Translate this page into:
Comparison of morphometric and gravimetric measurements of Common Skittering Frog (Euphlyctis cyanophlyctis) from paddy fields and urban wetlands
⁎Corresponding author. sahil@uaar.edu.pk (Muhammad Rais)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Frogs are common in aquatic habitats such as marshes, edges of water bodies and paddy fields. High prevalence of deformed frogs as well as reduction in body length, head length, limb size, growth rate and increase in liver weight are known from various frog species occurring in pesticide contaminated sites. We hypothesized that snout-vent length, liver, kidney, body weight and growth of Common Skittering Frog inhabiting areas contaminated by pesticides and fertilizers such as paddy fields of District Gujranwala, Punjab, Pakistan, do not differ than those from areas with less pesticide and fertilizer use such as urban wetlands in Rawalpindi-Islamabad Districts. Our results revealed statistically significant differences in measurements such as in snout-vent length, inter-orbital width, distance from front of eyes to the tip of snout, distance between the nostrils, inter-nasal space, distance from front of eyes to the nostrils, distance from nostrils to the tip of snout, distance from tympanum to the back of eyes, forelimb length and body weight of frogs from paddy fields and urban wetlands. We found a strong significant relationship between snout-vent length and body weight of female frogs collected from paddy fields and urban wetlands and of male frogs collected from paddy fields only. We found that Common Skittering Frog had a higher growth in paddy fields, presumably due to abundance of food. Our study documented first record of a deformity (micromelia) and an abnormality (gas bubble disease) in Common Skittering Frog from Pakistan. We suggest detailed studies on food availability and consumption by Common Skittering Frog in the paddy fields and urban wetlands to understand factors influencing the growth pattern.
Keywords
Dicroglossidae
Gujranwala
Micromelia
Herbicides
Malformations
Gas bubble disease
Eutrophication
1 Introduction
It is estimated that about 30% of the anurans species are threatened globally (Stuart et al., 2004). Studies suggest that more than 500 populations of frogs, toads, and salamanders are declining (Alford and Richards, 1999). The phenomenon of amphibian decline and underlying causes are yet not fully understood. However, it is believed that numerous factors such as climate change, increased exposure to ultraviolet radiation, pathogens, introduced species, habitat destruction and modification, acid rain, and chemical stressors such as pesticides and fertilizers are the main threats to amphibian populations (Blaustein et al., 2003; Boone and Bridges, 2003).
Frogs are closely linked to well watered areas such as marshes, edges of water bodies and croplands particularly paddy fields (Bambaradeniya et al., 2004; Rais et al., 2015). Extensive use of fertilizers and pesticides has greatly altered habitat quality of agricultural sites affecting anuran populations (Manna et al., 2009). Anuran fauna of Pakistan is represented by 25 species belonging to four families (Pratihar et al., 2014). Frog species such as Euphlyctis cyanophlyctis and Fejervarya limnocharis are quite abundant in the plain areas of Pakistan, and are associated with a variety of wetland habitats such as paddy fields (Khan, 2006).
Reductions in body length, head length, limbs size, growth rate and increase in liver weight are known from frog species occurring in pesticide contaminated sites (Crawshaw and Weinkle, 2000; Amor et al., 2009; Thammachoti et al., 2012). High prevalence of deformed frogs is reported from the vicinity of agricultural areas with extensive pesticide and fertilizer use (Hayes et al., 2002; Khan and Law, 2005; Taylor et al., 2005). It is believed that toxic effects of pesticides, fertilizers and heavy metals result in deformities in anurans (Alford et al., 2001; Story and Cox, 2001; Fort et al., 2006).
The most recent studies on anurans in Pakistan focus on estimating abundance of frog species. Yousaf et al. (2010) recorded mean population densities of Bull Frog (Hoplobatrachus tigerinus) and Common Skittering Frog (Euphlyctis cyanophlyctis) as 25.07 frogs ha−1 from the paddy fields of Gujranwala, Punjab Province. Tabassum et al. (2011) reported mean population density of Common Skittering Frog as 0.46 ± 0.11 frogs ha−1 from Rawal Lake, Islamabad. Shaikh et al. (2012) recorded variations in dorsal body color, body length and body weight of 23 specimens of Common Skittering Frog collected from different sites of Province Sindh, Pakistan.
Review of the literature revealed that farmers in areas under rice cultivation in Pakistan use chemical fertilizers to obtain a good yield of rice crop. About 81% of farmers used 100–150 Kilograms of chemical fertilizer per acre (GoP, 2008). Likewise, about 96% of farmers use pesticides such as fungicides: Topsin-M (thiopenate methyl); herbicides: Acetachlor (acetachlor), Butachlor (butachlor) and insecticides such as Cartap (cartap hydrochloride) in the paddy fields (Asghar, 2010). Further, it is estimated that cotton crop accounts for about 80% of Pakistan’s pesticide use (NFDC, 2002). On contrary, croplands of Potohar such as Chakwal, Attock and Rawalpindi-Islamabad receive very less pesticide application (Hussain et al., 2006). The cotton and rice crops are not grown in Rawalpindi-Islamabad.
Knowledge on the effect of pesticide and fertilizer on body measurements of frog species, inhabiting areas differing in level of pesticide and fertilizer usage, from Pakistan is lacking and not yet reported in the literature. We hypothesized that snout-vent length, liver, kidney and body weight and growth of Common Skittering Frog inhabiting areas contaminated by pesticides and fertilizers such as paddy fields of District Gujranwala, Punjab, Pakistan, do not differ than those from areas with less pesticide and fertilizer use such as urban wetlands in Rawalpindi-Islamabad Districts.
2 Methods
2.1 Study area
We collected frogs from the paddy fields (42 ♀, 33♂) of District Gujranwala, Punjab, Pakistan (Fig. 1A, B) and from ponds, referred to as urban wetlands from here onwards, (41 ♀, 34♂) in Pir Mehr Ali Shah Arid Agriculture University Rawalpindi (PMAS AAUR) campus, Rawalpindi, and Rawal Lake, Islamabad (Fig. 2C, D). The district is the second largest (over 500,000 tons) rice producer in Pakistan. Rice crop is sown during April to June and is harvested from October to December.
Paddy Fields in Gujranwala, Punjab, Pakistan (A&B); Urban Wetlands: Pond near tube well at PMAS Arid Agriculture University Rawalpindi (C) and irrigation canal (D) at Rawal Lake, Islamabad.
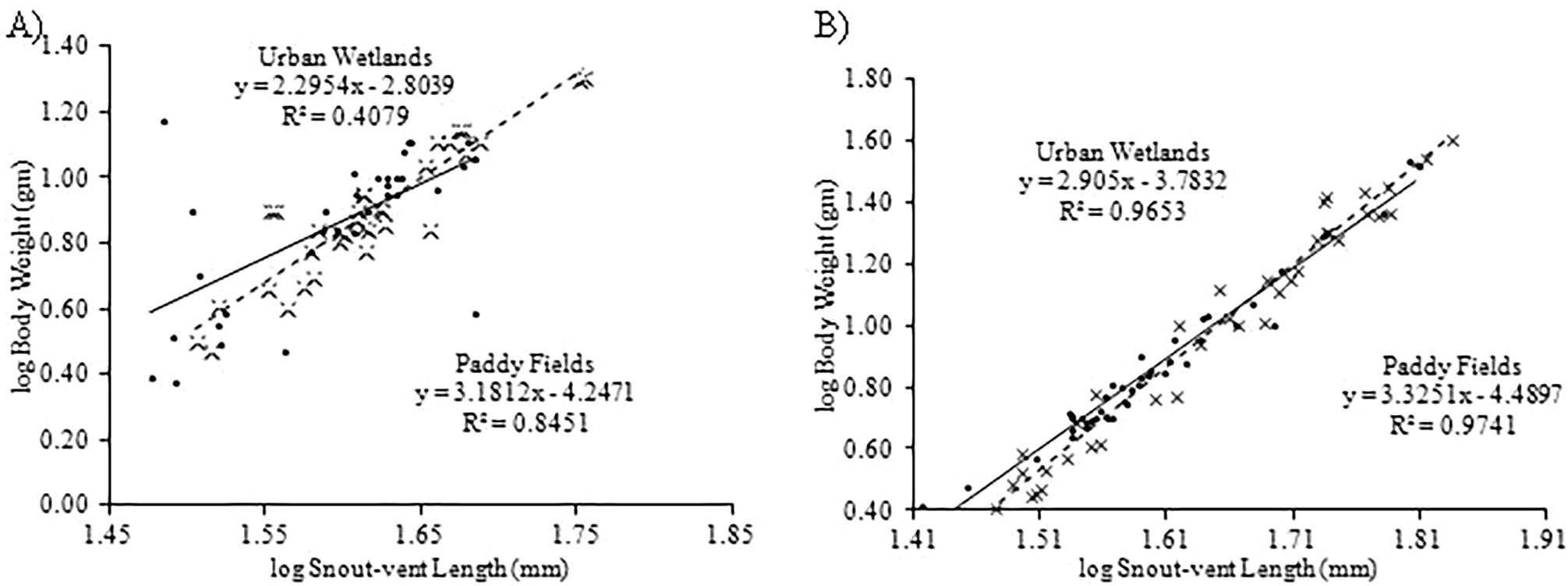
Regression analysis showing relationship between body weight and snout-vent length of females (A) and males (B) of Common Skittering Frog (Euphlyctis cyanophlyctis) collected from paddy fields (dotted line) and urban wetlands (bold line).
The areas of Rawalpindi-Islamabad experience a humid subtropical climate with long and very hot summers, a short monsoon and mild wet winters. The topography of the area is rugged, varying in elevation from 465 m to 1600 meters comprising mainly of steep slopes and gullies where the rock structure is basically limestone (Jabeen et al., 2009). The mean annual rain fall is about 1000 mm. Maximum temperature in summer may rise up to 45 °C while in winter may drop to a minimum of −3 °C (Malik et al., 2012). The notable wetlands of the area include River Kurrang and Soan with slow-flowing water during most part of the year; water storage reservoirs such as the Rawal Dam, Simly Dam and several other small dams with associated marshes (Ashraf et al., 2007).
2.2 Study design
We used Common Skittering Frog (Euphlyctis cyanophlyctis) as a model species. The frog prefers shallow water ponds and edges of other water bodies. It is a highly aquatic frog species, and is the most common frog species in the plains and sub-mountainous areas of Pakistan. The species exhibits a wide range of body coloration from light gray, light brown to olive-green with irregular black spots (Khan, 2006). The frog is abundant in district Gujranwala (Yousaf et al., 2010) and areas of Rawalpindi-Islamabad (Tabassum et al., 2011; Rais et al., 2012). No permit is required to collect or dissect the frog species since it is not listed in the list of protected species in Punjab Wildlife Acts and Rule (1974) and Islamabad Wildlife Protection, Preservation, Conservation and Management Ordinance (1979).
We collected adult specimens of Common Skittering Frogs from March, 2013 to June, 2014 (pre-breeding season). We captured frogs using dip nets, transferred into plastic buckets and transported to the laboratory of Wildlife Management, PMAS AAUR. We euthanized the frogs using Chloroform solution and took morphometric measurements with the help (to the nearest of 0.05 mm) using a non-digital vernier caliper as described in Ohler (1996) and Kok and Kalamandeen (2008). We measured 24 morphometric measurements: snout-vent length, head length, head width, width of upper eyelid, inter-orbital width, distance from back of mandible to the nostrils, distance from back of mandible to the front of eyes, distance from back of mandible to the back of eyes, distance between front of eyes, eye length, distance from front of eyes to the tip of snout, distance between the back of eyes, distance between the nostrils, inter-nasal space, distance from front of eyes to the nostrils, distance from nostrils to the tip of snout, distance from tympanum to the back of eyes, greatest tympanum diameter, forelimb length, hand length, femur length, tibia length, length of tarsus and foot and foot length . We weighed each specimen, dissected, took out liver and kidney and weighed them on a digital weighing balance (Ochaus, PA-214).
We compared means of morphometric and gravimetric measurements of male/female from paddy fields and urban wetlands using student’s t-test (α = 0.05). We run multivariate generalized linear model (one-way MANOVA) to examine if there were any differences (α= 0.05) between categorical independent variables i.e. sex (male and female frogs) or site (paddy fields and urban wetlands) on continuous dependent variables (24 morphometric and three gravimetric measurements). We used slope (scaling coefficient) of regression equation (logBW = b + logSVL∗a; BW = Body weight, SVL = Snout-vent length, b = slope and a = intercept) to compare growth rate of the frogs from paddy fields and urban wetlands. We also conducted linear regression to compare relationship between body weight (log) and liver (log)/kidney weight (log) of the frogs. We performed all statistical tests in SPSS 22.0. We followed Meteyer (2000) to identify and classify deformities and abnormalities in the specimens.
3 Results
The multivariate generalized linear model revealed statistically significant difference (F (27, 122) = 3.90, P < 0.0005; Wilk’s Λ = 0.537, partial η2 = 0.46) in the measurements of male and female frogs. This difference was attributed to distance between the nostrils and inter-nasal space. We recorded statistically significant difference (F (27, 122) = 7.42, P < 0.0005; Wilk’s Λ = 0.378, partial η2 = 0.62) in the measurements of frogs collected from paddy fields and urban wetlands. This difference was contributed by snout-vent length, inter-orbital width, distance from front of eyes to the tip of snout, distance between the nostrils, inter-nasal space, distance from front of eyes to the nostrils, distance from nostrils to the tip of snout, distance from tympanum to the back of eyes, forelimb length and body weight (Appendix A)
Of all the measurements we taken, four measurements such as snout-vent length, inter-nasal space, distance from nostrils to the tip of snout and forelimb length were significantly lower in males and females from urban wetlands and four measurements such as inter-orbital width, distance from front of eyes to the tip of snout, distance from tympanum to the back of eye and body weight were significantly lower in females from urban wetlands (Appendix A). We found strong significant relationship between snout-vent length and body weight of female frogs collected from urban wetlands (R2 = 0.96; F = 1111.18(1, 40); P < 0.05) and paddy fields (R2 = 0.97; F = 1469.55(1, 39); P < 0.05) (Fig. 2A) and of male frogs (R2 = 0.84; F = 174.62(1, 32); P < 0.05) collected from paddy fields (Fig. 2B). However, relationship between snout-vent length and body weight of male frogs collected from urban wetlands was weak but significant (R2 = 0.40; F = 21.35(1, 31); P < 0.05) (Fig. 2B). We found that scaling coefficient (slope of regression equation) of female (3.325) and male (3.181) frogs collected from paddy fields was higher than that of recorded from urban wetlands (2.905 and 2.295, respectively) (Fig. 2A, B) which showed high growth rate of frogs in paddy fields. On contrary, we obtained low growth rate in liver and kidney of females (Fig. 3A, B) and in liver of males (Fig. 3C) collected from paddy fields but higher in kidney of males (Fig. 3D) collected from paddy fields. However, the difference in the liver and kidney weight of males and females frogs collected from paddy fields and urban wetlands was non-significant (P > 0.05) (Appendix A).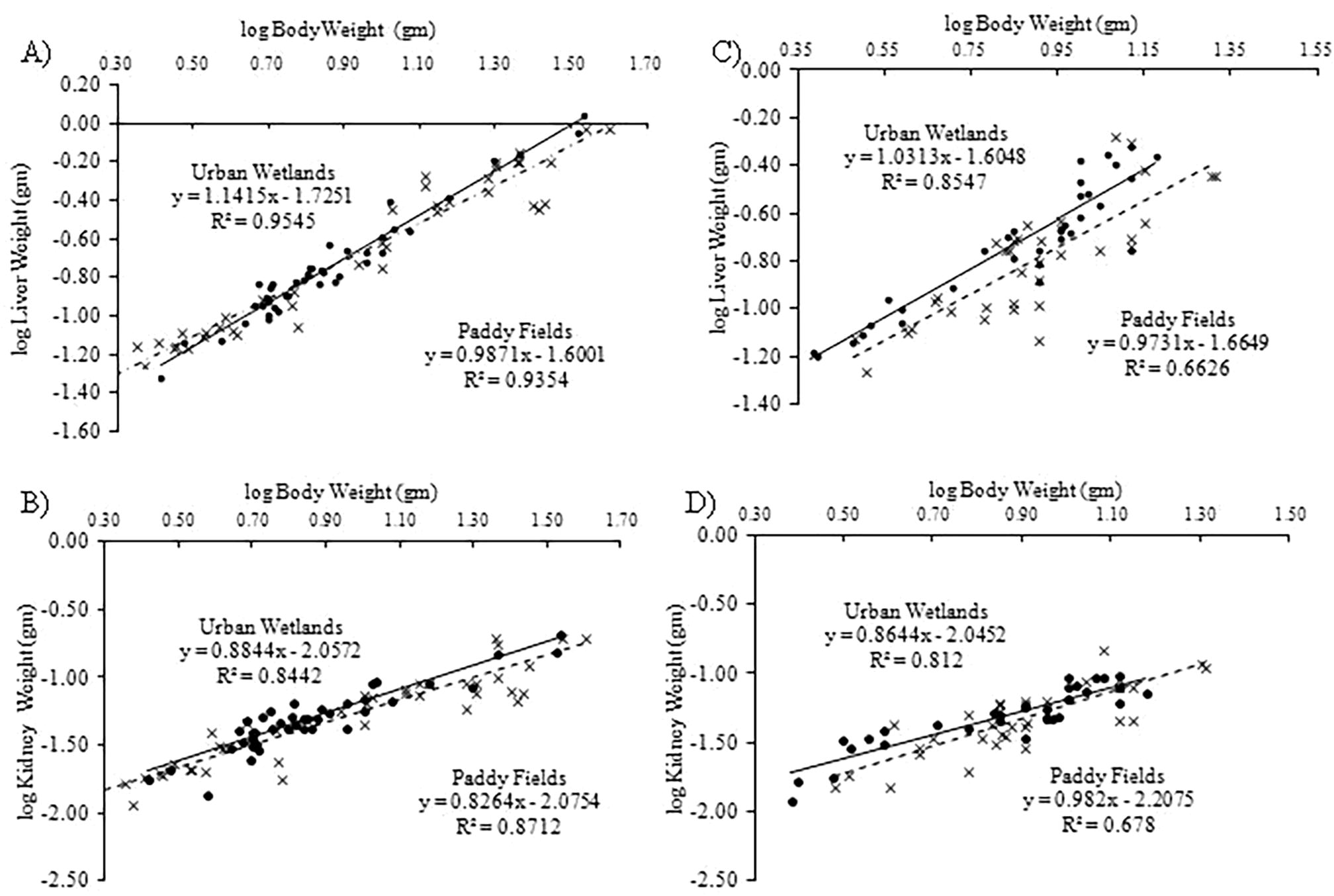
Regression analysis showing relationship between body weight and liver weight (A); body weight and kidney weight (B) of females; body weight and liver weight (C) and body weight and kidney weight (D) of males of Common Skittering Frog (Euphlyctis cyanophlyctis) collected from paddy fields (dotted line) and urban wetlands (bold line).
We did not find malformation or abnormality in any specimen of Common Skittering Frog from the paddy fields. However, we recorded micromelic individual (Fig. 4A, B; Table 1) and an abnormal specimen with the gas bubble disease (Fig. 4C, D) from the pond of PMAS AAUR Campus. The pond had a high algal growth (Fig. 1C), but had no connection with any other source of contamination such as pesticides and municipal waste.
Specimens of Common Skittering Frog (Euphlyctis cyanophlyctis) showing limb deformity (A, B) and gas bubble disease (C, D).
Measurements
ID: J-1401
(Fig. 4A&B)ID: J-1402
(Fig. 4C&D)
Snout-vent length
29.2
26.5
Head width
10.8
10
Head length
12.5
10.4
Width of upper eyelid
2
1.5
Inter-orbital width
2.4
2.7
Distance from back of mandible to the nostril
10.9
8.8
Distance from back of mandible to the front of eye
8.4
6.9
Distance from back of mandible to the back of eye
6.3
5.2
Distance between front of the eyes
5.7
5.7
Eye length
3.7
2.5
Distance from the front of the eyes to the tip of the snout
5.3
4.3
Distance between the back of the eyes
8
8
Distance between nostrils
2.5
2.2
Inter-nasal space
1.9
1.1
Distance from the front of eye to the nostrils
2.4
2.3
Distance from the nostril to the tip of snout
2.9
2
Greatest tympanum diameter
2.2
Not measurable
Distance from tympanum to the back of eye
1
Not measurable
Forelimb length
5.6
4.2
Hand length
8.4
5.7
Length of tarsus and foot
17.8
17.2
Foot length
14
13
Femur length
Not measurable
12.6
Tibia length
Not measurable
9.4
Deformity/abnormality
Reduced hind limbs
Gas bubble disease
4 Discussion
Reduction in body length, head length, limbs size, growth rate and increase in liver weight are known from frog species occurring in pesticide contaminated sites (Crawshaw and Weinkle, 2000; Amor et al., 2009; Thammachoti et al., 2012). Morphometric differences exist between different sexes of frogs (Shine, 1979). Our study reported two such measurements (distance between the nostrils and inter-nasal space) which caused the difference in male and female frogs. Based on statistical analysis, we rejected our hypothesis and concluded that morphometric measurements, body weight and growth of the frog species collected from paddy fields differed from frogs of urban wetlands. Our results showed that snout-vent length of specimens collected from paddy fields and urban wetlands differ significantly. Both female and male frogs collected from paddy fields were larger in size compared to those recorded from urban wetlands. However, only females from urban wetlands had significantly lower body weight. The liver and kidney weight of males and females frogs collected from paddy fields and urban wetlands did not differ.
We recorded higher mean values of measurements such as head length, head width, tympanic diameter, snout-vent length, femur length and foot length but lower mean value of inter-orbital width of Common Skittering Frog (Euphlyctis cyanophlyctis) than reported by Ramakrishna et al. (2013) for 10 specimens of the same species. We attributed this difference to two main reasons. First, our sample size was high and second we took separate measurements for male and female. We recorded lower mean body weight, tympanum diameter and forelimb length but higher mean snout-vent length than reported by Shaikh et al. (2012) for only 12 male and 11 female specimens of the same species. Our findings provided relatively better estimates of 24 morphometric and three gravimetric measurements for male and female of Common Skittering Frog.
Our results showed higher growth rate of Common Skittering Frog at paddy fields whereas Thammachoti et al. (2012) recorded lower growth rate in Cricket Frog (Fejervarya limnocahris) from paddy fields. We believed that different anuran species might respond differently in the same habitat and that population of Common Skittering Frog we recorded from paddy fields was healthy. We did not find any significant difference between liver and kidney weight of frogs collected from paddy fields and urban wetlands. Studies by Thammachoti et al., (2012) and Crawshaw and Weinkle (2000) showed that the liver of frogs from sites contaminated with pesticides such as croplands weighed more. However, our study showed that the difference in liver and kidney weight in frogs from paddy fields and urban wetlands was non-significant.
The phenomenon of malformations in frogs was first reported in 1995 from Minnesota, USA (Meteyer et al., 2000). Since then, a substantial body of research has been conducted on understating factors affecting amphibian populations across the world with almost no contribution from Pakistan. Up until now, no report on deformity and abnormality in any frog species of Pakistan is documented. We collected a deformed and an abnormal specimen from a highly eutrophic pond (Fig. 1C). Studies by Alford et al. (2001), Story and Cox (2001) and Fort et al., (2006) have suggested that the toxic effects of pesticides, fertilizers and heavy metals result in malformations and abnormalities in anurans. Taylor et al. (2005) and Khan and Law (2005) reported high frequency of anurans malformations in areas in the proximity of agricultural land. Our study recorded healthy population of Common Skittering Frog from paddy fields. We assumed that excess nutrients might have resulted in the malformations and abnormalities in Common Skittering Frog.
We concluded that the studied morphometric and gravimetric measurements of Common Skittering Frog differed significantly in male and female frogs and from paddy fields and urban wetlands. We found that Common Skittering Frog had a higher growth in paddy fields presumably due to abundance of food. Our study documented first record of a deformity (micromelia) and an abnormality (gas bubble disease) in Common Skittering Frog from urban wetlands. However, we believed that these incidences were not sufficient enough to draw a meaningful conclusion on the malformations and abnormalities in the frogs of the country. We suggest detailed studies on food availability and consumption by Common Skittering Frog in the paddy fields and urban wetlands to understand factors influencing growth pattern. We also recommend field and lab based studies on anuran populations in Pakistan particularly in eutrophic wetlands.
References
- Global amphibian declines: a problem in applied ecology. Ann. Rev. Ecol. Syst.. 1999;30:133-165.
- [Google Scholar]
- Morphometric variation in the Tunisian green frog, (Rana saharica) (Anura: Ranidae) Afr Zool.. 2009;44:194-203.
- [Google Scholar]
- Biodiversity of insects associated with rice (Oryza sativa) crop agroecosystem in the Punjab. Faisalabad: Faculty of Agriculture, University of Agriculture; 2010. (Pakistan. Ph. D dissertation) 260 pp
- Impact of small dams on agriculture and groundwater development: a case study from Pakistan. Agri. Water Manage. 2007;92:90-98.
- [Google Scholar]
- Biodiversity associated with an irrigated rice agro-ecosystem in Sri Lanka. Biodivers Conser.. 2004;13:1715-1753.
- [Google Scholar]
- Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib.. 2003;9:123-140.
- [Google Scholar]
- Effects of Pesticides on Amphibian Populations. Washington: Amphibian Conservation. Smithsonian Institution; 2003. p. :152-167.
- Clinical and Pathological Aspects of the Amphibian Liver. Seminar Avian Exotic Pet. Med.. 2000;9:165-173.
- [Google Scholar]
- Deformities in cane toad (Bufo marinus) populations in Bermuda: part II. Progress towards characterization of chemical stressors. Appl. Herpetol.. 2006;3:143-172.
- [Google Scholar]
- Government of Pakistan (GoP). 2008. Agricultural Survey of Gujranwala: Exploring District’s Rural Economy. State Bank of Pakistan. Banking Services Corporation (Bank). Development Finance Support Department. Head Office Karachi and SBP-BCS. Gujranwala. 40 pp.
- A preliminary study on risk analysis of pesticides to insectivorous birds inhabiting cotton based agro-ecosystem of Punjab, Pakistan. Pak. J. Zool.. 2006;38:255-260.
- [Google Scholar]
- Indigenous uses of economically important flora of Margallah Hills National Park, Islamabad, Pakistan. Afric J Biotech. 2009;8:763-784.
- [Google Scholar]
- Khan M.S., 2006. Amphibians and Reptiles of Pakistan [Malabar, Florida]: Krieger Publishing Company. 328 pp.
- Adverse effects of Pesticides and Related Chemicals on Enzyme and Hormone Systems of Fish, Amphibians and Reptiles: A Review. Proc. Pak. Acad. Sci.. 2005;42:315-323.
- [Google Scholar]
- Introduction to the taxonomy of Kaieteur National Park. Guyana: Belgian Development Cooperation; 2008.
- Linkages between spatial variations in riparian vegetation and floristic quality to the environmental heterogeneity a case study of river Soan and its associated streams. Pak. J. Bot.. 2012;44:187-197.
- [Google Scholar]
- Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ. Pollut.. 2009;157:2903-2927.
- [Google Scholar]
- Meteyer C.U., 2000. Field guide to malformations of frogs and toads with radiographic interpretations. Biological Science Report USGS/BRD/BSR–2000–0005. 20pp.
- Hind limb malformations in free-living northern leopard frogs (Rana pipiens) from Maine, Minnesota, and Vermont suggest multiple etiologies. Teratology. 2000;62:151-171.
- [Google Scholar]
- NFDC (National Fertilizer Development Center). 2002. Pesticide use and its impact: farm level survey. Street H-8/1, P.O. Box 3104, Islamabad.
- Systematics, morphometrics and biogeography of the genus Aubria (Ranidae, Pyxicephalinae) Alytes. 1996;13:141-166.
- [Google Scholar]
- Diversity and conservation of amphibibians in South and Southeast Asia. Sauria. 2014;36:9-59.
- [Google Scholar]
- Qualitative analysis of factors influencing diversity and spatial distribution of herpetofauna in Chakwal Tehsil (Chakwal District), Punjab, Pakistan. Herpetol. Conserv. Biol.. 2015;10(3):801-1110.
- [Google Scholar]
- Diversity and conservation of amphibians and reptiles in North Punjab, Pakistan. Herpetol. Bull.. 2012;122:16-25.
- [Google Scholar]
- Morphometric studies on Euphlyctis cyanophlyctis (schneider, 1799) Cibt. J. Zool.. 2013;3:5-8.
- [Google Scholar]
- A Study of Morphological Variations in Populations of Euphlyctis cyanophlyctis (Schneider, 1799) (Anura: Ranidae) From District Jamshoro, Sindh. Pak. J. Zool.. 2012;44:1450-1452.
- [Google Scholar]
- Review of the effects of organophosphorus and carbamate insecticides on vertebrates. Are there implications for locust management in Australia? Wildlife Res.. 2001;28:179-193.
- [Google Scholar]
- Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783-1786.
- [Google Scholar]
- Abundance and Breeding of the Common Skittering Frog (Euphlyctis cyanophlyctis) and Bull Frog (Hoplobatrachus tigerinus) at Rawal Lake, Islamabad, Pakistan. Asian Herpetol. Res.. 2011;2:245-250.
- [Google Scholar]
- Proximity to pollution sources and risk of amphibian limb malformation. Environ. Health Perspect.. 2005;113:1497-1501.
- [Google Scholar]
- Morphometric and Gravimetric Parameters of the Rice Frog (Fejervarya limnocharis) Living in Areas with Different Agricultural Activity. J. Environ. Protec.. 2012;3:1403-1408.
- [Google Scholar]
- Population variation and food habits of ranid frogs in the rice-based cropping system in Gujranwala Region, Pakistan. Asian Herpetol. Res.. 2010;1:123-130.
- [Google Scholar]
Appendix A
Morphometric (mm) and gravimetric (gm) measurements (mean ± SE) of female (♀) and male (♂) Common Skittering Frog (Euphlyctis cyanophlyctis) collected from paddy fields and urban wetlands
Urban wetlands ♀
Paddy fields ♀
Urban wetlands ♂
Paddy fields ♂
Sample size
42
41
33
34
Measurements
Body length
SVL
40.21∗∗ ± 1.28
44.34∗∗ ± 1.84
39.67∗ ± 0.98
41.49∗ ± 1.00
∗t = 2.83; df = 32; P < 0.05∗∗t = 2.18; df = 40; P = 0.03†F (1, 148) = 4.90; P = 0.02; Partial η2 = 0.032
Head structures
HW
14.82 ± 0.44
15.51 ± 0.63
14.74 ± 0.36
14.7 ± 0.39
HL
16.44 ± 0.41
17.16 ± 0.60
16.21 ± 0.30
15.73 ± 0.32
Orbital structures
WUE
2.86 ± 0.09
2.95 ± 0.10
3.03 ± 0.10
2.96 ± 0.10
IW
2.52∗ ± 0.05
2.84∗ ± 0.13
2.65 ± 0.11
2.98 ± 0.16
∗t = 2.21; df = 40; P = 0.03†F (1, 148) = 6.78; P = 0.01; Partial η2 = 0.04
Mandible structures
MN
13.64 ± 0.35
13.96 ± 0.50
13.01 ± 0.33
13.34 ± 0.33
MFE
10.53 ± 0.29
11.04 ± 0.45
10.06 ± 0.25
10.32 ± 0.24
MBE
7.19 ± 0.22
7.51 ± 0.37
7 ± 0.19
6.78 ± 0.17
Eye structures
IFE
7.08 ± 0.15
7.45 ± 0.24
7.08 ± 0.16
6.97 ± 0.18
EL
4.10 ± 0.10
4.26 ± 0.12
4.20 ± 0.11
4.25 ± 0.14
SL
6.68∗ ± 0.16
7.25∗ ± 0.23
6.45 ± 0.17
7.14 ± 0.20
∗t = 2.66; df = 40; P = 0.01†F (1, 148) = 9.60; P = 0.002; Partial η2 = 0.061
IBE
9.60 ± 0.20
10.16 ± 0.30
9.59 ± 0.24
9.52 ± 0.20
Nasal structures
IN
1.85∗∗ ± 0.06
3.09∗∗ ± 0.11
2.76∗ ± 0.08
2.89∗ ± 0.10
∗t = -2.91; df = 32; P < 0.05∗∗t = 2.39; df = 40; P = 0.02‡F (1, 122) = 35.03; P < 0.05; Partial η2 = 0.191†F (1, 148) = 8.65; P = 0.004; Partial η2 = 0.05
DBN
2.88 ± 0.08
2.09 ± 0.09
1.74 ± 0.07
2.03 ± 0.07
‡ F (1, 122) = 8.86; P = 0.003; partial η2 = 0.057†F (1, 148) = 46.11; P = 0.00; Partial η2 = 0.23
EN
3.55 ± 0.09
3.76 ± 0.12
3.41 ± 0.08
3.66 ± 0.12
†F (1, 148) = 4.11; P = 0.04; Partial η2 = 0.027
SN
3.12∗∗ ± 0.10
3.49∗∗ ± 0.13
3.06∗ ± 0.09
3.47∗ ± 0.09
∗t = 3.21; df = 32; P < 0.05∗∗t = 3.15; df = 40; P < 0.05†F (1, 148) = 11.98; P = 0.001; Partial η2 = 0.075
Tympanum
TYD
3.14 ± 0.09
3.28 ± 0.14
2.99 ± 0.08
3.28 ± 0.11
TYE
1.65∗ ± 0.06
2.04∗ ± 0.10
1.74 ± 0.10
1.84 ± 0.10
∗t = 3.38; df = 40; P < 0.05†F (1, 148) = 7.15; P = 0.008; Partial η2 = 0.046
Forelimb
FLL
8.23∗∗ ± 0.26
9.18∗∗ ± 0.39
8.02∗ ± 0.21
8.70∗ ± 0.27
∗t = 2.36; df = 32; P = 0.02∗∗t = 2.31; df = 40; P = 0.02†F (1, 148) = 7.18; P = 0.008; Partial η2 = 0.046
HAL
10.68 ± 0.27
11.25 ± 0.41
10.06 ± 0.18
10.62 ± 0.26
Hind limb
FL
19.94 ± 0.58
20.91 ± 0.81
20.15 ± 0.55
20.13 ± 0.51
TL
16.93 ± 0.53
17.64 ± 0.68
17.69 ± 0.43
16.87 ± 0.37
TFOL
30.14 ± 0.80
30.69 ± 1.12
29.51 ± 0.54
29 ± 0.70
FOL
21.91 ± 0.61
22.92 ± 0.88
21.21 ± 0.45
21.82 ± 0.56
BW
8.63∗ ± 1.06
12.45∗ ± 1.56
8.05 ± 0.61
8.38 ± 0.73
∗t = 2.51; df = 40; P = 0.01†F (1, 148) = 4.10; P = 0.04; Partial η2 = 0.027
Liver
WL
0.26 ± 0.03
0.31 ± 0.04
0.22 ± 0.02
0.18 ± 0.02
Kidney
KW
0.05 ± 0.007
0.06 ± 0.008
0.05 ± 0.004
0.05 ± 0.005
∗ & ∗∗ show significant difference (α = 0.05); † multivariate generalized linear model (one-way MANOVA) shows significant difference (α = 0.05) using sites as independent variables, ‡ multivariate generalized linear model shows significant difference (α = 0.05) using sex as independent variables.







