Translate this page into:
Preparation, characterization of CoxMn1−xO2 nanowires and their catalytic performance for degradation of methylene blue
⁎Corresponding author at: Department of Chemistry, Faculty of Science and Education, Taif University, P.O. Box: 888 Postal Code: 5700, Saudi Arabia. khalidgnad@hotmail.com (Khalid Abdelazez Mohamed Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
CoxMn1−xO2 nanowires and microspheres (0.15 ⩽ x ⩽ 0.5) catalysts were synthesized, and their catalytic performance in oxidative degradation of methylene blue (MB) in water under oxygen air bubbles pumping was investigated. X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FT-IR), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HR-TEM) and N2 adsorption–desorption techniques were used to characterize the structure, morphology and SBET of CoxMn1−xO2 nanostructures. Nucleation–dissolution–recrystallization and reduction migration species mechanism was suggested for the growth of the nanowires. The effect of molar ratios of reactants and morphology of products were investigated in terms of MB degradation. The catalyst characterization was performed by mass spectra, chemical oxygen demand (COD), total organic carbon (TOC), the Langmuir and Freundlich isotherms. The results revealed the CoxMn1−xO2 nanowires exhibited excellent catalytic efficiency for the degradation of MB than CoxMn1−xO2 microspheres.
Keywords
CoxMn1−xO2
Nanomaterials
Hydrothermal
Crystal growth
Degradation
1 Introduction
Organic dyes have received particular attention as eminent environmental contaminants because of their non-biodegradability and carcinogenic impacts on humans (Priya et al., 2009). Among organic dyes, MB as a type of cationic dye is widely used in many fields such as dyeing, monitoring and printing. The hazardous effects of MB dye can be a cause for health problems, such as skin irritation, increased heart rate on inhalation and cancer (Choi et al., 2007). Many chemical processes were employed to treat dye from wastewater (Munaf et al., 1997; Aksu and Yener, 1998; Bertoncini et al., 2003; Khalid et al., 2004; Denizli et al., 2005). As one of them, manganese oxide has a great deal of attention to remove organic dye pollutants due to their reactivity with contaminants under environmentally relevant conditions (Chen et al., 2013; Remucal and Ginder-Vogel, 2014; Luo et al., 2015).
Metal dopant material oxide nanostructures are of interest in numerous industrial applications due to their unique and often advantageous properties (Cremades et al., 2014). In particular, the selection of transition metals inserted in the framework of manganese oxides can improve the properties of materials (Brousse et al., 2004; Zhang et al., 2004; Yin et al., 2011; Sawangphruk et al., 2012). The synthesis of metal incorporated nanocrystals has made great progress in the past few years (Heiligtag and Niederberger, 2013). The crystal growth of the nanostructures, an electrostatic interaction between two differently charged ions makes possible the incorporation of cobalt ion into the manganese oxide lattice and to cause the improvement of their catalytic activity with respect to olefin oxidation and degradation of RhB (Lee et al., 2007; Ahmed et al., 2013).
In this work, a one-step hydrothermal synthesis of CoxMn1−xO2 nanowires was carried out through the reduction potassium permanganate with cobalt nitrate under hydrothermal process. The catalytic degradation of MB is investigated in a reflux reactor using CoxMn1−xO2 nanowires under O2-air bubble pumping. The effect of molar ratios of products was estimated in terms of the degradation, TOC and COD removal, catalytic stability, the Langmuir and Freundlich isotherms adsorption of catalysts surface and reaction rate constant were also determined.
2 Experimental method
2.1 Synthesis of CoxMn1−xO2 nanowires
CoxMn1−xO2 were obtained by an in-situ redox precipitation hydrothermal synthesis method. In a typical experiment, 1 mmol of KMnO4 was added to an aqueous solution of 0.5 mmol Co(NO3)2 under magnetic stirring for 10 min. The homogeneous solution was transferred into a 40 mL Teflon-lined stainless steel autoclave, which was subsequently sealed at 140 °C for 18 h. After the desired time, the system was allowed to cool down naturally and the resulting precipitation was collected, washed several times with distilled water and absolute ethanol, centrifuged, and dried under vacuum at 60 °C for 12 h.
2.2 Measurements
The morphology and structures of the samples were characterized using a field emission scanning electron microscope (FEI Sirion, 200, Netherlands). The transmission electron microscopy (TEM) images were investigated using a Tecnai G220, Netherlands. A high-resolution transmission electron microscopic (HR-TEM) image was investigated by JEM-2010 FEF TEM at an acceleration voltage of 200 kV. XRD data were obtained on an X-ray diffractometer (Panalytical X’ Pert Pro; Netherlands). The IR spectrum was recorded with an EQUINOX55, Bruker FT-IR spectrometer within the range 400–4000 cm−1. EDAX Eagle III energy-dispersive micro-XRF (mXRF) spectrometer was employed by Agilent 6510 in positive ionization mode between mass ranges of 50 and 600 Da.
2.3 Test of the catalytic activity
The catalytic degradation of MB process was studied under in reflux route, magnetic stirring, oxygen air bubble pumping and room light (250 lux or 23foot-candle) in three-neck of ground glass. 1 mmol of catalyst powders were replaced in 150 mg/L of MB solution containing. At regular intervals, samples are taken from reactor and the catalytic powder was removed by centrifuging route. Total organic carbon (TOC) was examined by employing a Vario TOC Cube Elementar (Varian). The COD analysis of the degradation dye was obtained by following by potassium dichromate in 50% sulfuric acid solution at reflux temperature. UV–vis spectrophotometer of decomposition of dye was analyzed using a Varian Cary 50 Bio. The degradation rate of MB was estimated by [D% = (1 − At/Ao)/100] equation. The mass spectra were recorded by Agilent 6510 in positive ionization mode between mass ranges of 50–600 Da.
3 Results and discussion
The crystalline phase of CoxMn1−xO2 nanowires was determined by XRD (Fig. 1(a)). Almost diffraction peaks indicated to tetragonal α-MnO2 with lattice parameter of a = 9.7847 and c = 2.8630 nm, space group of I4/m, corresponding to JCPDS card No. 44-141 (Fig. 1(b)). The peaks obtained at 43, 48, 55o is constituted of Co doped in α-MnO2 phase (JCPDS card No. 1-1254). The diffraction peak observed at 23° which can be identified for K-birnessite type of layer structured MnO2 phase (JCPDS 86-666). The EDX analysis of the samples shown in Fig. 1(c) indicates a wire form has Co0.5Mn0.5O2 or CoO–MnO structures. Fig. 2(a) reveals the FT-IR spectra of CoxMn1−xO2 nanowires have the tetrahedral and octahedral sites of Mn–O stretching modes are associated at 626 and 572 cm−1. The peak at 463 cm−1 is attributed to the band-stretching mode of the octahedral sites. Moreover, the peaks at 723 and 1350 cm−1 can be due to the Co stretching vibration. The O–H stretching of water molecules is observed at 1639 and 3432 cm−1. The SBET of CoxMn1−xO2 nanowires were obtained from an analysis of the desorption branch of N2 gas isotherms method. Fig. 2(b) shows that the isotherms are typical for a slightly mesoporous material with a small hysteresis loop at high partial pressures (Sing et al., 1985). The BET surface area of CoxMn1−xO2 nanowires is calculated to be 342 m2/g.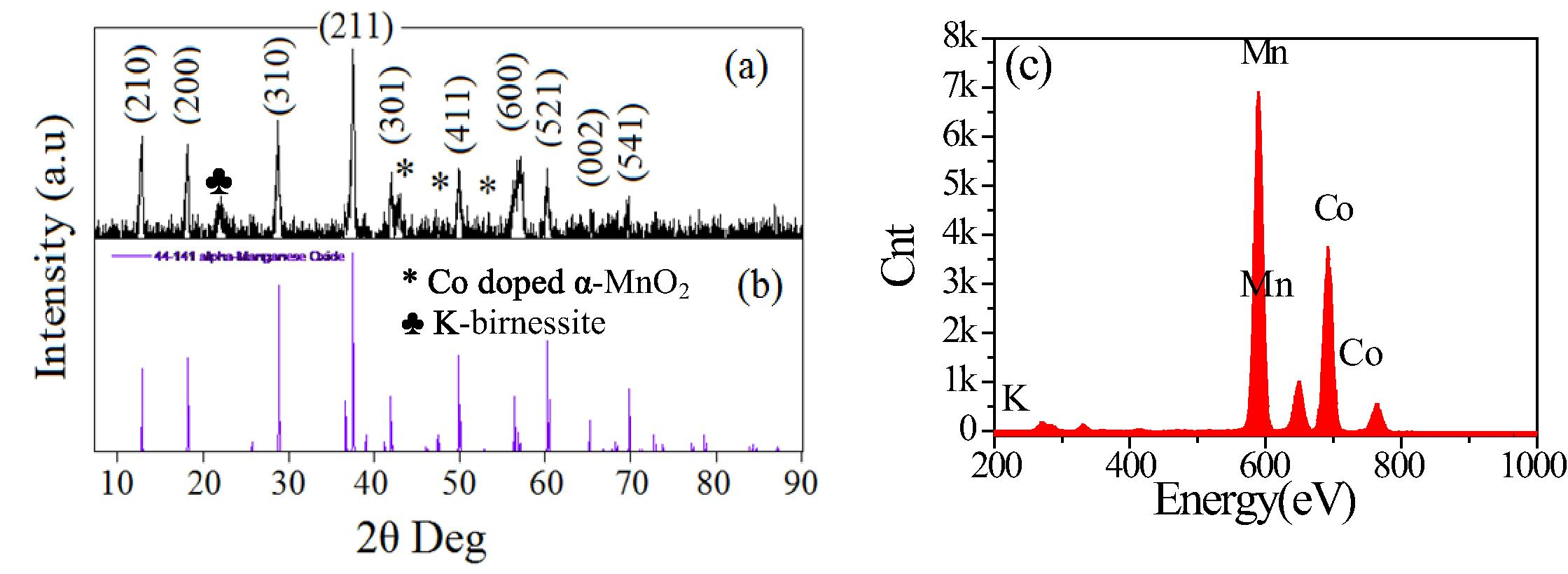
(a) XRD pattern, (b) the standard data from JCPDS card No. 44-0141 and (c) EDAX spectrum of the prepared CoxMn1−xO2 nanowires.
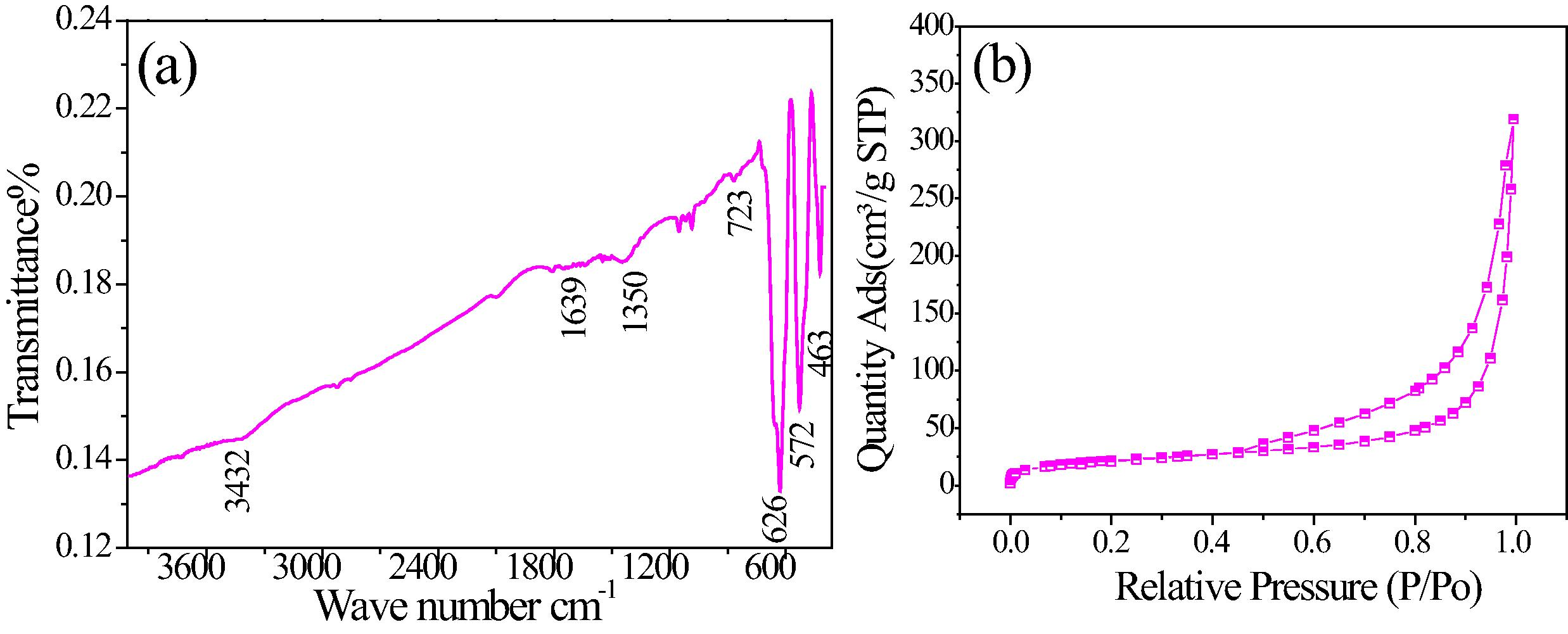
(a) FT-IR spectra and (b) N2 adsorption–desorption isotherm curve of CoxMn1−xO2 nanowires.
FESEM, TEM and HRTEM of the as-synthesized Co0.5Mn0.5O2 sample. Fig. 3(a) is a low magnification, face-on image showing the uniformity of the nanowires. At a high magnification (Fig. 3(b)), the nanowires with typical sizes range from 50 to 80 nm and more than ten micrometers in a length. Fig. 3(c) shows the TEM image of Co0.5Mn0.5O2 nanowire is in agreement with the FE-SEM observation. The corresponding SA-ED pattern (inset in a Fig. 3(c)), shows Co0.5Mn0.5O2 is a single crystal. The HR-TEM image of Co0.5Mn0.5O2 nanowires (Fig. 3(d)) shows the interplanar has a distance of 0.238 nm and miller index of {2 1 1} particle.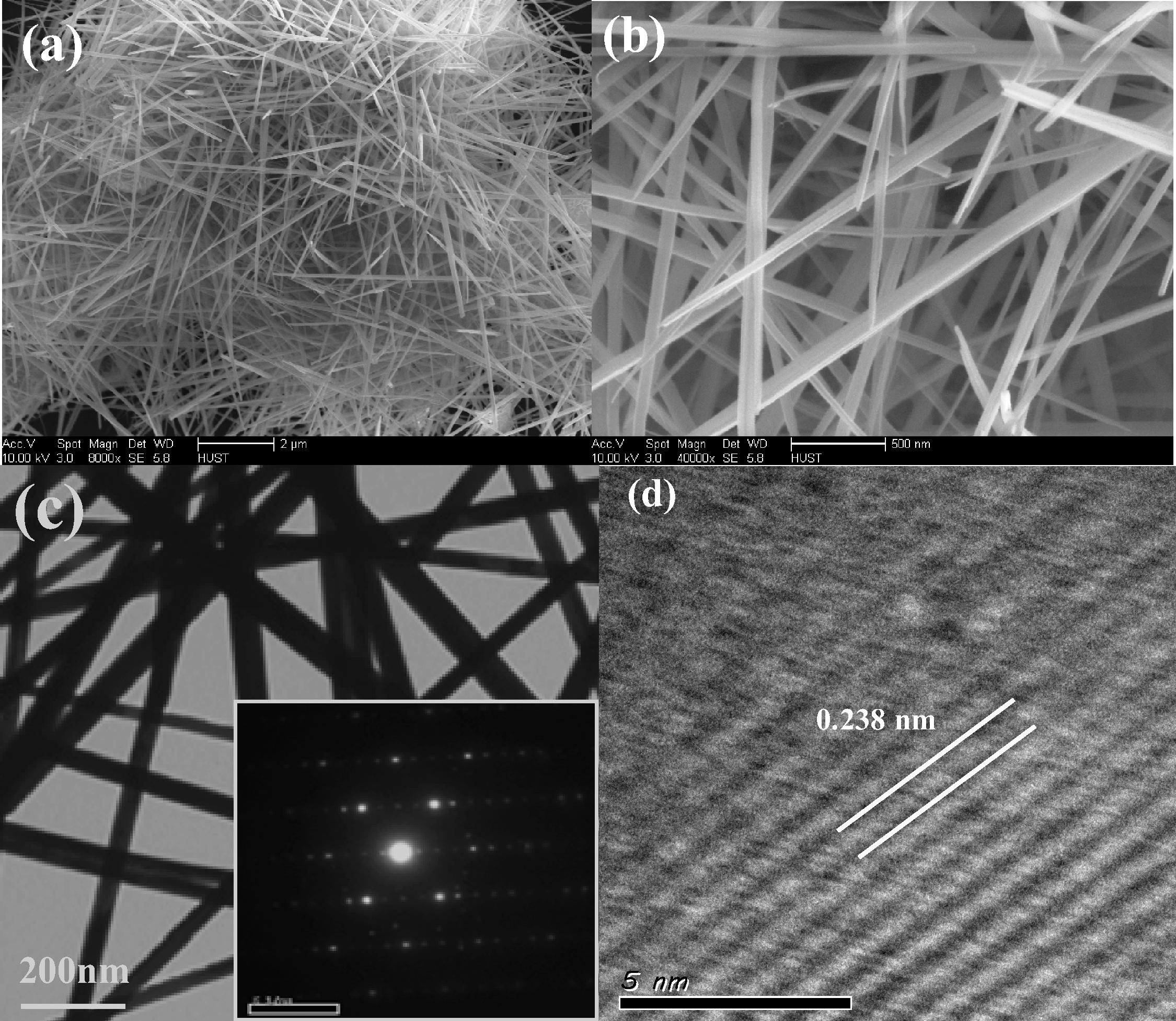
(a) Low-magnification SEM image, (b) high magnification SEM image, (c) TEM image (the inset shows the corresponding SAED pattern) and (d) HR-TEM image of as-prepared CoxMn1−xO2 nanowires by hydrothermal route with Co:Mn molar ratio of 1:2 at 140 °C for 18 h.
In order to obtain a complete view of the CoxMn1−xO2 nanowire formation process and their growth mechanism, the clear time-dependent morphology evolution process from octahedron to tubular shapes was evaluated thoroughly by FE-SEM. At the early reaction stage (4 h), the birnessite particles may be obtained through potassium permanganate reduction species in initial nucleation stage Fig. 4(a). As the reaction proceeded to 8 h, birnessite particles gradually disappeared and the three dimensional nanoflowers of plate surfaces were obtained (Fig. 4(b)). Thus, when the reaction sealed to 12 h, the one dimensional CoxMn1−xO2 nanowires began to grow up out from topic plate-like hierarchical structures (Fig. 4(c)). On the basis of the above results, we hypothesize that the formation of CoxMn1−xO2 nanowires may be obtained by nucleation–dissolution recrystallization process. It is similar to that of CdTe, tungsten bronze, Co3O4 nanowires (Volkov et al., 2004; Liu et al., 2013; Varghese et al., 2007). They believed that the evolution of a wire structure seeded from liquid phase involves fundamentals steps: nucleation and growth. In nucleation step, the birnessite particles may be occurred by KMnO4 reduction with water. With the holding time, the building blocks, the birnessite can be nuclei served as seeds for further growth to form flowerlike structures. Through the dissolution re-crystallization process, the plate-like crystal went to wires and the cobalt substitute of potassium in structures formula.
FE-SEM images of CoxMn1−xO2 fabricated through hydrothermal route using Co:Mn of 1:2 mol ratio at 140 °C for various times (a) 4 h, (b) 8 h and (c) 12 h; (d) Co:Mn molar ratios of 1:4 (inserted of molar ratios of 1:6).
To compare the impact of morphology faces and cobalt constitution on the catalytic efficacy, other CoxMn1−xO2 nanocrystals with different molar ratios have, therefore been prepared. When the reaction precursor performed with Co:Mn molar ratios of 1:4, the microspheres consist of 2D nanoplates with the thickness of 10–20 nm were raised (Fig. 4(d)). Whereas the molar ratio is deficiency to 1:6 (insert of Fig. 4(d)), microspheres composed of needle-like nanostructure assembled to 3D microspheres should be bring out.
4 Catalytic activities
Fig. 5(a) shows the UV–vis spectra absorption of MB before and after degradation of MB catalyzed by CoxMn1−xO2 nanowires at room temperature and natural pH. Before therapy, the MB has contained two peaks of 653 and 281 nm, revealed the visible and UV regions. The visible region is displaced azine linkage contains and UV region is assigned of aromatic rings. When treated by catalyst, the absorption intensity of both peaks is decreased with time. Fig. 5(b) shows the catalytic degradation of MB over O2-air or CoxMn1−xO2 nanowires only and with both catalyst and air, respectively. In absence of a catalyst no degradation occurred (curve I). With present of catalyst and absence air bubbles, the degree of degradation was less than 30% (curve II). However, with the presence of both (catalyst and air), fast and efficient degradation of MB was achieved, and nearly 97% of MB was degraded in 40 min, indicating that MB was degraded in Co0.5Mn0.5O2 nanowire-O2pumping (curve III). In order to investigate the role of room light irradiation in the catalytic degradation of MB by CoxMn1−xO2 nanowires (Fig. S1(a)), we compared the degradation efficiency in the same condition with and without light irradiation. The percentages of degradation results in absent light are very close with light irradiation occurs. The calculate the energy gap of as-prepared CoxMn1−xO2 (Fig. S1(b)), showing the absorption edges of the nanowires, microspheres are around of 540, 560 and 605 nm, respectively. The band gap (Eg) of the samples can be evaluated from the following equation: [αhc⧹λ = A(hc⧹λ − Eg)n/2]; where α, h, c, λ and Eg are absorption coefficient, Planck constant, light velocity, wavelength and band gap energy, respectively. Constants A and n depend on the characteristics of the transition in a semiconductor. The band gap (Eg) of wire phase is 2.00 and microspheres are about 1.8 and 1.7 eV, respectively. To compare the potential environmental impacts of manufactured CoxMn1−xO2 microspheres with molar ratio of 1:4 and 1:6 on MB degradation under same reaction conditions Fig. 5(c). The catalytic studies for the degradation of MB dye over wire morphology have high catalytic activity than microspheres. The reaction kinetics of MB degradation is described by pseudo-first-order as follows: [K = 2.303 log(Ao/At)/t]. Fig. 5(d) shows the rate constant by CoxMn1−xO2 nanowires-O2 system is being about 16 and 19 folds than obtained with CoxMn1−xO2 microspheres due to surface area and catalytic properties with these crystal defects (Franklin et al., 1991). On other hand, the surface characterization results is described by the Freundlich and Langmuir isotherms (Eqs. (1) and (2))
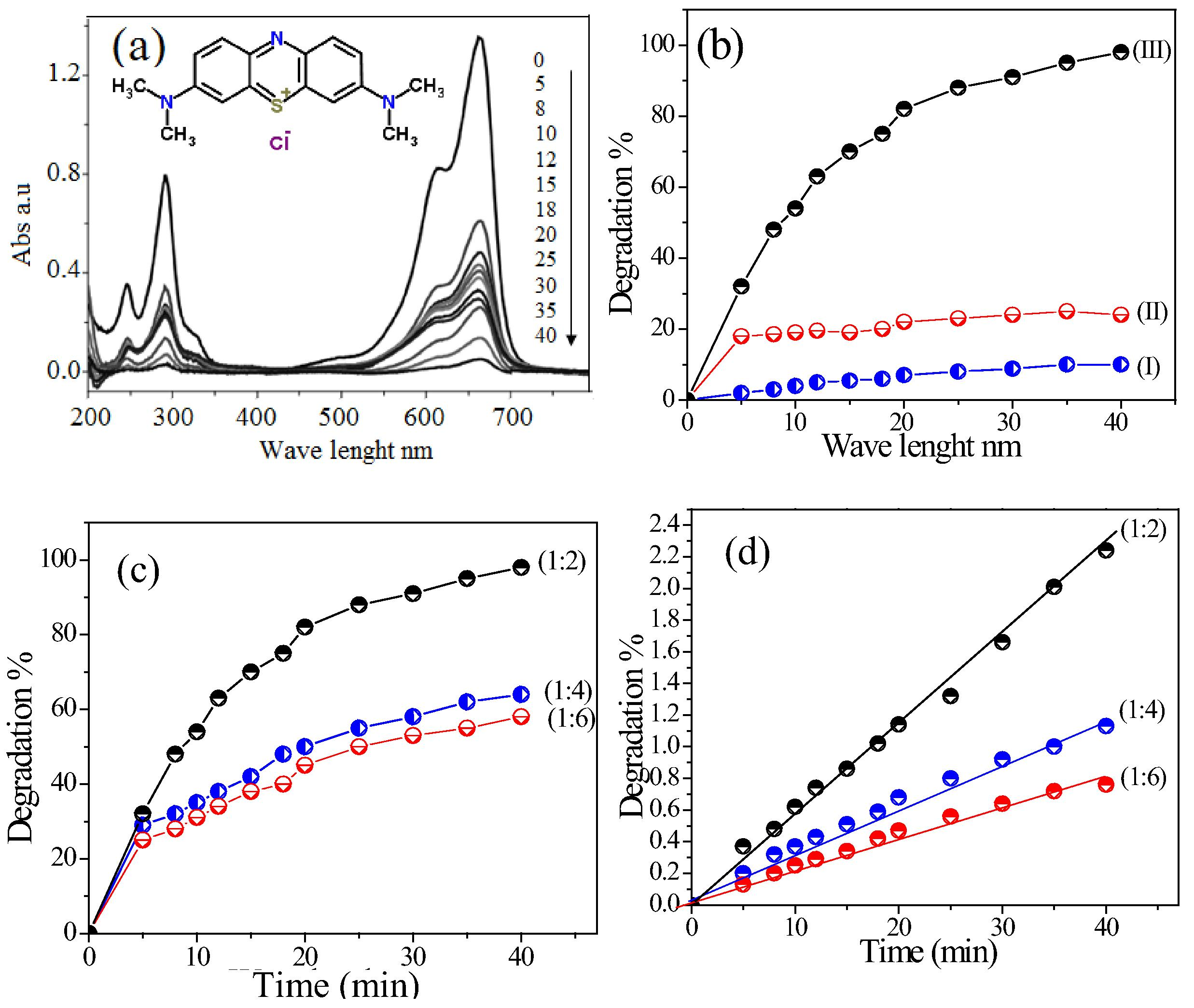
(a) UV–vis absorptions of MB degradation through CoxMn1−xO2 nanowires with times and (b) Degradation% of MB under various conditions: (I) without catalyst; (II) in existence of catalyst and absence of air pumping; (III) with catalyst and O2-air, (c) the degradation and (d) degradation rate constant of MB catalyzed over CoxMn1−xO2 with different Co:Mn molar ratios.
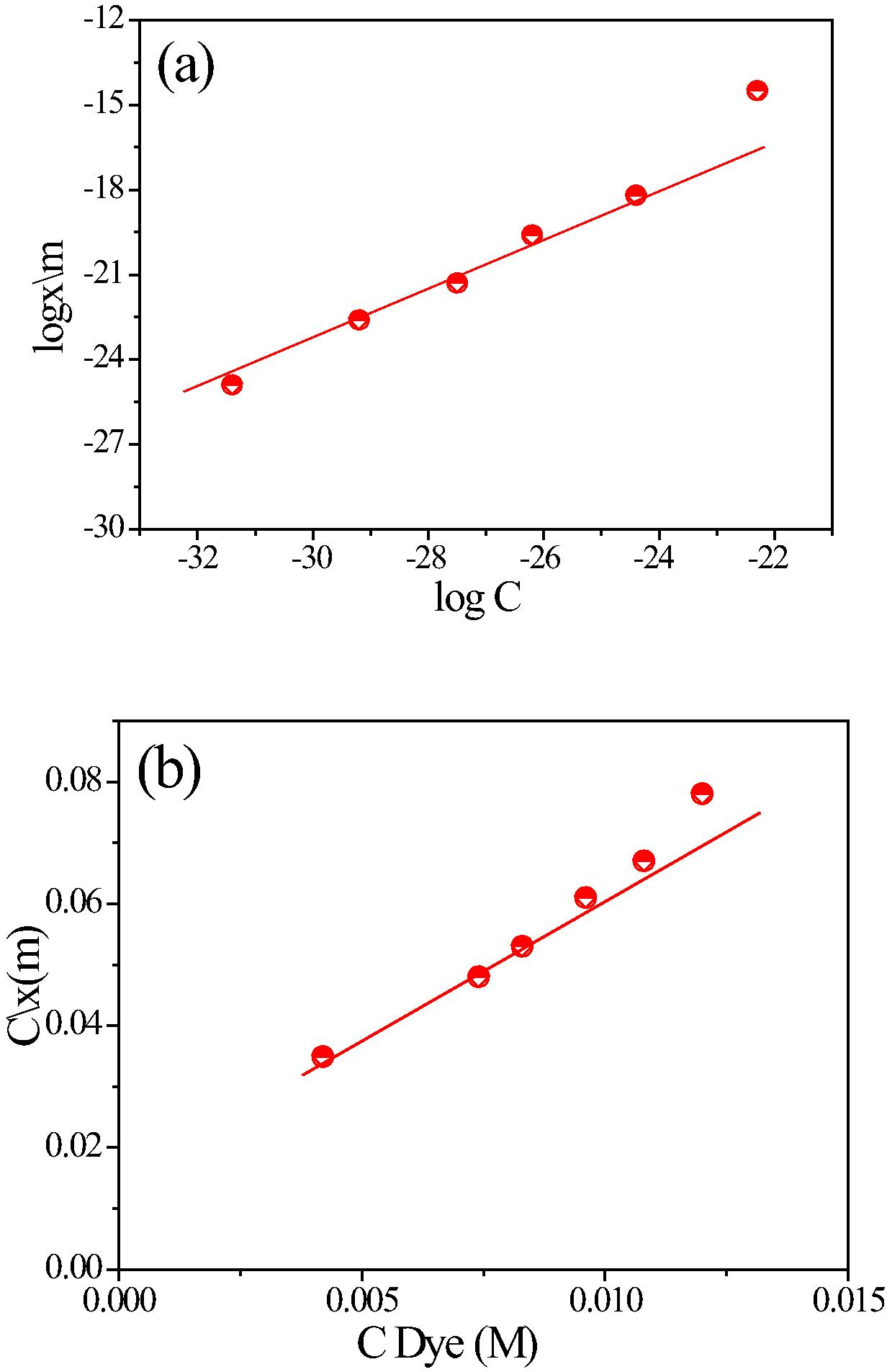
(a) Isotherms of the Langmuir and (b) Freundlich on CoxMn1−xO2 nanowires catalyst surface.
Catalysts
Rate constant (min−1)
Degradation times (min)
References
β-MnO2 nanorods
0.00125
125
Zhang et al. (2006)
α-MnO2 nanorods
0.00312
90
Cao et al. (2010)
α-Mn2O3 nanorods
0.0012
150
Yang et al. (2006)
Mn3O4 octahedral structures
0.0014
180
Zhang et al. (2010)
δ-MnO2-coated montmorillonite
0.0068
720
Zhu et al. (2010)
Co doped α-MnO2 nanowires
0.122
30
This work
Mn-oxide loaded hollow silica particles
0.046
60
Meng et al. (2013)
K-OMS-2
0.0036
120
Sriskandakumar et al. (2009)
Mo-K-OMS-2
0.0076
120
Sriskandakumar et al. (2009)
Fig. 7(a) depicts the mineralization of organic carbon of MB followed by TOC disappearance for CoxMn1−xO2 nanowires catalysis, whereas the catalytic reaction gives first order kinetics with a diversion of 85% MB dye for 40 min. The kinetic curve of COD explained the reduction of MB with time (Fig. 7(b)). Based on the identification of aromatic intermediates by MS spectra (Fig. S2) and TOC removal results, a reasonable reaction pathway for the complete mineralization of MB is postulated by hydroxyl radical process. Recently, they investigated the catalytic reaction of dye solution in oxygen air can be produced of superoxide radicals, hole and hydroxyl radical. (Houas et al., 2001; Gnaser et al., 2005; Rashad et al., 2014). Typical of this mechanism process, sulfoxide group can further react with hydroxyl radical to propagate a sulfone that can afterward undergo a ring-opening reaction. Furthermore, MB in aqueous solution was enriched continuously on the surface of CoxMn1−xO2 nanowires and broken down to NH4+ and SO42−. In order to estimate the stability and reusability, the CoxMn1−xO2 nanowire catalyst was recycled 4 times for the degradation of MB dye in the presence of O2-air pumping (Fig. 8). The catalytic activity of the NWs decreases after each run and only 62.8% of MB dye was degraded in the 4th run.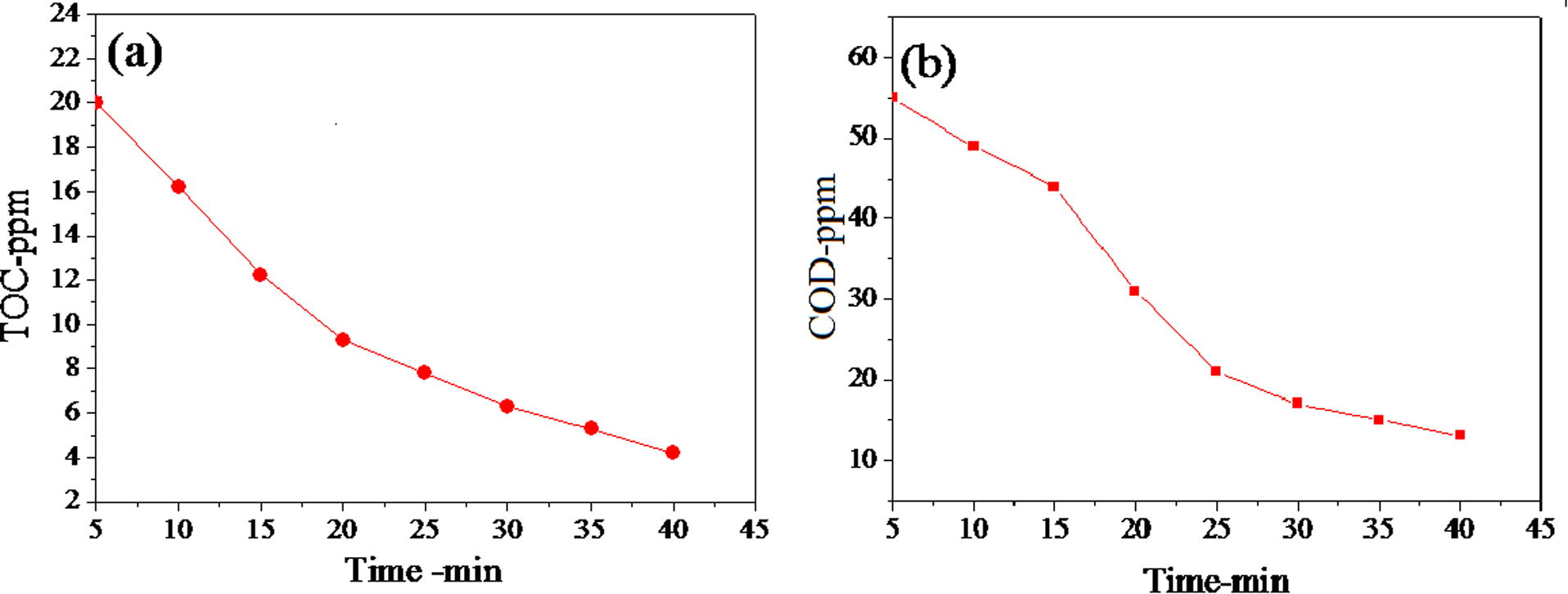
(a) TOC and (b) COD reduction of MB over CoxMn1−xO2 nanowires.
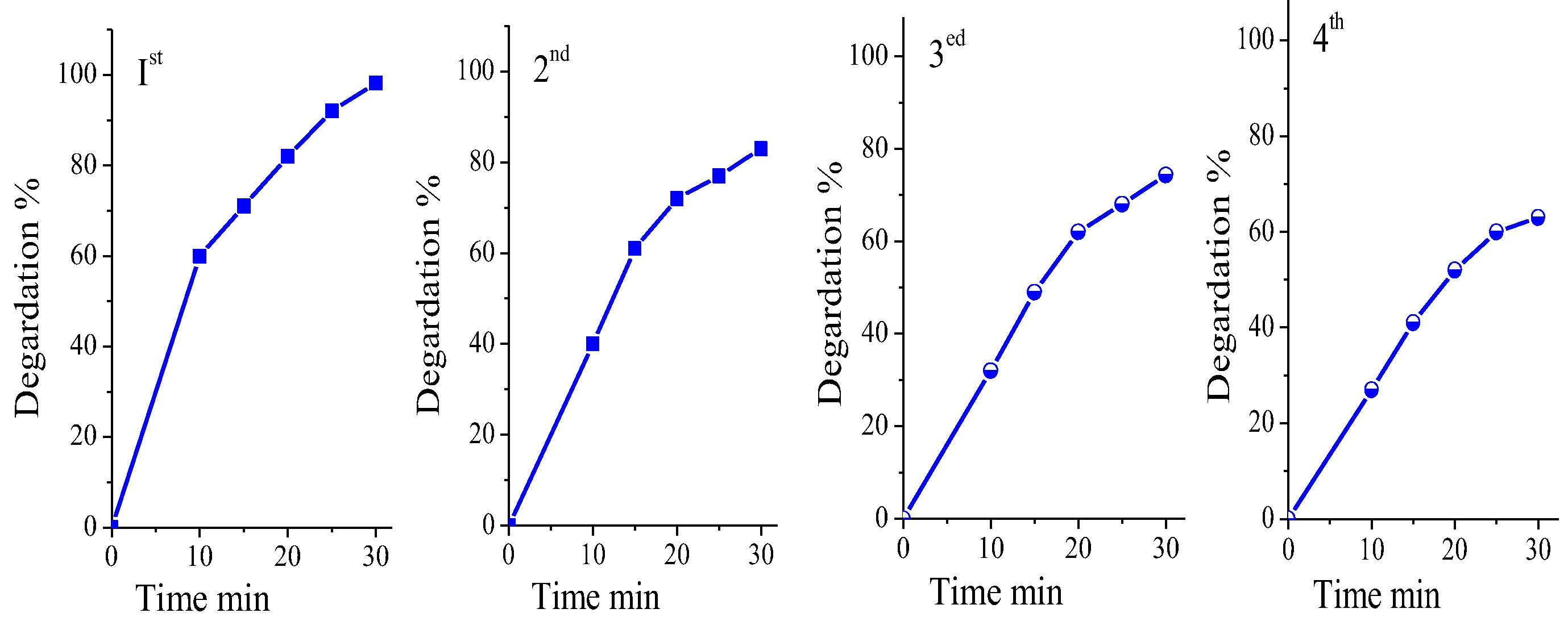
Degradation of MB on CoxMn1−xO2 nanowires catalyst against time at 1, 2, 3 and 4 rounds.
5 Conclusion
The hydrothermal method showed to be fast, simple and efficient for preparing nanosized CoxMn1−xO2 in nanowires phase. Our results revealed that it is possible to control the growth of the nanowires by nucleation–dissolution–recrystallization and reduction mechanism. Degradation MB aqueous solution was completely achieved with CoxMn1−xO2 nanowires, which shows the possible application for water treatment. Investigation of MS spectra, TOC, COD, catalytic stability, the Langmuir and Freund-lich isotherm analysis revealed that the CoxMn1−xO2 nanowires exhibit significantly enhanced catalytic activity. The results showed that the degradation efficiency of methylene blue catalyzed by the hydrothermal products is remarkably enhanced due to Co doping, suggesting that CoxMn1−xO2 nanowires are a good candidate for room-light-driven catalysts.
Acknowledgments
The authors would like to thank the Faculty of Science and Education, Department of Chemistry, Taif University for partially supporting this research and allowing sufficient time to write this article. Also great thanks to faculty from the Analysis and Test Center of Huazhong University of Science and Technology for the technical assistance on characterization (2006CB705606a).
References
- Urchin-like cobalt incorporated manganese oxide OMS-2 hollow spheres: synthesis, characterization and catalytic degradation of RhB dye. Solid State Sci.. 2013;15:66-72.
- [Google Scholar]
- Investigation of biosorption of phenol and monochlorinated phenols on the dried activated sludge. Process Biochem.. 1998;33:649-655.
- [Google Scholar]
- A hybrid activated carbon-manganese dioxide capacitor using a mild aqueous electrolyte. J. Electrochem. Soc.. 2004;151:614A-622A.
- [Google Scholar]
- Hydrothermal synthesis and catalytic properties of α- and β-MnO2 nanorods. Mater. Res. Bull.. 2010;45:425-428.
- [Google Scholar]
- Hierarchically porous MnO2 microspheres with enhanced adsorption performance. J. Mater. Chem. A. 2013;1:11682-11690.
- [Google Scholar]
- Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination. 2007;202:199-206.
- [Google Scholar]
- On the thermal growth and properties of doped TiO2 and In2O3 elongated nanostructures and nanoplates. Phys. B: Condens. Matter. 2014;453:92-99.
- [Google Scholar]
- Removal of chlorophenols from aquatic systems using the dried and dead fungus Pleurotus sajor caju. Bioresour. Technol.. 2005;96:59-62.
- [Google Scholar]
- Stabilisation and catalytic properties of high surface area zirconia. Catal. Today. 1991;10:405-407.
- [Google Scholar]
- Photocatalytic degradation of methylene blue on nanocrystalline TiO2: surface mass spectrometry of reaction intermediates. Int. J. Mass Spectrom.. 2005;245:61-67.
- [Google Scholar]
- Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B: Environ.. 2001;31:145-157.
- [Google Scholar]
- Removal of phenol from water by adsorption using zeolites. Ind. Eng. Chem. Res.. 2004;43:5275-5280.
- [Google Scholar]
- Electrodeposition of manganese and molybdenum mixed oxide thin films and their charge storage properties. Chem. Mater.. 2007;19:5010-5017.
- [Google Scholar]
- Electro-static-induced synthesis of tungsten bronze nanostructures with excellent photo-to-thermal conversion behavior. J. Mater. Chem.. 2013;A1:10120-10129.
- [Google Scholar]
- Manganese oxide octahedral molecular sieve (OMS-2) as an effective catalyst for degradation of organic dyes in aqueous solutions in the presence of peroxymonosulfate. Appl. Catal. B Environ.. 2015;164:92-99.
- [Google Scholar]
- Facile synthesis of manganese oxide loaded hollow silica particles and their application for methylene blue degradation. J. Colloid Interface Sci.. 2013;405:28-34.
- [Google Scholar]
- The use of rice husk for removal of phenol from waste water as studied using 4-aminoantipyrine spectrophotometric method. Environ. Technol.. 1997;18:355-358.
- [Google Scholar]
- LbL fabricated poly(styrene sulfonate)/TiO2 multilayer thin films for environmental applications. ACS Appl. Mater. Interf.. 2009;1:2684-2693.
- [Google Scholar]
- Photocatalytic decomposition of dyes using ZnO doped SnO2 nanoparticles prepared by solvothermal method. Arabian J. Chem.. 2014;7:71-77.
- [Google Scholar]
- A critical review of the reactivity of manganese oxides with organic contaminants. Environ. Sci.: Process. Impacts. 2014;16:1247-1266.
- [Google Scholar]
- Surfactant-assisted electrodeposition and improved electrochemical capacitance of silver-doped manganese oxide pseudocapacitor electrodes. J. Solid State Electrochem.. 2012;2012(16):2623-2629.
- [Google Scholar]
- Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem.. 1985;57:603-619.
- [Google Scholar]
- Green decomposition of organic dyes using octahedral molecular sieve manganese oxide catalysts. J. Phys. Chem. A. 2009;113:1523-1530.
- [Google Scholar]
- Co3O4 nanostructures with different morphologies and their field-Emission properties. Adv. Funct. Mater.. 2007;17:1932-1939.
- [Google Scholar]
- In-situ observation of nanowire growth from luminescent CdTe nanocrystals in a phosphate buffer solution. Chem. Phys. Chem.. 2004;5:1600-1602.
- [Google Scholar]
- Nanorods of manganese oxides: synthesis, characterization and catalytic application. J. Solid State Chem.. 2006;179:679-684.
- [Google Scholar]
- Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3−xO4) for Fenton-Like reaction in water. J. Hazard. Mater.. 2012;296:128-137.
- [Google Scholar]
- Lead adsorption and arsenite oxidation by cobalt doped birnessite. J. Hazard. Mater.. 2011;2011(188):341-349.
- [Google Scholar]
- Anew air electrode based on carbon nanotubes and Ag–MnO2 for metal air electrochemical cells. Carbon. 2004;42:3097-3102.
- [Google Scholar]
- Large-scale synthesis of β-MnO2 nanorods and their rapid and efficient catalytic oxidation of methylene blue dye. Catal. Commun.. 2006;7:408-412.
- [Google Scholar]
- Shape-controlled synthesis of Mn3O4 nanocrystals and their catalysis of the degradation of methylene blue. Nano Res.. 2010;3:235-243.
- [Google Scholar]
- Decolorization of methylene blue by δ-MnO2-coated mont-morillonite complexes: emphasizing redox reactivity of Mn-oxide coatings. J. Hazard. Mater.. 2010;2010(181):57-64.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jksus.2016.11.004.
Appendix A
Supplementary data







