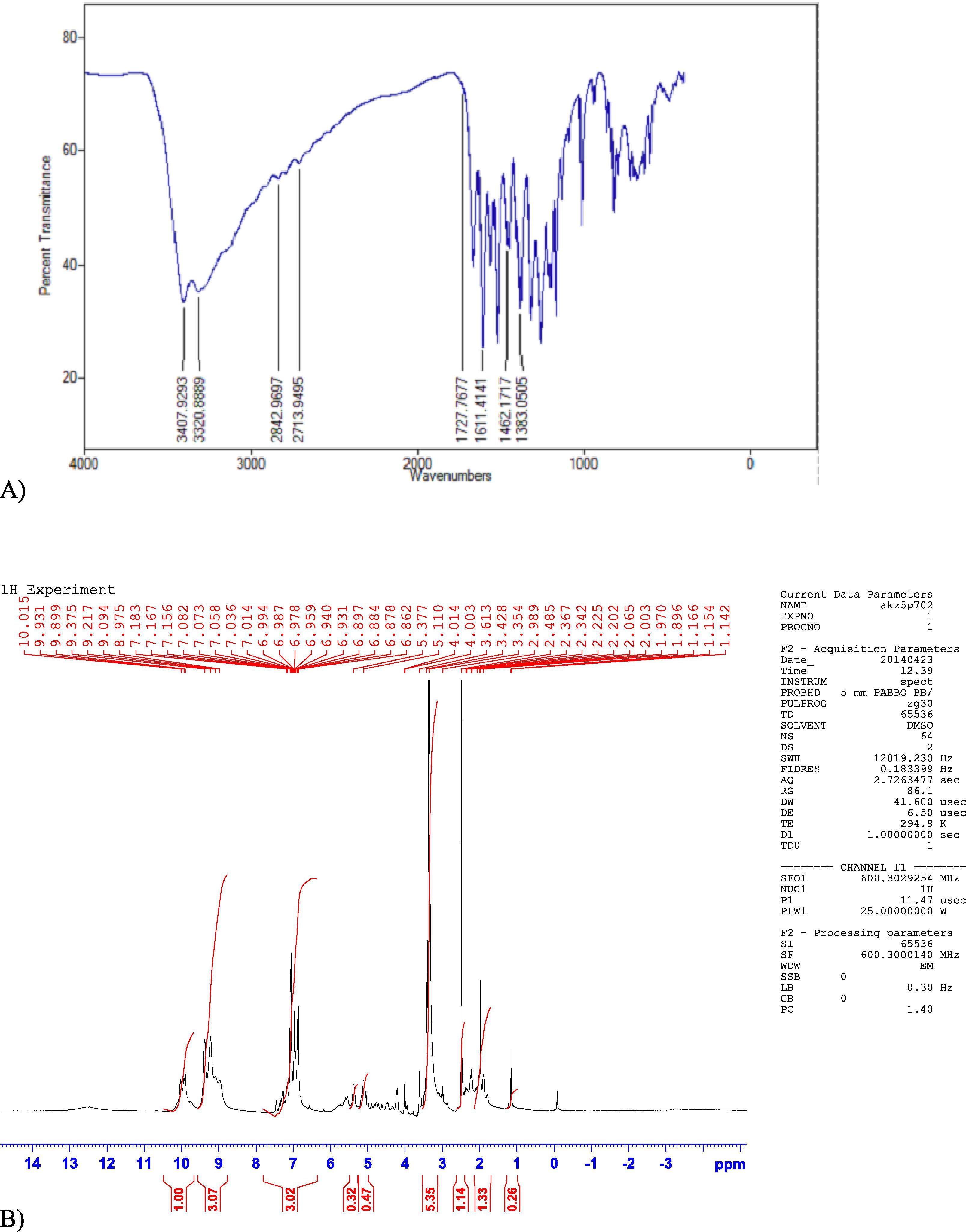
(A): FTIR spectrum of Rutin. (B): 1H NMR spectrum showed peaks with (δ/ppm) at value 7.82 ppm which was due to benzene ring and a cluster of signals at value range 4.2–4.9 is due to presence of hydroxyl group.
3.3 DPPH assay
The flavonoids from C. tenuifolia exhibited notable concentration-dependent DPPH radical scavenging activity. The scavenging activity of quercetin (86.1%) was higher than the ruitn (73.2%), as shown in Fig 4. These results indicate that flavonoids extracted by MFE had a strong DPPH activity, with an IC50 value of 39.10 µg /ml. These results indicate that flavonoids extracted by MFE had a strong DPPH radical-scavenging activity. The uses of these flavonoids are clearly a long way to clinical application.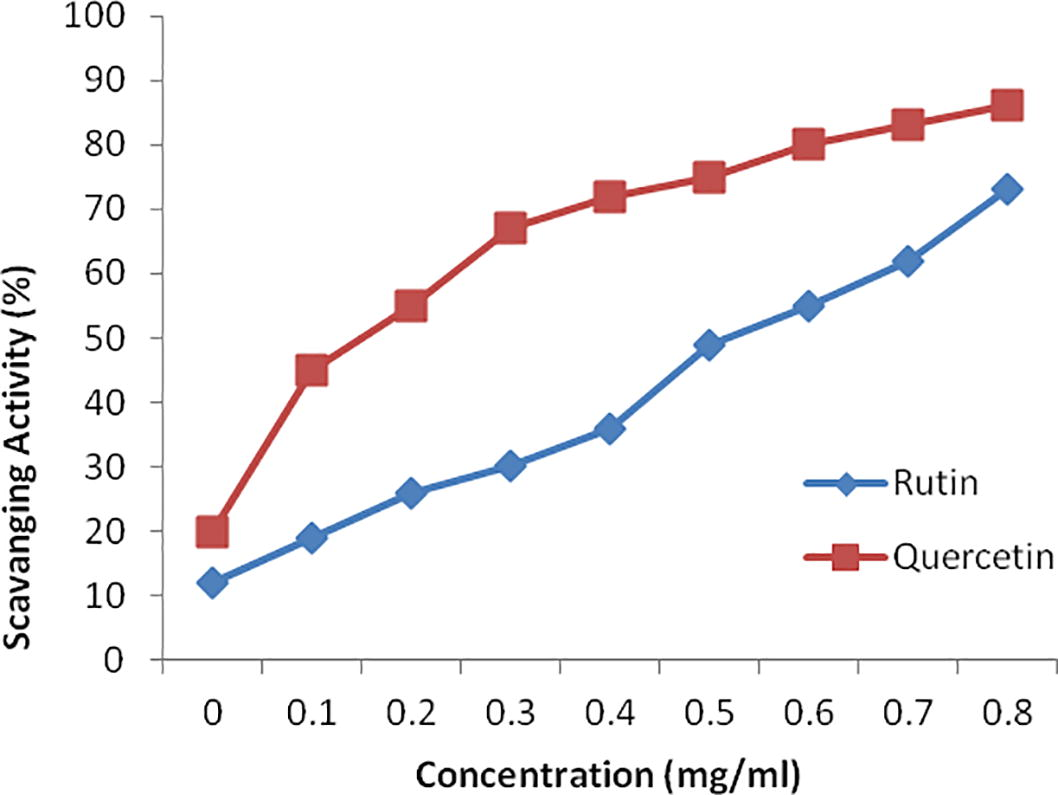
Antioxidant activity of flavonoids (quercetin and rutin) from Cheilanthes, tenuifolia assessed by the DPPH radical-scavenging assay. Data represent means ± SD of three independent experiments.
3.4 MIC values of C. Tenuifolia against selected bacterial strains
The MIC values of purified flavonoids from C. tenuifolia were recorded against selected common pathogenic bacteria. The MIC of compounds 1–2 against S. typhi, S. aureus, E. coli, Enterobacter sp, S. paratyphi and S. mutans are outlined in Table 2. Amongst the tested flavonoids, quercetin showed the strongest antimicrobial activity with an MIC value of 2.25 µg/ml, as shown in Table 2(a) against S. aureus and Enterobacter sp. Rutin had relatively weak antimicrobial activity with MIC values of 4.50 and 9.0 µg/ml as shown in Table 2(b), against S. aureus and Enterobacter sp. in the tested concentration range.
(a)
Microorganism
Tested Conc. of Quercetin
MIC mg/ml
18.0
9.0
4.5
2.25
1.12
0.56
0.28
0.14
S. typhi
−
−
−
+
+
+
+
+
4.50
S. paratyphi
−
−
−
+
+
+
+
+
4.50
E. coli
−
−
−
+
+
+
+
+
4.50
Enterobacter sp.
−
−
−
−
+
+
+
+
2.25
S. mutans
−
−
+
+
+
+
+
+
9.0
S. aureus
−
−
−
−
+
+
+
+
2.25
(b)
Microorganism
Tested Conc. of Rutin (µg/ml)
MIC µg/ml
18.0
9.0
4.5
2.25
1.12
0.56
0.28
0.14
S. typhi
−
−
+
+
+
+
+
+
4.50
S. paratyphi
−
−
+
+
+
+
+
+
4.50
E. coli
−
−
+
+
+
+
+
+
4.50
Enterobacter sp.
−
+
+
+
+
+
+
+
9.0
S. mutans
−
−
+
+
+
+
+
+
4.50
S. aureus
−
−
+
+
+
+
+
+
4.50
3.5 Cytotoxicity assay
The observed results of MTT assay suggested that both purified flavonoids (rutin and quercetin) at all the selected concentrations (240 to 600 µg/ml) had marked cytotoxicity / inhibitory effect on the growth of HepG2 and HeLa cells (Table 3). The maximum viability of the mammalian cells was observed at 600 µg/ml of quercetin (HepaG2 78.16 ± 0.04) and HeLa (80.91 ± 1.93). At enhanced concentration of quercetin (600 µg/ml) a marked decline in the viability of the cells was noticed that highlighted the inhibitory effect of C. tenuifolia. These results indicate that flavonoids extracted by MFE had a strong cytotoxicity activity, with an IC50 value of 507 µg/ml. Sylvestre et al., 2007 showed that the bioactive compounds did not affect cell viability at concentrations <100 µg/mL, thus validating its safety within this range. It is interesting to note that in the current study, the compound was active at concentrations <1 µg/mL. The high IC50 of the two compounds indicate that they may be safe for human consumption.
Concentration (µg/mL)
Cytotoxicity %
Quercetin
Rutin
HepG2
HeLa
HepG2
HeLa
0
100
100
100
100
240
−31.64 ± 4.86
19.99 ± 3.33
−31.64 ± 4.86
19.99 ± 3.33
360
−5.67 ± 2.59
11.10 ± 2.10
−5.67 ± 2.59
11.10 ± 2.10
480
46.53 ± 5.58
37.77 ± 5.34
−5.67 ± 2.59
11.10 ± 2.10
600
78.16 ± 0.04
80.91 ± 1.93
−5.67 ± 2.59
11.10 ± 2.10
4 Discussion
In the present era, given the abundance of botanical and herbal plant resources, a large number of studies have been used to obtain a wide variety of purified phytochemicals. However, few screening approaches have been attempted for the purification of crude plant materials. Flavonoids are secondary metabolite known to rich in pharmacological properties such as anti-oxidative, anti-fungal, anti-inflammatory and diuretic actions (Adil et al., 2010; Bagiu and Butnariu, 2012; Butnariu et al., 2012). Flavonoids are considered favoured bio compounds as chemotaxonomic markers in plants because they show large structural diversity and are chemically stable (Butnariu and Coradini, 2012). Contrary to the widespread belief that ferns are unwanted plants, a number of pharmaceutical surveys have indicated that ferns have their own importance. There have been reports of the anti-bacterial effects of fern extracts both in vitro and in vivo, primarily against enteric pathogens (Benjamin and Manickam, 2007). Besides, antiviral and antimicrobial activities of flavonoids extracted from ferns have been reported (Akabori and Hasegawa, 1970).
In the present study, we have isolated and purified the flavonoids from fern extract by silica gel column chromatography, a well-documented technique of extraction in previous studies (Hazra et al., 2007; Krishnan et al., 2015). Active compounds of C. tenuifolia were extracted by methanol and purified by Sephadex LH-20 column resulted into purification of two unknown flavonoids. After spectral analyses, these flavonoids were recognized as rutin (C27H30O16) and quercetin (C15H10O7). The aforementioned 1H NMR spectra match well with the reported values given in paper published by Qiu et al., 2000. The protons of quercetin are appearing well in the region of 6–13 ppm, whereas the peaks between 2 and 3.5 correspond to the peaks of deuterated DMSO. The 1HNMR spectrum of the compound in DMSO-D6 exhibited two sets of ortho- and meta-coupling aromatic protons at δH, 7.67 (1H, d, J = 2 Hz, H-2′), 7.64 (1H, dd, J = 8.0, 2.0 Hz,), 6.92 (1H, d, J = 8.0 Hz) as well as 6.23 (1H, d,) and 6.42 (1H, d, J = 2.0 Hz, H-8). The 1HNMR spectrum also supported the presence of two sugar moieties with the anomeric proton signals at δH 5.12 (1H, d, J = 7.5 Hz, Glc–H-1″) and δH 4.59 (1H, s, Ram-H-1‴) related to glucose and rhamnose respectively. The spectra also showed corresponding peaks with the well reported spectras. The results are supported by previous reports (Qiu et al., 2000). Putative therapeutic effects of many traditional medicines might be ascribed to the presence of flavonoids (Wang et al., 2016). The presence of anti-carcinogenic (Zhang et al., 2012) and antioxidative properties of extract of C. tenuifolia can be attributed due to the presence of flavonoids.
Significant in vitro anti-oxidant activity were observed for both the purified flavonoids, in this regards, the DPPH radical scavenging activity of quercetin (86.1%) was higher than the ruitn (73.2%). Quercetin has more hydroxyl groups that could be responsible for its higher scavenging activity of as compare with rutin. Previous studies have demonstrated that anti-oxidant activity of phenolics could be determined by the hydroxyl groups (number and positions), the groups’ glycosylation and ortho-dihydroxy were the inevitable structural features for the anti-oxidant activity of phenolics (Zhang et al., 2012). A similar finding was reported in C. officinalis flowers (Butnariu and Coradini, 2012). The presence and the position of the hydroxyl group amongst the phenolic acids like carvacrol and thymol affects to a wide extent the degree of the antibacterial action (Butnariu and Bostan, 2011). The presence of the o-dihydroxy structure in B-ring (3′4′-OH), the 2, 3-double bond in combination with a 4-oxo group and the existence of both hydroxyl groups in positions 3 and 5 are the reason of anti-oxidant activity of any flavonoid. Quercetin has an identical number of hydroxyl groups but also contains a 2, 3-double bond in the combination with a 4-oxo group in the C ring: this structure provides an enhancement of anti-oxidant activity, as demonstrated by the results. The anti-oxidant activity of quercetin was much higher than rutin, which lacks the 3-OH group. All these results confirmed the importance of 3-OH group in the C ring. In our present study, the MIC values for MFE were determined by the micro-dilution method. It is noteworthy that purified quercetin of C. tenuifolia showed remarkable MIC values against S. aureus and Enterobacter (2.25 and 2.25 µg/ml) compared with rutin (4.50 and 9.0 µg/ml), respectively. A similar finding was reported for Dryopteris erythrosora (6.25 µg/ml) (Lee et al., 2008). (Rashed and Butnariu, 2014) also showed excellent value of Bauhinia racemosa against Bacillus subtilis. There are some contradictions over precisely which bacterial species are inhibited by C. tenuifolia as previously S. typhimurium have been reported to be resistant and susceptible to the MEF (Alcaraz et al., 2000). These differences in the observations might have arisen due to variations in bacterial strain variation, the sources and infusions/ extracts strengths of the species used and the definitions of susceptibility and of resistance. Our study also provided solid evidence of ability of the quercetin exhibited high anti-cancer activity: it was found that the value of quercetin on HepG2 and Hela cancer cells were 78.16 ± 0.04 and 80.91 ± 1.93, respectively, These were higher than the best value hitherto documented in previous studies the EC50 values of quercetin on HepG2 and Hela cancer cells have been reported to be 69.50 and 22.64 lM (43.21 and 12.31 lg/mL), respectively (Alia et al., 2006). As for rutin, anti-cancer activity against HepG2 and Hela cancer cells were −5.67 ± 2.59 and 11.10 ± 2.10, respectively. Previous studies have shown that quercetin had a significant stimulatory effect on human leukemia LH-60, OCM-1 melanoma, carcinoma of human squamous cells and colon cancer lines (Richter et al., 1999). The quercetin alone exerts cytotoxic effects against the human cancer cell line (Mario et al., 2006).In essence, rutin and quercetin two bioactive flavonoids have been purified from C. tenuifolia, in an affordable route and the fern have a potent source for the isolation of these bioactive flavonoids. These analyses support that the potential of this fern constituting a potent source of health-protective polyphenolic compounds with natural anti-oxidants and anti-bacterial activities of considerable biomedical values.
The plausible mechanism of action of the rutin and quercetin could be the radical scavenging properties that are typically involved in the genesis of oxidative stress and DPPH activity. Given that chronic inflammation has been found to contribute to the genesis of cancer, the potential anti-cancer activity of the extract is evident.
5 Conclusion
We have successfully extracted two bioactive flavonoids, quercetin and rutin from C. tenuifolia using column Sephdex LH-20 and identified by NMR spectroscopy and FTIR. Quercetin exhibited not only remarkable anti-oxidant activities but also noteworthy antimicrobial activities against S. aureus. Furthermore, quercetin was found to exhibit higher cytotoxicities than rutin against HepG2 and human carcinoma Hela. The purification of the above described flavonoids would be useful in making plant-based pharmaceutical to treat many complicated human diseases. Further studies on determining other chemical compounds and bioactivity of C. tenuifolia are worthwhile in later for better elucidating and exploiting the phytochemicals of this fern.
Funding
This work was financially supported by University Malaysia Pahang under Internal Research Scheme Grant (GRSI 50368). We are also thankful to Ministry of Higher Education Malaysia for Common wealth fellowship.
Conflicts of interest
The authors declare that no conflict of interest exists amongst them or with the parent institution.
References
- First isolation of a flavonoid from Juniperus procera using ethyl acetate extract. Arab J Chem.. 2010;3:85-88.
- [Google Scholar]
- Flavonoid pattern in the Pteridaceae. III. Flavonoid constituents in the fronds of Dennstaedtia wilfordii. Bot. Mag.. 1970;83:63-269.
- [Google Scholar]
- Antibacterial activity of flavonoids against methicillin -resistant Staphylococcus aureus strains. J. Theor. Biol.. 2000;205:231-240.
- [Google Scholar]
- Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG) Eur. J. Clin. Nutr.. 2006;45:19-28.
- [Google Scholar]
- Antibacterial evaluation and preliminary phytochemical screening of selected ferns from Iran. RPJ. 2015;2(2):53-59.
- [Google Scholar]
- Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol.. 2016;120(3):630-637.
- [Google Scholar]
- Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae) Int. J. Mol. Sci.. 2012;13:1426-1436.
- [Google Scholar]
- Antioxidant activity and phenolic content in methanol crude extracts from three Lamiaceae grown in southwestern. Algeria J. Nat. Prod. Plant Resour.. 2012;2(1):175-181.
- [Google Scholar]
- Medicinal pteridophytes from western Ghats. Indian J. Tradit. Know.. 2007;6:611-618.
- [Google Scholar]
- Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem. Cent. J.. 2012;35:2-7.
- [Google Scholar]
- Reverse phase chromatographic behaviour of major components in Capsicum Annuum extract. Chem. Cent. J.. 2012;6(146):1-6.
- [Google Scholar]
- Antimicrobial and anti-inflammatory activities of the volatile oil compounds from Tropaeolum majus L.(Nasturtium) AJOB. 2011;10(31):5900-5909.
- [Google Scholar]
- Hot topic: natural polyphenols properties: chemopreventive and chemosensitizing activities. Anticancer Agents Med. Chem.. 2012;12:835-841.
- [Google Scholar]
- Isolation of antibacterial pentahydroxy flavones from the seeds of Mimusops elengi Linn. Afr. J. Biotechnol.. 2007;6:1446-1449.
- [Google Scholar]
- Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab. J. Chem.. 2015;8:218-221.
- [Google Scholar]
- Chalcones and flavonols from the Chinese species, Saruma henryi (Aristolochiaceae) Biochemical systematics and ecology.. 2002;30:1101-1103.
- [Google Scholar]
- Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium nidus. J. King Saud Univ. Sci.. 2018;30:185-192.
- [Google Scholar]
- Optimization of monoclonal antibodies purification expressed in H- 192 cells using preparative native- polyacrylamide gel electrophoresis. J. Biochem. Microbiol. Biotechnol.. 2015;3(1):21-25.
- [Google Scholar]
- Chemistry and biological activities of flavonoids: an overview. Sci. World J.. 2013;50:1-16.
- [Google Scholar]
- Antibacterial effects of S-(-)-tulipalin B isolated from Spiraea thunbergii Sieb on Escherichia coli, a major food borne pathogenic microorganism. J. Med. Plant Res.. 2008;2:059-065.
- [Google Scholar]
- Identification of Flavonoids from Cheilanthes albomarginata Clarke and their simultaneous determination and quantification by UPLC/DAD Method. J. Liq. Chrom. Relat. Tech.. 2015;38:1713-1721.
- [Google Scholar]
- Diterpenoid alkaloids and flavonoids from Delphinium trichophorum. Phytochemistry. 2014;97:88-95.
- [Google Scholar]
- Isolation and Identification of flavonoid “Quercetin” from Citrullus colocynthis (Linn.) Schrad. Asian J. Exp. Biol. Sci.. 2008;22:137-142.
- [Google Scholar]
- The Screening of phytochemical and antioxidant activity of two endemic ferns, Mycodium excertum (Wall.Ex Hook) Copel and Tectaria zeilanica (Holtt) Sledge. PhOL. 2009;1:56-63.
- [Google Scholar]
- Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall.. 2005;40:255-260.
- [Google Scholar]
- Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2) Eur. J. Nutr.. 2006;45:19.
- [Google Scholar]
- Antibacterial activity of rhizome extracts of four pteridophytes from Southern Assam, North East India. AJPCR. 2016;4(1):1-5.
- [Google Scholar]
- Antioxidant activity, total phenol, flavonoid, alkaloid, tannin and saponin contents of leaf extracts of Salvinia Molesta D. S. Mitchell (1972) Asian J. Pharm. Clin. Res.. 2016;9(1):200-203.
- [Google Scholar]
- A study on chemical constituents of the stems of Opuntia dillenii (Ker-Gawl.) Haw. J. Shenyang Pharm. Univ.. 2000;17(4):267-268.
- [Google Scholar]
- Antimicrobial and Antioxidant Activities of Bauhinia racemosa Lam. and Chemical Content. Iran. J. Pharm. Res.. 2014;13:1073-1080.
- [Google Scholar]
- Quercetin-induced apoptosis in colorectal tumor cells: possible role of EGF receptor signaling. Nutr. Cancer. 1999;34:88-99.
- [Google Scholar]
- Isolation and structural elucidation of flavonoids from aquatic fern azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Sci.. 2013;5:743-749.
- [Google Scholar]
- Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrina(L.) Coulter. Phytother. Res.. 2007;21:536-540.
- [Google Scholar]
- Antimicrobial activity of some important Adiantum species used traditionally in indigenous systems of medicine. J. Ethnopharmacol.. 2008;9:327-329.
- [Google Scholar]
- Flavonoids, antioxidant potential, and acetylcholinesterase inhibition activity of the extracts from the gametophyte and archegoniophore of marchantia polymorpha L. Molecules. 2016;12:1-13.
- [Google Scholar]
- Natural polyphenols and diabetes: understanding their mechanism of action. Curr. Med. Chem.. 2015;22:2-3.
- [Google Scholar]
- Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:1-15.
- [Google Scholar]
- Antimicrobial screening of some medicinal plants. Phytother. Res.. 1997;11:368-371.
- [Google Scholar]
- Flavonoids contents and free radical scavenging activity of extracts from leaves, stems, rachis and roots of Dryopteris erythrosora. Iran J. Pharm. Res.. 2012;11:991-995.
- [Google Scholar]
- Antimicrobial activities of Cynara scolymus L. leaf, head, and stem extracts. J. Food Sci.. 2005;70:149-152.
- [Google Scholar]







